-
PDF
- Split View
-
Views
-
Cite
Cite
Mossaab Maaloum, Cheikh Ibrahima Lo, Sokhna Ndongo, Marine Makoa Meng, Rachid Saile, Stéphane Alibar, Didier Raoult, Pierre-Edouard Fournier, Ottowia massiliensis sp. nov., a new bacterium isolated from a fresh, healthy human fecal sample, FEMS Microbiology Letters, Volume 369, Issue 1, 2022, fnac086, https://doi.org/10.1093/femsle/fnac086
Close - Share Icon Share
Abstract
The culturomics method enabled isolation of a new member of the Ottowia genus from the stool sample of a healthy volunteer. Strain Marseille-P4747T exhibited a 96.18% 16S rRNA sequence identity with Ottowia beijingensis strain GCS-AN-3 (NR_133803.1), the closest species with standing in nomenclature. It is a Gram-stain-negative, nonmotile, and aerobic bacterium. It does not possess catalase and oxidase activities. Its genome has a size of 2 830 447 bp and a G + C content of 63.5 mol%. Based on the phylogenic, phenotypic, and genomic analyses, we conclude that Ottowia massiliensis sp. nov. is a new species, represented by Marseille-P4747T ( = CSUR P4747 = CECT 30348) as type strain.
Abbreviations
- COG
Clusters of orthologous groups
- CSUR
Collection de Souches de l'Unité des Rickettsies (Rickettsia Unit Strain Collection)
- FAME
Fatty acid methyl ester
- GC/MS
Gas chromatography/mass spectrometry
- MALDI-TOF MS
Matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry
- RPS-BLAST
Reverse position specific BLAST
Introduction
The description of the genus Ottowia dates back to 2004 when Spring et al. (2004) proposed Ottowia thiooxydans as a type species. Taxonomically, the genus Ottowia belongs to the family Comamonadaceae, described by Willems et al. in 1991 (Willems et al. 1991). Members of this genus are defined as Gram-stain-negative, nonmotile, and rod-shaped cells. Most Ottowia species contain C16:1ω6c, C16:1ω7c, C16:0, and C18:1ω6c, C18:1ω7c as the major fatty acids.
At the moment of writing, the genus Ottowia includes six species with validly published names (https://lpsn.dsmz.de/search?word = ottowia), including Ottowia pentelensis (Felföldi et al. 2011), Ottowia beijingensis (Cao et al. 2014), and O. thiooxydans (Spring et al. 2004), isolated from activated sludge, Ottowia flava (Shi et al. 2019) isolated from fish intestines, Ottowia oryzae (Heo et al. 2018) isolated from a Korean traditional rice beverage and Ottowia konkukae (Yi et al. 2018) isolated from rotten biji.
In this article, we describe a new strain of the genus Ottowia isolated from human feces. Strain Marseille-P4747 is closely related to the O. beijingensis type strain, GCS-AN-3, with which it displays a 96.18% 16S rRNA sequence identity. We, therefore, suggest strain Marseille-P4747T as type strain from a new Ottowia species for which the name Ottowia massiliensis sp. nov. is proposed.
Materials and methods
Organism information and collection
Strain Marseille-P4747T was isolated from the fecal microbiome of a healthy donor at the Méditerrannée-Infection institute (Marseille, France) as part of the MEGAGUT culturomics project. The project consists of exploring and studying the composition of the human microbiome using the culturomics approach. The patient provided informed and written consent, and the stool sample was frozen at −80°C. The study was authorized by the IHU Méditerranée-Infection ethics committee under number 2016–011.
