-
PDF
- Split View
-
Views
-
Cite
Cite
Brandon D Pickett, Elizabeth M Wallace, Perry G Ridge, John S K Kauwe, Lingering Taxonomic Challenges Hinder Conservation and Management of Global Bonefishes, Fisheries, Volume 45, Issue 7, July 2020, Pages 347–358, https://doi.org/10.1002/fsh.10438
Close - Share Icon Share
Abstract
Despite expanding research on the popular recreational fishery, bonefish taxonomy remains murky. The genus Albula, comprising these iconic circumtropical marine sportfishes, has a complex taxonomic history driven by highly conserved morphology. Presently, 12 putative species are spread among 3 species complexes. The cryptic morphology hinders visual identification, requiring genetic species identification in some cases. Unclear nomenclature can have unintended consequences, including exacerbating taxonomic uncertainty and complicating resolution efforts. Further, ignoring this reality in publications may erode management and conservation efforts. In the Indian and Pacific oceans, ranges and areas of overlap are unclear, precluding certainty about which species support the fishery and hindering conservation efforts. Species overlap, at both broad and localized spatial scales, may mask population declines if one is targeted primarily (as demonstrated in the western Atlantic fishery). Additional work is necessary, especially to increase our understanding of spatiotemporal ecology across life history stages and taxa. If combined with increased capacity to discern between cryptic species, population structure may be ascertained, and fisheries stakeholders will be enabled to make informed decisions. To assist in such efforts, we have constructed new range maps for each species and species complex. For bonefishes, conservation genomic approaches may resolve lingering taxonomic uncertainties, supporting effective conservation and management efforts. These methods apply broadly to taxonomic groups with cryptic diversity, aiding species delimitation and taxonomic revisions.

BACKGROUND
Bonefish (Albulidae) Albula spp. are tropical, marine, benthivorous fish found principally in sand flats, sea grasses, and mangroves. They are characterized by an inferior mouth with the snout extending beyond the mandible (Hildebrand 1963; Datovo and Vari 2014; Figure 1). Although bonefish are a source of food in some parts of the world (Breder 1948; Scott and Scott 1988), the principal interests to humans are fishing and tourism as bonefish are prized sportfish, since they are elusive and difficult to land. The sportfishing tourism industry for bonefish in the Bahamas was estimated at US$141 million (Fedler 2010), while the flats fishery (bonefish and other flats species) in the Florida Keys was estimated at $465 million (Fedler 2013). Despite a culture, sometimes enforced by law, of catch‐and‐release fishing (Adams and Cooke 2015; Adams 2016), bonefish catch rates appear to be declining around the globe (Friedlander and Rodgers 2008; Santos et al. 2019a). Preserving bonefish diversity and the flats fisheries depends on increasing our understanding of each species’ ecology and life history; however, most research has focused on a single species, Albula vulpes (Linnaeus 1758). In part, this is a result of the complicated taxonomy that is currently under revision. Much of the difficulty emanates from several cryptic species—species that are effectively impossible to discern visually due to high morphological similarity. After providing a brief background in bonefish life history and ecology, global depletions of bonefish populations, and cryptic species, we discuss bonefish taxonomic history and the resulting implications for conservation and management.
Illustration of Albula vulpes. Copyright Diane Rome Peebles, used with permission.
Ecology and Life History
Bonefish are circumtropical shorefish with an interesting life history. Although the bulk of our knowledge comes from A. vulpes and is, in some cases, based on a single site or region, most characteristics and behaviors may be similar across the genus, except perhaps for the A. nemoptera complex. Additional research for all species, including A. vulpes, is still required to fill in the gaps in our understanding of bonefish spatiotemporal ecology.
Like all elopomorphs, bonefishes spend time in development as transparent, ribbon‐like larvae called leptocephali (Hollister 1939; Rasquin 1955; Inoue et al. 2004). The leptocephali feed principally on plankton as they grow in length to about 6–9 cm (Hollister 1936; Pfeiler 1984; Vásquez‐Yeomans et al. 2009). Exact pelagic larval duration may vary considerably across taxa (Pfeiler et al. 1988), however in A. vulpes ranges 41–71 days (Mojica et al. 1994; Adams and Cooke 2015). They then undergo a fascinating metamorphosis in which they shrink to about 2 cm, resulting in individuals reaching the same length three times during development. During the approximately 10‐day metamorphosis, the leptocephalus transitions to a miniature of the adult form (Hollister 1936; Pfeiler 1984). Pre‐metamorphic larvae have some swimming capacity; however, considering ocean currents, they may disperse hundreds of kilometers away from their spawning site (Zeng et al. 2019).
Post‐metamorphic larvae move into shallower water to utilize mangroves and estuaries as nurseries for 2–4 years. Evidence from Florida (USA) and Cuba, based on A. vulpes and A. sp. cf. vulpes (Wallace and Tringali 2010), suggests that juveniles prefer the less saline waters in estuaries compared to the more saline environment of the flats where adults are typically found (Santos et al. 2019b). However, Channel Bonefish A. goreensis (Cuvier and Valenciennes 1847) appears to also utilize more exposed beach habitat (Haak et al. 2019), and preferred juvenile habitat for other species is unknown. The juvenile diet consists primarily of amphipods and carideans, though diet analyses are limited (Griffin et al. 2019). Despite the importance of early life history to population stability and resilience (Lefcheck et al. 2019), relatively little is known of juvenile behavior and ecology.