Strain isolation and identification by MALDI-TOF MS and 16S rRNA sequencing
Strain Marseille-P4747T was isolated on 5% sheep blood-enriched Columbia agar (bioMérieux, Marcy l'Etoile, France) after 2 weeks of preincubation at 37°C in an aerobic blood culture bottle containing 5% sheep blood and 5% sterile-filtered rumen fluid (bioMérieux),
Grown colonies were subcultured separately for purification for 3 days in an aerobic atmosphere at 37°C and studied using MALDI-TOF mass spectrometry (MALDI-TOF MS) type Microflex LT (Bruker Daltonics, Leipzig, Germany) as described by Seng et al. (2009). The collected spectra were then analyzed and compared against those included in the local database (https://www.mediterranee-infection.com/urms-data-base). As MALDI-TOF MS did not correctly identify the colonies, 16S rRNA was amplified and sequenced as previously reported (Drancourt et al. 2000). Subsequently, a 16S rRNA-based phylogenetic analysis of strain Marseille-P4747T and closely related species was conducted in MEGA X (Kumar et al. 2018) using the Maximum Likelihood method and Tamura–Nei model (Tamura and Nei 1993), sequences were aligned using CLUSTALW (Thompson et al. 1994). Initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura–Nei model, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites.
Bacterial morphology and growth conditions
The morphology of strain Marseille-P4747T was observed by spreading the isolate directly on microscopy slides. Slides were then processed to images acquisition, where some of them were not stained and others were stained with PTA (phosphotungstic acid 1%) in order to check any difference or morphological changes. The sample was then sputtered with a 5-µm thick platinum layer (ion sputter MC1000) to reduce charging of the imaged samples. To evaluate bacterial structures, we used a scanning electron microscope (SEM, Hitachi SU5000; Figure S1, Supporting Information).
The ability of strain Marseille-P4747 to grow was tested on 5% sheep blood-enriched Columbia agar (bioMérieux) at various temperatures (4, 10, 25, 28, 30, 37, and 42°C), then in different atmospheres [aerobic, anaerobic, and microaerophilic (Becton Dickinson, Le Pont de Claix, France)], varied pH conditions (5, 6, 6.5, 7.5, 8.5, and 9), and NaCl concentrations (0, 10, 25, 50, 75, and 100 g/l).
Phenotypic and chemotaxonomic features
Biochemical characteristics of strain Marseille-P4747 were tested using the API Rapid 32A, API 50 CH and API ZYM strips (bioMérieux) according to the manufacturer’s instructions. Susceptibility to antibiotics was tested using E-test strips (bioMérieux) by studying the Minimal Inhibitory Concentrations (MICs) of benzylpenicillin, oxacillin, amoxicillin, ampicillin, ceftriaxone, imipenem, gentamicin, amikacin, tobramycin, ciprofloxacin, ofloxacin, erythromycin, clindamycin, colistin, doxycycline, fosfomycin, nitrofurantoin, and trimethoprim-sulfamethoxazole as recommended by EUCAST (Citron et al. 1991). Cellular fatty acid methyl ester (FAME) analysis was performed by GC/MS. A total of two samples were prepared with approximately 40 mg of bacterial biomass per tube harvested from several culture plates. FAMEs were prepared as previously described by Sasser (2006). GC/MS analyses were carried out as previously described (Dione et al. 2016). Briefly, FAMES were separated using an Elite 5-MS column and monitored by mass spectrometry (Clarus 500–SQ 8 S, Perkin Elmer, Courtaboeuf, France). A spectral database search was performed using MS Search 2.0 with the Standard Reference Database 1A (NIST, Gaithersburg, USA) and the FAME mass spectral database (Wiley, Chichester, UK).
Genome extraction, sequencing, and analysis
The genomic DNA (gDNA) of O. massiliensis was extracted using the EZ1 biorobot (Qiagen, Hilden, Germany) with the EZ1 DNA Tissue kit and then normalized by a Qubit assay with the high sensitivity kit (Life Technologies, Carlsbad, CA, USA) to 0.2 ng/µl. gDNA was then sequenced on the MiSeq Technology (Illumina, San Diego, CA, USA) with the paired-end strategy and barcoded to be mixed respectively with 21 other genomic projects prepared with the Nextera XT DNA sample prep kit (Illumina).
To prepare the paired-end library, dilutions were performed to obtain 1 ng of each genome as input. DNA was fragmented and tagged at the tagmentation step. Then, limited cycle PCR amplification (12 cycles) completed the tag adapters and introduced dual-index barcodes. After purification on AMPure XP beads (Beckman Coulter, Fullerton, CA, USA), libraries were normalized on specific beads according to the Nextera XT protocol (Illumina). Normalized libraries were pooled into a single library for sequencing on the MiSeq sequencer. The pooled single strand library was loaded onto the reagent cartridge and then onto the instrument along with the flow cell. Automated cluster generation and paired-end sequencing with dual index reads were performed in a single 39-hour run in 2 × 250 bp.
Total information of 10.5 Gb was obtained from a 1160 K/mm2 cluster density, with a cluster passing quality control filters of 91.7%. Within this run, the index representation for O. massiliensis was determined to 5%. The 22 101 000 paired-end reads were filtered and trimmed according to the read qualities. The Spades program (version 3.14.1) was used with default parameters for the assembly (Bankevich et al. 2012).
Annotation was performed using the Prokaryotic Genome Annotation Pipeline (PGAP; Tatusova et al. 2016) proposed by NCBI as part of the RefSeq (Reference Sequence) project (Haft et al. 2018). Protein sequences were predicted using NCBI’s Reverse Position Specific BLAST (RPS-BLAST; Yang et al. 2020) algorithm against the clusters of orthologous groups (COG; Tatusov et al. 2001) database from the NCBI conserved domain database (CDD). Genes coding for signal peptides and transmembrane helices were predicted using SignalP (Nielsen et al. 1997) and TMHMM (Krogh et al. 2001), respectively. The CGView Server was used for the visualization of sequence features (Grant and Stothard 2008). Digital DNA–DNA hybridization (dDDH) and OrthoANI values were calculated using the GGDC and AOT softwares, respectively.
Results and discussion
Phylogenetic analysis
Based on 16S rRNA-based sequence similarity and taxonomic classification, strain Marseille-P4747 was found to represent a potential new species, with an identity of 96.18% with O. beijingensis strain GCS-AN-3 (NR_133803.1), the phylogenetically closest species with standing in nomenclature (Fig. 1). This percentage is lower than the threshold value (98.65%) recommended by Kim et al. (2014) to define the taxonomic novelty of a prokaryote. Therefore, we classified strain Marseille-P4747 as a representative of a putative new species of the genus Ottowia. The 16S rRNA sequence from strain Marseille-P4747T was deposited in GenBank under accession number LT960589.

Phylogenetic analysis based on the comparison of 16S RNA gene sequences highlighting the position of O. massiliensis sp. nov. strain Marseille-P4747 among closely related species. GenBank accession numbers are indicated in parentheses. Evolutionary analyses were conducted in MEGA X, sequences were aligned using CLUSTALW and the phylogenetic analysis was inferred using the Maximum Likelihood method and Tamura–Nei model. The scale bar represents a 2% nucleotide sequence divergence.
Phenotypic description
Cells from strain Marseille-P4747T were Gram-stain-negative, nonmotile, and rod-shaped bacteria with a mean length of 1.3 µm and a mean width of 0.7 µm. The bacterium exhibited negative activity for catalase and oxidase. Optimal growth was observed on 5% sheep blood-enriched Columbia agar (COS, bioMérieux) at 30°C in aerobic atmosphere after 72 hours of incubation. Cells were able to grow on COS agar with pH values ranging from 7.5 to 10, with an optimal growth at 7.5. However, no growth was observed on the same medium with an NaCl concentration higher than 10 g/l. On COS agar, colonies of strain Marseille-P4747T appeared smooth, beige, small and circular, with a mean diameter of 1 mm.
Using API ZYM strips (bioMérieux), we observed positive reactions for esterase (C4), esterase lipase (C8), leucine arylamidase, trypsin, and acid phosphatase, but negative reactions for alkaline phosphatase lipase (C14), valine arylamidase, cystine arylamidase, α-chymotrypsin, naphthol-AS-BI-phosphohydrolase, α-galactosidase, β-galactosidase, β-glucuronidase, α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, and α-fucosidase. Using API 20A strips (bioMérieux), only gelatin was hydrolyzed; potassium nitrate, ʟ-tryptophane, ᴅ-glucose, ʟ-arginine, urea, esculin ferric citrate, 4-nitrophenyl-βᴅ-galactopyranoside, glucose, ʟ-arabinose, ᴅ-mannose, ᴅ-mannitol, N-acetyl-glucosamine, ᴅ-maltose, potassium gluconate, capric acid, adipic acid, malic acid, trisodium citrate, and phenylacetic acid were negative. Using API 50 CH strips, none of the carbon sources was fermented. Biochemical and physiological comparisons of strain Marseille-P4747T and type strains of the closest phylogenetically related neighbor species with standing in the nomenclature are summarized in Table 1. By comparison with other Ottowia species, strain Marseille-P4747T differed in a combination of malic acid metabolism and lipase production.