Adults grow to lengths of 100 cm (Scott and Scott 1988) and up to 8 kg in weight (Robins and Ray 1986), though size reports vary among species and locations; a typical adult is probably half as long and heavy (Donovan et al. 2015; Kamikawa et al. 2015). Bonefish lifespans can extend past 20 years, though the average is shorter (Posada et al. 2008). Their diet consists primarily of mollusks and crustaceans, but other benthic fauna is not unusual (Warmke and Erdman 1963; Colton and Alevizon 1983; Liston et al. 2013). Some evidence suggest they forage nomadically, changing location every few days (Ault et al. 2008), though they have high site fidelity for a general area (Murchie et al. 2013; Boucek et al. 2019; Moxham et al. 2019). In A. vulpes, spawning migrations of varied distances (over 80 km documented) occur October through May (Murchie et al. 2015), sometimes near the full or new moons (Adams et al. 2019). Large pre‐spawning aggregations with hundreds to thousands of fish form in relatively shallow water, and they then move to deep water drop‐offs at dusk to spawn (Danylchuk et al. 2011, 2019). Though other bonefishes may exhibit similar spawning behaviors to A. vulpes, timing likely varies across taxa, and reproductive ecology has not been evaluated in other species. This information is important for conservation and management globally, as pre‐spawning aggregations are vulnerable to harvest and coastal migratory corridors are susceptible to human disturbance.
Relative to A. vulpes, the literature on the ecology and life history of other bonefish species is sparse. Differences have been identified between species complexes and some individual species. Of particular importance is research to determine fishery species composition at local scales in areas of known species overlap and further elucidate spawning behaviors and locations for species supporting fisheries. Without this fundamental information, population declines within a particular fishery (i.e., island or nation) may be masked due to the presence of cryptics, and conservation efforts may be confounded due to interspecific variability.
Population Declines
Decreases in bonefish catch rates and instances of shifting baselines have been reported around the globe. However, accurate data from all relevant components of the fishery (recreational catch‐and‐release, subsistence harvest, and targeted and incidental commercial harvest) are often lacking. Anthropogenic habitat loss is suspected as the primary contributor to population declines in most areas, but exploitation in under‐regulated fisheries is also a significant problem (Bunce et al. 2008; Adams et al. 2012; Filous et al. 2019a). Even in catch‐and‐release fisheries, the negative impact to the target species may be larger than previously thought (Dallas et al. 2010; Raby et al. 2014; Brownscombe et al. 2015; Cook et al. 2015), and recent research has focused on understanding and mitigating the effects of catch‐and‐release practices (Hannan et al. 2015; Adams 2016; Brownscombe et al. 2017). Regardless of the precise cause, The International Union for the Conservation of Nature (IUCN) Red List of Threatened Species reports Roundjaw Bonefish A. glossodonta (Forsskål 1775) as “Vulnerable,” A. vulpes as “Near Threatened,” and Eastern Pacific Bonefish A. esuncula (Garman 1899) as “Least Concern” (Nielsen et al. 2010; Adams et al. 2014). Six other species are listed as “Data Deficient” and the remaining three have not yet been evaluated (see Table 1). Insufficient data is clearly a bottleneck for ecological work with most bonefish species. Yet, even for A. vulpes, where information is relatively plentiful, data is still deficient to determine (1) how much population decline is caused by overfishing as opposed to anthropogenic habitat loss and (2) which species in the A. vulpes species complex may be most vulnerable (Adams et al. 2014). Indeed, information is not available for many areas and species, but available data does raise concerns: (1) catch rates are decreasing in the southwestern Indian Ocean and the Florida Keys according to fishers (Bunce et al. 2008; Frezza and Clem 2015; Santos et al. 2019a); (2) demand from recreational tourist fishers is increasing in the Bahamas (Danylchuk et al. 2008); (3) data from the National Oceanic and Atmospheric Administration Marine Recreational Information Program suggest population declines in the western Atlantic Ocean (National Marine Fisheries Service, Fisheries Statistics Division, personal communication); (4) data from Hawai'i's Department of Land and Natural Resources/Division of Aquatic Resources and the United States Fish Commission demonstrate precipitous declines in landings in Hawaiian waters (Friedlander and Rodgers 2008); and (5) unsustainable fishing practices and extirpation of spawning groups have been documented in the southern Pacific Ocean (Johannes and Yeeting 2000; Ram‐Bidesi 2011; Ram‐Bidesi and Petaia, 2010). The clear consensus is that population declines are occurring; the uncertainties are to what extent they are occurring, specific causes, and which species are at the highest risk.