Differential characteristics of strain Marseille-P4747 compared to phylogenetically closely related neighbor species with standing in nomenclature.
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Colony colors | Beige | Beige | Beige | Yellow | Yellow | Beige | Light |
| Cell size (width–length) | 0.5–0.7 | 0.7–1.3 | 0.5–0.7 | 0.8–2 | 0.7–0.8 | 0.9–1.4 | 0.5–1.0 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Facultatively anaerobic | Aerobic | Aerobic | Aerobic |
| Catalase | − | + | + | − | + | + | + |
| Oxidase | − | + | − | + | + | + | + |
| Lipase (C14) | − | + | w | + | + | − | + |
| Esterase lipase (C8) | + | + | + | + | + | + | + |
| Acid phosphatase | + | + | + | w | + | + | + |
| β-Glucuronidase | − | − | + | − | − | − | − |
| Assimilation from | |||||||
| ᴅ-mannitol | − | − | − | − | − | − | NR |
| Urease | − | − | − | − | − | + | + |
| β-galactosidase | − | − | − | − | − | − | + |
| ᴅ-glucose | − | − | − | − | − | − | + |
| Maltose | − | w | − | − | − | − | − |
| Adipic acid | − | + | w | − | + | + | − |
| Malic acid | − | + | + | + | − | + | + |
| DNA G + C content (%) | 63.5 | 67.6 | 68.5 | 59 | 62.9 | 66.8 | 65.8 |
| Source isolation | Human feces | Activated sludge | Activated sludge | Activated sludge | Fish intestines | Andong sikhye | Rotten biji |
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Colony colors | Beige | Beige | Beige | Yellow | Yellow | Beige | Light |
| Cell size (width–length) | 0.5–0.7 | 0.7–1.3 | 0.5–0.7 | 0.8–2 | 0.7–0.8 | 0.9–1.4 | 0.5–1.0 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Facultatively anaerobic | Aerobic | Aerobic | Aerobic |
| Catalase | − | + | + | − | + | + | + |
| Oxidase | − | + | − | + | + | + | + |
| Lipase (C14) | − | + | w | + | + | − | + |
| Esterase lipase (C8) | + | + | + | + | + | + | + |
| Acid phosphatase | + | + | + | w | + | + | + |
| β-Glucuronidase | − | − | + | − | − | − | − |
| Assimilation from | |||||||
| ᴅ-mannitol | − | − | − | − | − | − | NR |
| Urease | − | − | − | − | − | + | + |
| β-galactosidase | − | − | − | − | − | − | + |
| ᴅ-glucose | − | − | − | − | − | − | + |
| Maltose | − | w | − | − | − | − | − |
| Adipic acid | − | + | w | − | + | + | − |
| Malic acid | − | + | + | + | − | + | + |
| DNA G + C content (%) | 63.5 | 67.6 | 68.5 | 59 | 62.9 | 66.8 | 65.8 |
| Source isolation | Human feces | Activated sludge | Activated sludge | Activated sludge | Fish intestines | Andong sikhye | Rotten biji |
Strains : (1) O. massiliensis sp. nov., strain Marseille-P4747; (2) O. beijingensis strain GCS-AN-3T(Cao et al. 2014); (3) O. pentelensis strain RB3-7T (Felföldi et al. 2011); (4) O. thiooxydans strain DSM 14619T(Spring et al. 2004); (5) O. flava strain GY511T (Shi et al. 2019); (6) O. oryzae strain KADR8-3T (Heo et al. 2018); (7) O. konkukae strain SK3863T (Yi et al. 2018); all strains were negative for N-acetyl-β-glucosaminidase, β-glucosidase, and α-galactosidase activity, and assimilation of indole. +, positive; −, negative; w, weakly positive; and NR, not reported.