Taxonomic and conservation statuses of each bonefish species. All species, except A. sp. cf. vulpes, are recognized in Eschmeyer's Catalog of Fishes (Fricke et al. 2019). Near Threatened, Vulnerable, Least Concern, and Data Deficient are formal classifications of the International Union for the Conservancy of Nature (IUCN); the term Unevaluated indicates the IUCN has not yet evaluated the status of that species. Common names all include bonefish (e.g., Smallscale Bonefish)
| Scientific name | Common name | Taxonomic status | Conservation status |
| Albula argentea complex | |||
| A. argentea (Forster in Bloch and Schneider 1801) | NA | Described species | Data Deficient |
| A. oligolepis (Hidaka et al. 2008) | Smallscale | Described species | Data Deficient |
| A. virgata (Jordan and Jordan 1922) | NA | Described species | Data Deficient |
| Albula nemoptera complex | |||
| A. nemoptera (Fowler 1911) | Threadfin | Described species | Data Deficient |
| A. pacifica (Beebe 1942) | Pacific Shafted | Described species | Unevaluated |
| Albula vulpes complex | |||
| A. vulpes (Linnaeus 1758) | Bonefish | Described species | Near Threatened |
| A. glossodonta (Forsskål 1775) | Roundjaw | Described species | Vulnerable |
| A. esuncula (Garman 1899) | Eastern Pacific | Described species | Least Concern |
| A. sp. cf. vulpes (Wallace and Tringali 2010) | NA | Provisional species | Unevaluated |
| A. koreana (Kwun and Kim 2011) | Korean | Described species | Data Deficient |
| A. gilberti (Pfeiler et al. 2011) | Cortez | Described species | Unevaluated |
| A. goreensis (Cuvier and Valenciennes 1847) | Channel | Described species | Data Deficient |
| Scientific name | Common name | Taxonomic status | Conservation status |
| Albula argentea complex | |||
| A. argentea (Forster in Bloch and Schneider 1801) | NA | Described species | Data Deficient |
| A. oligolepis (Hidaka et al. 2008) | Smallscale | Described species | Data Deficient |
| A. virgata (Jordan and Jordan 1922) | NA | Described species | Data Deficient |
| Albula nemoptera complex | |||
| A. nemoptera (Fowler 1911) | Threadfin | Described species | Data Deficient |
| A. pacifica (Beebe 1942) | Pacific Shafted | Described species | Unevaluated |
| Albula vulpes complex | |||
| A. vulpes (Linnaeus 1758) | Bonefish | Described species | Near Threatened |
| A. glossodonta (Forsskål 1775) | Roundjaw | Described species | Vulnerable |
| A. esuncula (Garman 1899) | Eastern Pacific | Described species | Least Concern |
| A. sp. cf. vulpes (Wallace and Tringali 2010) | NA | Provisional species | Unevaluated |
| A. koreana (Kwun and Kim 2011) | Korean | Described species | Data Deficient |
| A. gilberti (Pfeiler et al. 2011) | Cortez | Described species | Unevaluated |
| A. goreensis (Cuvier and Valenciennes 1847) | Channel | Described species | Data Deficient |
Taxonomic and conservation statuses of each bonefish species. All species, except A. sp. cf. vulpes, are recognized in Eschmeyer's Catalog of Fishes (Fricke et al. 2019). Near Threatened, Vulnerable, Least Concern, and Data Deficient are formal classifications of the International Union for the Conservancy of Nature (IUCN); the term Unevaluated indicates the IUCN has not yet evaluated the status of that species. Common names all include bonefish (e.g., Smallscale Bonefish)
| Scientific name | Common name | Taxonomic status | Conservation status |
| Albula argentea complex | |||
| A. argentea (Forster in Bloch and Schneider 1801) | NA | Described species | Data Deficient |
| A. oligolepis (Hidaka et al. 2008) | Smallscale | Described species | Data Deficient |
| A. virgata (Jordan and Jordan 1922) | NA | Described species | Data Deficient |
| Albula nemoptera complex | |||
| A. nemoptera (Fowler 1911) | Threadfin | Described species | Data Deficient |
| A. pacifica (Beebe 1942) | Pacific Shafted | Described species | Unevaluated |
| Albula vulpes complex | |||
| A. vulpes (Linnaeus 1758) | Bonefish | Described species | Near Threatened |
| A. glossodonta (Forsskål 1775) | Roundjaw | Described species | Vulnerable |
| A. esuncula (Garman 1899) | Eastern Pacific | Described species | Least Concern |
| A. sp. cf. vulpes (Wallace and Tringali 2010) | NA | Provisional species | Unevaluated |
| A. koreana (Kwun and Kim 2011) | Korean | Described species | Data Deficient |
| A. gilberti (Pfeiler et al. 2011) | Cortez | Described species | Unevaluated |
| A. goreensis (Cuvier and Valenciennes 1847) | Channel | Described species | Data Deficient |
| Scientific name | Common name | Taxonomic status | Conservation status |
| Albula argentea complex | |||
| A. argentea (Forster in Bloch and Schneider 1801) | NA | Described species | Data Deficient |
| A. oligolepis (Hidaka et al. 2008) | Smallscale | Described species | Data Deficient |
| A. virgata (Jordan and Jordan 1922) | NA | Described species | Data Deficient |
| Albula nemoptera complex | |||
| A. nemoptera (Fowler 1911) | Threadfin | Described species | Data Deficient |
| A. pacifica (Beebe 1942) | Pacific Shafted | Described species | Unevaluated |
| Albula vulpes complex | |||
| A. vulpes (Linnaeus 1758) | Bonefish | Described species | Near Threatened |
| A. glossodonta (Forsskål 1775) | Roundjaw | Described species | Vulnerable |
| A. esuncula (Garman 1899) | Eastern Pacific | Described species | Least Concern |
| A. sp. cf. vulpes (Wallace and Tringali 2010) | NA | Provisional species | Unevaluated |
| A. koreana (Kwun and Kim 2011) | Korean | Described species | Data Deficient |
| A. gilberti (Pfeiler et al. 2011) | Cortez | Described species | Unevaluated |
| A. goreensis (Cuvier and Valenciennes 1847) | Channel | Described species | Data Deficient |
| Species | Other applied names | Distribution |
| Albula argentea complex | ||
| Albula argentea (Forster in Bloch and Schneider 1801) | A. forsteri, A. neoguinaica | Western and central Pacific |
| Albula oligolepis (Hidaka et al. 2008) | A. sp. D | Indian and western Pacific |
| Albula virgata (Jordan and Jordan 1922) | A. neoguinaica | Hawai‘i |
| Albula nemoptera complex | ||
| Albula nemoptera (Fowler 1911) | A. sp. E, Dixonina nemoptera | Western Atlantic and Caribbean |
| Albula pacifica (Beebe 1942) | A. nemoptera | Tropical eastern Pacific |
| Albula vulpes complex | ||
| Albula vulpes (Linnaeus 1758) | NA | Western Atlantic and Caribbean |
| Albula glossodonta (Forsskål 1775) | NA | Indian, western and central Pacific |
| Albula esuncula (Garman 1899) | A. sp. C, A. neoguinaica | Tropical eastern Pacific, southern Gulf of California |
| Albula sp. cf. vulpes Wallace and Tringali (2010) | A. sp. F | Western Atlantic and Caribbean |
| Albula koreana (Kwun and Kim 2011) | NA | Western Pacific (East China Sea) |
| Albula gilberti (Pfeiler et al. 2011) | A. sp. A | Eastern Pacific, Gulf of California |
| Albula goreensis (Cuvier and Valenciennes 1847) | A. sp. B, A. garcia, A. nova sp. | Tropical Atlantic and Caribbean |
| Species | Other applied names | Distribution |
| Albula argentea complex | ||
| Albula argentea (Forster in Bloch and Schneider 1801) | A. forsteri, A. neoguinaica | Western and central Pacific |
| Albula oligolepis (Hidaka et al. 2008) | A. sp. D | Indian and western Pacific |
| Albula virgata (Jordan and Jordan 1922) | A. neoguinaica | Hawai‘i |
| Albula nemoptera complex | ||
| Albula nemoptera (Fowler 1911) | A. sp. E, Dixonina nemoptera | Western Atlantic and Caribbean |
| Albula pacifica (Beebe 1942) | A. nemoptera | Tropical eastern Pacific |
| Albula vulpes complex | ||
| Albula vulpes (Linnaeus 1758) | NA | Western Atlantic and Caribbean |
| Albula glossodonta (Forsskål 1775) | NA | Indian, western and central Pacific |
| Albula esuncula (Garman 1899) | A. sp. C, A. neoguinaica | Tropical eastern Pacific, southern Gulf of California |
| Albula sp. cf. vulpes Wallace and Tringali (2010) | A. sp. F | Western Atlantic and Caribbean |
| Albula koreana (Kwun and Kim 2011) | NA | Western Pacific (East China Sea) |
| Albula gilberti (Pfeiler et al. 2011) | A. sp. A | Eastern Pacific, Gulf of California |
| Albula goreensis (Cuvier and Valenciennes 1847) | A. sp. B, A. garcia, A. nova sp. | Tropical Atlantic and Caribbean |
| Species | Other applied names | Distribution |
| Albula argentea complex | ||
| Albula argentea (Forster in Bloch and Schneider 1801) | A. forsteri, A. neoguinaica | Western and central Pacific |
| Albula oligolepis (Hidaka et al. 2008) | A. sp. D | Indian and western Pacific |
| Albula virgata (Jordan and Jordan 1922) | A. neoguinaica | Hawai‘i |
| Albula nemoptera complex | ||
| Albula nemoptera (Fowler 1911) | A. sp. E, Dixonina nemoptera | Western Atlantic and Caribbean |
| Albula pacifica (Beebe 1942) | A. nemoptera | Tropical eastern Pacific |
| Albula vulpes complex | ||
| Albula vulpes (Linnaeus 1758) | NA | Western Atlantic and Caribbean |
| Albula glossodonta (Forsskål 1775) | NA | Indian, western and central Pacific |
| Albula esuncula (Garman 1899) | A. sp. C, A. neoguinaica | Tropical eastern Pacific, southern Gulf of California |
| Albula sp. cf. vulpes Wallace and Tringali (2010) | A. sp. F | Western Atlantic and Caribbean |
| Albula koreana (Kwun and Kim 2011) | NA | Western Pacific (East China Sea) |
| Albula gilberti (Pfeiler et al. 2011) | A. sp. A | Eastern Pacific, Gulf of California |
| Albula goreensis (Cuvier and Valenciennes 1847) | A. sp. B, A. garcia, A. nova sp. | Tropical Atlantic and Caribbean |
| Species | Other applied names | Distribution |
| Albula argentea complex | ||
| Albula argentea (Forster in Bloch and Schneider 1801) | A. forsteri, A. neoguinaica | Western and central Pacific |
| Albula oligolepis (Hidaka et al. 2008) | A. sp. D | Indian and western Pacific |
| Albula virgata (Jordan and Jordan 1922) | A. neoguinaica | Hawai‘i |
| Albula nemoptera complex | ||
| Albula nemoptera (Fowler 1911) | A. sp. E, Dixonina nemoptera | Western Atlantic and Caribbean |
| Albula pacifica (Beebe 1942) | A. nemoptera | Tropical eastern Pacific |
| Albula vulpes complex | ||
| Albula vulpes (Linnaeus 1758) | NA | Western Atlantic and Caribbean |
| Albula glossodonta (Forsskål 1775) | NA | Indian, western and central Pacific |
| Albula esuncula (Garman 1899) | A. sp. C, A. neoguinaica | Tropical eastern Pacific, southern Gulf of California |
| Albula sp. cf. vulpes Wallace and Tringali (2010) | A. sp. F | Western Atlantic and Caribbean |