Differential characteristics of strain Marseille-P4747 compared to phylogenetically closely related neighbor species with standing in nomenclature.
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Colony colors | Beige | Beige | Beige | Yellow | Yellow | Beige | Light |
| Cell size (width–length) | 0.5–0.7 | 0.7–1.3 | 0.5–0.7 | 0.8–2 | 0.7–0.8 | 0.9–1.4 | 0.5–1.0 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Facultatively anaerobic | Aerobic | Aerobic | Aerobic |
| Catalase | − | + | + | − | + | + | + |
| Oxidase | − | + | − | + | + | + | + |
| Lipase (C14) | − | + | w | + | + | − | + |
| Esterase lipase (C8) | + | + | + | + | + | + | + |
| Acid phosphatase | + | + | + | w | + | + | + |
| β-Glucuronidase | − | − | + | − | − | − | − |
| Assimilation from | |||||||
| ᴅ-mannitol | − | − | − | − | − | − | NR |
| Urease | − | − | − | − | − | + | + |
| β-galactosidase | − | − | − | − | − | − | + |
| ᴅ-glucose | − | − | − | − | − | − | + |
| Maltose | − | w | − | − | − | − | − |
| Adipic acid | − | + | w | − | + | + | − |
| Malic acid | − | + | + | + | − | + | + |
| DNA G + C content (%) | 63.5 | 67.6 | 68.5 | 59 | 62.9 | 66.8 | 65.8 |
| Source isolation | Human feces | Activated sludge | Activated sludge | Activated sludge | Fish intestines | Andong sikhye | Rotten biji |
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| Colony colors | Beige | Beige | Beige | Yellow | Yellow | Beige | Light |
| Cell size (width–length) | 0.5–0.7 | 0.7–1.3 | 0.5–0.7 | 0.8–2 | 0.7–0.8 | 0.9–1.4 | 0.5–1.0 |
| Oxygen requirement | Aerobic | Aerobic | Aerobic | Facultatively anaerobic | Aerobic | Aerobic | Aerobic |
| Catalase | − | + | + | − | + | + | + |
| Oxidase | − | + | − | + | + | + | + |
| Lipase (C14) | − | + | w | + | + | − | + |
| Esterase lipase (C8) | + | + | + | + | + | + | + |
| Acid phosphatase | + | + | + | w | + | + | + |
| β-Glucuronidase | − | − | + | − | − | − | − |
| Assimilation from | |||||||
| ᴅ-mannitol | − | − | − | − | − | − | NR |
| Urease | − | − | − | − | − | + | + |
| β-galactosidase | − | − | − | − | − | − | + |
| ᴅ-glucose | − | − | − | − | − | − | + |
| Maltose | − | w | − | − | − | − | − |
| Adipic acid | − | + | w | − | + | + | − |
| Malic acid | − | + | + | + | − | + | + |
| DNA G + C content (%) | 63.5 | 67.6 | 68.5 | 59 | 62.9 | 66.8 | 65.8 |
| Source isolation | Human feces | Activated sludge | Activated sludge | Activated sludge | Fish intestines | Andong sikhye | Rotten biji |
Strains : (1) O. massiliensis sp. nov., strain Marseille-P4747; (2) O. beijingensis strain GCS-AN-3T(Cao et al. 2014); (3) O. pentelensis strain RB3-7T (Felföldi et al. 2011); (4) O. thiooxydans strain DSM 14619T(Spring et al. 2004); (5) O. flava strain GY511T (Shi et al. 2019); (6) O. oryzae strain KADR8-3T (Heo et al. 2018); (7) O. konkukae strain SK3863T (Yi et al. 2018); all strains were negative for N-acetyl-β-glucosaminidase, β-glucosidase, and α-galactosidase activity, and assimilation of indole. +, positive; −, negative; w, weakly positive; and NR, not reported.