| Albula koreana (Kwun and Kim 2011) | NA | Western Pacific (East China Sea) |
| Albula gilberti (Pfeiler et al. 2011) | A. sp. A | Eastern Pacific, Gulf of California |
| Albula goreensis (Cuvier and Valenciennes 1847) | A. sp. B, A. garcia, A. nova sp. | Tropical Atlantic and Caribbean |
Cryptic Species
In bonefishes, the presence of morphologically cryptic species creates challenges to conservation and management (Colborn et al. 2001; Pfeiler et al. 2002; Wallace and Tringali 2016). Correct identification of cryptic species is a prerequisite to examinations of biogeographic and ecological processes as well as conservation applications (Jörger and Schrödl 2013). Cryptic species are relatively widespread, and their recognition is generally considered nontrivial (Bickford et al. 2007; Trontelj and Fišer 2009; Reist et al. 2013). Black basses Micropterus spp. and charrs Salvelinus spp., iconic sportfishes themselves, are similarly under active taxonomic revision (Reist et al. 2013; Taylor et al. 2019). The conservation and management challenges for any group with cryptic species are inherently similar. In bonefishes, the presence of cryptic species and broadly overlapping ranges make it very difficult to determine the species composition in various fisheries. Occurrences of secondary contact (Pfeiler et al. 2008b) and hybrids (Wallace and Tringali 2016; Rennert et al. 2019) have been documented among bonefish. While the extent and frequency of hybrids are unknown, they further challenge efforts to understand bonefish relationships and ecology. Unsurprisingly, Albula is too often described as monotypic and placeholder names are perpetuated after formal descriptions have updated the terms for a given species (Galdino Brandão et al. 2016; Joshi et al. 2016; Abdussamad 2017). Without distinguishing between cryptic species of bonefish in areas of overlap, conservation and management decisions will remain difficult. Increased understanding of spatiotemporal ecology for the various life stages and ability to discriminate between the various cryptic species are necessary to discern population structure and make effective policy decisions.
TAXONOMIC HISTORY
Bonefish were initially described by Linnaeus (1758) as Albula vulpes. Twenty‐three independent discoveries of bonefish were described under various names, but they were eventually synonymized into a circumglobal A. vulpes by 1940 (Whitehead 1986; Colborn et al. 2001; Bowen et al. 2008) as no significant characters were able to consistently delineate species (Hildebrand 1963). However, a second bonefish species, Threadfin Bonefish A. nemoptera (Fowler 1911), was recognized at this time; it is both rarely encountered by anglers due to its deep water habitat and easily distinguished by an elongated caudal ray of the dorsal fin (Fowler 1911; Rivas and Warlen 1967). This new status quo was later broken by Shaklee and Tamaru (1981), when they demonstrated by molecular analysis that two species of bonefish are present in Hawaiian waters, A. glossodonta and A. neoguinaica (Cuvier and Valenciennes 1847). Albula neoguinaica was subsequently renamed to A. forsteri (Bloch and Schneider 1801) and then A. argentea (Forster in Bloch and Schneider 1801; see Bowen et al. 2008 for further details). Colborn et al. (2001) confirmed and extended the results of Shaklee and Tamaru's study with additional molecular analyses, screening 174 specimens from 26 globally distributed sites for a portion of the mitochondrial DNA (mtDNA) cytochrome b gene. They concluded that the three species (A. vulpes, A. glossodonta, and A. neoguinaica [now A. argentea]) are distinct and that up to five additional species may be present, which they labeled as A. spp. A–E. Since then, these and additional species have been described resulting in 12 putative species spread across 3 species complexes (see Table 2 for a summary of species names and distributions and Figures 3–6 and Supplementary Figures 1–16 for maps of their distributions).
Some morphological traits enable distinction between the complexes, but expertise is usually required. The currently accepted phylogeny, based on portions of the mtDNA cytochrome b gene, is represented in Figure 2. The three complexes form distinct clades, with the A. vulpes and A. argentea complexes as sisters relative to the A. nemoptera complex. Given the currently accepted relationships (Figure 2), we summarize each of the three complexes. Note that we are not reviewing the two deep water bonefish species in the genus Pterothrissus. See Wallace (2014) for a discussion on whether Pterothrissus belongs in the order Albuliformes and Hidaka et al. (2017) for more recent taxonomic reclassification.
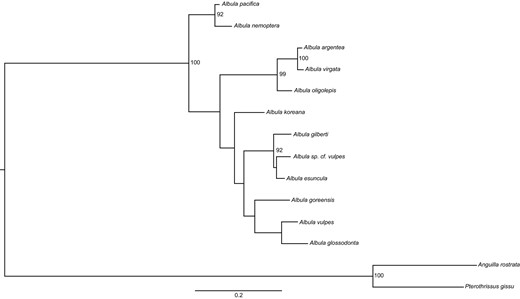
Relationships among all species of Albula. Tree topology was inferred using RAxML (Stamatakis 2014) with a portion of the cytochrome b mitochondrial gene. Branch lengths represent sequence divergence between taxa, and bootstrap support values are shown when above 90%. For additional details, see Wallace (2014). A text‐based version of the tree can be found in Supplementary File 1.
Albula argentea Complex
Bonefish in the A. argentea complex are distributed throughout the Indian Ocean and western and central Pacific Ocean (Pfeiler et al. 2011; Wallace 2014; Figure 4; Supplementary Figures 1–4). This species complex is well reviewed by Hidaka et al. (2008). In brief, the complex is comprised of three species: A. argentea, Smallscale Bonefish A. oligolepis (Hidaka et al. 2008), and Longjaw Bonefish A. virgata (Jordan and Jordan 1922). The species in this complex were resurrected from synonymy with A. vulpes, beginning with Shaklee and Tamaru's study (1981). The Hawaiian specimens they identified as A. neoguinaica are now known as A. virgata as a result of Hidaka et al. (2008); their work clarified A. forsteri as a junior synonym of A. argentea, accounting for the non‐endemic specimens that Shaklee and Tamaru (1981) identified as A. neoguinaica. Albula oligolepis was described as a new species in the same paper (Hidaka et al. 2008). These are distinct from A. glossodonta (in the A. vulpes complex), whose range overlaps in the Indian and western Pacific oceans (Supplementary Figure 10), due to molecular differences and because A. oligolepis has a more pointed lower jaw. All species in the A. argentea complex share this trait relative to those in the A. vulpes complex. Albula oligolepis is A. sp. D from Colborn et al. (2001).
Albula nemoptera Complex
The Threadfin Bonefish A. nemoptera was first described by Fowler (1911) in the genus Dixonina, but later synonymized with Albula (Rivas and Warlen 1967). The range for the species in this complex is the western Atlantic and eastern Pacific oceans and they are typically found in deeper water (often in estuaries [Robins and Ray 1986]) than bonefish in the A. argentea and A. vulpes complexes (Bowen et al. 2008; Figure 5; Supplementary Figures 5–7). Albula nemoptera spp. (A. sp. E from Colborn et al. 2001) are further distinguished by shorter total length, elongated anal fin and caudal ray of the dorsal fin, mouth reaching a point below the eye, small scales, and a few differences in dentition and meristic characters (Rivas and Warlen 1967; Robins and Ray 1986). The western Atlantic Ocean form is designated A. nemoptera, and the Pacific Shafted Bonefish A. pacifica (Beebe 1942) is the eastern Pacific Ocean form (Pfeiler et al. 2006; Pfeiler 2008). Based on cytochrome b sequence data, they were designated sister species (Pfeiler 2008); additional nuclear gene sequence data supports this (Wallace 2014). We will discuss neither A. nemoptera nor A. pacifica further in this review as they are easily distinguished morphologically from other bonefish and not the target of a large sportfishing industry.
Albula vulpes Complex
Bonefish in the A. vulpes complex can be found around the globe (Figure 6; Supplementary Figure 8). Presently, seven species are recognized: A. vulpes, A. glossodonta, A. esuncula, A. sp. cf. vulpes, Korean Bonefish A. koreana (Kwun and Kim 2011), Cortez Bonefish A. gilberti (Pfeiler et al. 2011), and A. goreensis.
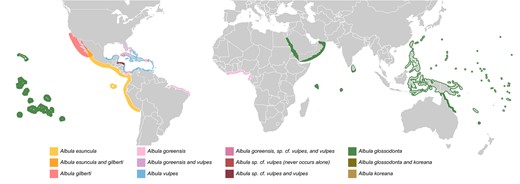
Distribution map of species in the Albula vulpes species complex. A non‐specific map showing this complex can be found in Supplementary Figure 8. Individual maps for each species can be found in Supplementary Figures 9–15. A subset of this map showing only A. esuncula and A. gilberti may be found in Supplementary Figure 16. Please see the note on distribution maps.
Bonefish A. vulpes
This is the original bonefish, described by Linnaeus (1758), with which all other species were synonymized by 1940 (Whitehead 1986; Colborn et al. 2001; Bowen et al. 2008). As additional species were later recognized or resurrected, the range of this species has decreased from worldwide to only the Caribbean, Gulf of Mexico, and western Atlantic Ocean (Wallace 2014; Supplementary Figure 9).
Roundjaw Bonefish A. glossodonta
Albula glossodonta was identified in Hawaiian waters by Shaklee and Tamaru (1981) based on molecular data. It possesses the largest range of any bonefish species, encompassing the Indian Ocean and western and central Pacific Ocean (Wallace 2014; Supplementary Figure 10). Recent studies suggest that A. glossodonta individuals are larger, on average, than A. vulpes (Donovan et al. 2015). Though lifespan and spawning timing have not been evaluated across it’s extensive range, current data suggest similar lifespan and seasonality to A. vulpes (Filous et al. 2019b).
Eastern Pacific Bonefish A. esuncula
Albula esuncula occurs in the eastern Pacific Ocean; it was previously identified as A. sp. C in Colborn et al. (2001) and later clarified in Pfeiler et al. (2008a). Its range stretches south to Panama and reaches north to Sinaloa, Mexico, where it occurs sympatrically with A. gilberti (Supplementary Figures 11 and 16). Albula gilberti (A. sp. A in Colborn et al. 2001) is found northward in the Gulf of California, stretching south to Sinaloa, Mexico. Thus, these two species occur principally in parapatry, except in the southern Gulf of California, where they are found in sympatry. Albula esuncula was formally described by Pfeiler et al. (2011) as a necessary step in the description of A. gilberti. They are morphological cryptics; however, they may be distinguished genetically (Pfeiler et al. 2008a; Díaz‐Viloria et al. 2017).