FAME analysis showed that the major fatty acids were C16:0 (36%), C18:1n7 (24%), and C14:0 (12%). A specific 3-hydroxy-decanoic acid (C10:0 3-OH) structure was also identified. No branched fatty acids were detected. Minor amounts of other unsaturated and saturated fatty acids were also detected and are detailed in Table 2. Regarding the antibiotic susceptibility, strain Marseille-P4747 was susceptible to benzylpenicillin (0.003 μg/ml), oxacillin (0.38 μg/ml), amoxicillin (0.016 μg/ml), ampicillin (0.016 μg/ml), ceftriaxone (0.016 μg/ml), imipenem (MIC 0.047 μg/ml), gentamicin (0.5 μg/ml), amikacin (4 μg/ml), tobramycin (0.38 μg/ml), ciprofloxacin (0.003 μg/ml), ofloxacin (0.012 μg/ml), erythromycin (0.19 μg/ml), clindamycin (1 μg/ml), doxycycline (0.5 μg/ml), fosfomycin (0.25 μg/ml), nitrofurantoin (0.064 μg/ml), and trimethoprim–sulfamethoxazole (0.064 μg/ml), but exhibited high resistance to colistin (64 μg/ml).
Cellular fatty acid composition of O. massiliensis sp. nov., strain Marseille-P4747 compared with other closest Ottowia species.
| Fatty acids . | Name . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|---|
| C16:0 | Hexadecanoic acid | 36.3 | 23.9 | 32.8 | 23.3 | 29.5 | 30.2 | 30.5 |
| C18:1n7 | 11-octadecenoic acid | 24.3 | 25.2 | 18.1 | 20.8 | 15.4 | 16.5 | 17.9 |
| C14:0 | Tetradecanoic acid | 11.7 | TR | TR | TR | 3.7 | 3.3 | 3.2 |
| C16:1n7 | 9-hexadecenoic acid | 9.9 | 35.2 | 14.1 | 40.8 | 39.5 | 34.7 | 33.9 |
| C12:0 | Dodecanoic acid | 8.5 | 2.7 | 3.5 | 3.0 | 2.9 | 1.8 | TR |
| C10:0 3-OH | 3-hydroxy-decanoic acid | 4.5 | 3.6 | 4.0 | 1.9 | 2.7 | 2.1 | TR |
| C18:0 | Octadecanoic acid | 2.0 | 2.3 | 1.2 | 2.5 | TR | TR | NR |
| C18:2n6 | 9,12-octadecadienoic acid | TR | NR | NR | NR | NR | NR | NR |
| C18:1n9 | 9-Octadecenoic acid | TR | 1.8 | NR | TR | TR | TR | TR |
| C10:0 | Decanoic acid | TR | TR | 1.0 | 1.8 | TR | TR | TR |
| C17:0 | Heptadecanoic acid | TR | 1.5 | TR | TR | TR | TR | TR |
| C15:0 | Pentadecanoic acid | TR | NR | NR | NR | NR | NR | NR |
| Fatty acids . | Name . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|---|
| C16:0 | Hexadecanoic acid | 36.3 | 23.9 | 32.8 | 23.3 | 29.5 | 30.2 | 30.5 |
| C18:1n7 | 11-octadecenoic acid | 24.3 | 25.2 | 18.1 | 20.8 | 15.4 | 16.5 | 17.9 |
| C14:0 | Tetradecanoic acid | 11.7 | TR | TR | TR | 3.7 | 3.3 | 3.2 |
| C16:1n7 | 9-hexadecenoic acid | 9.9 | 35.2 | 14.1 | 40.8 | 39.5 | 34.7 | 33.9 |
| C12:0 | Dodecanoic acid | 8.5 | 2.7 | 3.5 | 3.0 | 2.9 | 1.8 | TR |
| C10:0 3-OH | 3-hydroxy-decanoic acid | 4.5 | 3.6 | 4.0 | 1.9 | 2.7 | 2.1 | TR |
| C18:0 | Octadecanoic acid | 2.0 | 2.3 | 1.2 | 2.5 | TR | TR | NR |
| C18:2n6 | 9,12-octadecadienoic acid | TR | NR | NR | NR | NR | NR | NR |
| C18:1n9 | 9-Octadecenoic acid | TR | 1.8 | NR | TR | TR | TR | TR |
| C10:0 | Decanoic acid | TR | TR | 1.0 | 1.8 | TR | TR | TR |
| C17:0 | Heptadecanoic acid | TR | 1.5 | TR | TR | TR | TR | TR |
| C15:0 | Pentadecanoic acid | TR | NR | NR | NR | NR | NR | NR |
Strains : (1) O. massiliensis sp. nov., strain Marseille-P4747; (2) O. beijingensis GCS-AN-3T [data from Cao et al. (2014)]; (3) O. pentelensis RB3-7T (Felföldi et al. 2011); (4) O. thiooxydans DSM 14619T (Spring et al. 2004); (5) O. flava GY511T (Shi et al. 2019); (6) O. oryzae KADR8-3T (Heo et al. 2018); and (7) O. konkukae SK3863T (Yi et al. 2018).