A. sp. cf. vulpes
Continuing the nomenclature of Colborn et al. (2001), A. sp. F was postulated as another species by Valdez‐Moreno et al. (2010). Further identification was then provided by Wallace and Tringali (2010) and the species is presently referred to by the placeholder A. sp. cf. vulpes. A formal description is forthcoming. This species is a morphological cryptic of A. vulpes; it's range is the western Atlantic Ocean, Gulf of Mexico, and Caribbean (Wallace and Tringali 2010; Wallace 2014; Supplementary Figure 12).
Korean Bonefish A. koreana
This species was described by Kwun and Kim (2011) after morphological and molecular comparison with A. argentea; it has a restricted range in the southern Sea of Japan and East China Sea (Supplementary Figure 13). They differ based on vertebrae count and tooth patch distributions on the parasphenoid and mesopterygoid bones. Molecular differences (nuclear and mitochondrial) were also identified (Kwun et al. 2011; Wallace 2014).
Cortez Bonefish A. gilberti
Albula gilberti occurs in the eastern Pacific Ocean (previously A. sp. A from Colborn et al. 2001). Its range extends northward in the Gulf of California, stretching south around Sinaloa, Mexico—where it is sympatric, likely through secondary contact, with A. esuncula (Pfeiler et al. 2008b; Supplementary Figures 14 and 16).
Channel Bonefish A. goreensis
Wallace (2014) resurrected A. goreensis, a morphological cryptic, from synonymy with A. vulpes. Albula goreensis is A. sp. B from Colborn et al. (2001) and has previously been referred to as A. garcia (Bowen et al. 2008; Valdez‐Moreno et al. 2010; Galdino Brandão et al. 2016). Its range extends across the tropical western and eastern Atlantic Ocean, Gulf of Mexico, and the Caribbean (Whitehead 1990; Bowen et al. 2008; Wallace 2014; Supplementary Figure 15). Recent work suggests A. goreensis adults are smaller than A. vulpes and may occupy a different hydrodynamic niche (Haak et al. 2019; Rennert et al. 2019).
A Note on Distribution Maps
We generated new distribution maps for each of the bonefish species. Much of this information was derived from the IUCN reports, when available. The remaining information resulted from sieving the literature and the personal knowledge of the authors. Deviations from IUCN reported ranges are based on genetically verified collections. While uncertainties exist, these maps represent the best information currently available regarding bonefish species ranges. The full extent of ranges remains unknown for many species—absence on a map indicates no recorded and genetically verified collections. In areas with appropriate habitat, bonefish may occur there—we simply lack data. Alternately, the coastline of a country may be indicated, though appropriate bonefish habitat likely has a patchy distribution. Further, the exact width of highlighted areas is not intended to carry meaning—highlighted areas are simply wide enough to see easily. In some areas, the highlighted width is thinner to avoid overlapping other areas. All maps were generated by hand using Adobe Illustrator CC 2019 (https://adobe.ly/3blIK16); native vector graphics files are available in multiple formats on The Open Science Framework at https://osf.io/j4ksw/.
CONSERVATION AND MANAGEMENT IMPLICATIONS
Pursuing the goals of conserving bonefish diversity and ensuring the long‐term sustainability of recreational fisheries is a complicated challenge. For the global fishery, a primary impediment is the dearth of necessary biological and ecological information. Bonefish taxonomy remains under active revision, many life history and ecological traits are unknown, and the presence of cryptics creates additional conservation challenges. The focus of this review has been the current state of the taxonomic revisions, which have been hampered by divergent lineages with highly conserved morphology. The difficulties regarding species identification have also impeded our understanding of basic life history characteristics and behaviors. Recent research suggests differences between (1) cryptic species in the western Atlantic Ocean and Caribbean Sea (Adams et al. 2008; Haak et al. 2019; Rennert et al. 2019) and (2) cryptic species in the Pacific Ocean (Donovan et al. 2015). However, life history traits for many taxa remain unknown.
Research efforts have broached topics such as juvenile habitat (Szekeres 2017; Santos et al. 2019b), energy dynamics (Murchie et al. 2011; Szekeres et al. 2014; Nowell et al. 2015), spawning (Luck et al. 2019; Mejri et al. 2019a, 2019b), habitat use (Brownscombe et al. 2019) and threats (Steinberg 2015; Cissell and Steinberg 2019; Sweetman et al. 2019), migration (Murchie et al. 2015; Boucek et al. 2019; Perez et al. 2019), anthropogenic exploitation (Filous et al. 2019a), leptocephalus larval dispersion (Zeng et al. 2019), gear restriction (Donovan et al. 2016), light pollution (Szekeres et al. 2017), and local ecological knowledge (Kamikawa et al. 2015; Rehage et al. 1096; Santos et al. 2019a). Research efforts have begun to expand beyond A. vulpes, especially into A. glossodonta. Nevertheless, additional research is still needed; of principle importance is understanding species composition of fisheries at local scales.
Future Directions
The continuation of research efforts on the aforementioned variety of topics in fisheries around the globe is crucial, as is clarifying the taxonomic status of bonefishes. The designation of species and evolutionarily significant units provides the necessary foundation for conservation efforts and protections afforded through the Endangered Species Act, IUCN Red List, and Convention on International Trade in Endangered Species of Wild Fauna and Flora. Taxonomic clarity can further aid prioritization of conservation and management actions, given the realities of increasing anthropogenic ecosystem alterations and limited resources for conservation. Since relatively few morphological characters are capable of distinguishing between only some species, bonefish research will continue to require a large genetic component. Identification has routinely been accomplished based on mitochondrial cytochrome b sequence identity (Colborn et al. 2001; Pfeiler et al. 2002, 2006; Pfeiler 2008; Valdez‐Moreno et al. 2010; Kwun and Kim 2011; Kwun et al. 2011; Wallace 2014, 2015; Díaz‐Viloria et al. 2017), though some bonefishes may also be identified using microsatellite markers (Seyoum et al. 2008; Wallace 2015; Wallace and Tringali 2016). To resolve interspecific relationships, a robust phylogenetic analysis of the family will require more data because single‐gene methods—especially from mtDNA—provide an incomplete picture of evolutionary history (Pamilo and Nei 1988; Nichols 2001; Song et al. 2008). A multi‐locus approach, especially at the whole‐genome or transcriptome scale, would improve confidence in species delimitation and could provide higher‐resolution insights into population structure.
In combination with other biological and ecological studies, genetic/genomic approaches can illuminate a wide range of biodiversity issues necessary for conservation goals at population, species, and higher taxonomic levels. Remaining information needs regarding how bonefish species are distributed, such as evolutionarily significant units, species delimitation, stock identification, adaptation, bottlenecks, introgressive hybridization, and phylogenetic relationships, can be addressed with advanced genomics techniques. To meet these needs, pooled sequencing of specimens will allow the identification of orders of magnitude more markers and will help assess variation and perform accurate identification. In addition, at least one assembled and annotated genome from each species complex would be a valuable resource and would facilitate additional research on Elopomorpha (Breder and Rosen 1966; Chen et al. 2015). Efforts are currently underway with the goal of improved ability to identify species and further study the life history and ecology of the various bonefish species.
Further, protection of presumed endangered species of bonefish is impossible without a multidisciplinary approach. Albula glossodonta, Red List Vulnerable and targeted by consumptive fisheries, may be at greatest risk of regional extirpation and many others in the genus remain data deficient. Larger‐scale genetic or genomic analyses may provide key information necessary to make important management decisions. Conservation of bonefishes must include actions at multiple spatial and temporal scales. Effectively managed reserves (e.g., spawning sites) play an important role; however, additional consideration must be given to migration corridors, as well as larval settlement and juvenile nursery habitats—all of which will vary among species. These areas extend beyond the scale practical for formal reserve status and will require proactive management largely focused on mitigation of coastal habitat degradation. As we learn more about the distinct larval settlement and juvenile nursery habitat requirements among sympatric bonefishes, it will aid comprehensive and proactive habitat protections and mitigation efforts. Habitat conservation efforts will necessarily include limitations on coastal development. In consumptive fisheries, determination of sustainable harvest levels and enforcement of regulations remain high priorities. Clarification of taxonomic status, species boundaries, and areas of overlap are foundational to all of these directed conservation efforts.
Ultimately, fisheries managers and conservationists remain in a quandary over bonefish preservation until additional data are obtained. Presently, 12 putative species are distributed across 3 species complexes. The geographic extent, size, and species composition of global fisheries remains unelucidated. Studies with higher‐density genetic variation data from populations around the globe will greatly aid clarification of relationships among these iconic sportfishes. Such approaches are invaluable conservation tools, especially among sympatric cryptic species. These methods will assist ongoing bonefish conservation efforts, and similar genomic techniques will aid species and population delineation in other groups containing morphological cryptics.
ACKNOWLEDGMENTS
We thank Derek Olthuis, Jocelyn Curtis‐Quick, and Christopher Haak for providing photos. Suggestions that improved this manuscript were provided by two anonymous reviewers. We also thank Diane Rome Peebles for providing the illustration. There is no conflict of interest declared in this article.
References
Supplementary data
Supplementary Figure 5. Distribution map of the A. nemoptera species complex. A map showing each of the species in this complex can be found in Figure 5. Individual maps for each species can be found in Supplementary Figures 6 and 7. To see how the distribution of this complex compares with other complexes, see Figure 3. Please see the note on distribution maps.
Supplementary Figure 8. Distribution map of the A. vulpes species complex. A map showing each of the species in this complex can be found in Figure 6. Individual maps for each species can be found in Supplementary Figures 9–15. To see how the distribution of this complex compares with other complexes, see Figure 3. Please see the note on distribution maps.
Supplementary Figure 11. Distribution map of A. esuncula. View Supplementary Figure 16 to see the areas of sympatry and parapatry with A. gilberti. To see how the distribution of A. esuncula compares with other species in the A. vulpes species complex, see Figure 6. Please see the note on distribution maps.
Supplementary Figure 14. Distribution map of A. gilberti. View Supplementary Figure 16 to see the areas of sympatry and parapatry with A. esuncula. To see how the distribution of A. gilberti compares with other species in the A. vulpes species complex, see Figure 6. Please see the note on distribution maps.
Supplementary Figure 16. Distribution map of A. esuncula and A. gilberti. This map shows the approximate areas of sympatry and parapatry between these two species. View Supplementary Figures 11 and 14 to see individual maps for these species. To see how the distribution of A. esuncula and A. gilberti compares with other species in the A. vulpes species complex, see Figure 6. Please see the note on distribution maps.