TR = trace amounts < 1%; NR, not reported.
Cellular fatty acid composition of O. massiliensis sp. nov., strain Marseille-P4747 compared with other closest Ottowia species.
| Fatty acids . | Name . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|---|
| C16:0 | Hexadecanoic acid | 36.3 | 23.9 | 32.8 | 23.3 | 29.5 | 30.2 | 30.5 |
| C18:1n7 | 11-octadecenoic acid | 24.3 | 25.2 | 18.1 | 20.8 | 15.4 | 16.5 | 17.9 |
| C14:0 | Tetradecanoic acid | 11.7 | TR | TR | TR | 3.7 | 3.3 | 3.2 |
| C16:1n7 | 9-hexadecenoic acid | 9.9 | 35.2 | 14.1 | 40.8 | 39.5 | 34.7 | 33.9 |
| C12:0 | Dodecanoic acid | 8.5 | 2.7 | 3.5 | 3.0 | 2.9 | 1.8 | TR |
| C10:0 3-OH | 3-hydroxy-decanoic acid | 4.5 | 3.6 | 4.0 | 1.9 | 2.7 | 2.1 | TR |
| C18:0 | Octadecanoic acid | 2.0 | 2.3 | 1.2 | 2.5 | TR | TR | NR |
| C18:2n6 | 9,12-octadecadienoic acid | TR | NR | NR | NR | NR | NR | NR |
| C18:1n9 | 9-Octadecenoic acid | TR | 1.8 | NR | TR | TR | TR | TR |
| C10:0 | Decanoic acid | TR | TR | 1.0 | 1.8 | TR | TR | TR |
| C17:0 | Heptadecanoic acid | TR | 1.5 | TR | TR | TR | TR | TR |
| C15:0 | Pentadecanoic acid | TR | NR | NR | NR | NR | NR | NR |
| Fatty acids . | Name . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|---|
| C16:0 | Hexadecanoic acid | 36.3 | 23.9 | 32.8 | 23.3 | 29.5 | 30.2 | 30.5 |
| C18:1n7 | 11-octadecenoic acid | 24.3 | 25.2 | 18.1 | 20.8 | 15.4 | 16.5 | 17.9 |
| C14:0 | Tetradecanoic acid | 11.7 | TR | TR | TR | 3.7 | 3.3 | 3.2 |
| C16:1n7 | 9-hexadecenoic acid | 9.9 | 35.2 | 14.1 | 40.8 | 39.5 | 34.7 | 33.9 |
| C12:0 | Dodecanoic acid | 8.5 | 2.7 | 3.5 | 3.0 | 2.9 | 1.8 | TR |
| C10:0 3-OH | 3-hydroxy-decanoic acid | 4.5 | 3.6 | 4.0 | 1.9 | 2.7 | 2.1 | TR |
| C18:0 | Octadecanoic acid | 2.0 | 2.3 | 1.2 | 2.5 | TR | TR | NR |
| C18:2n6 | 9,12-octadecadienoic acid | TR | NR | NR | NR | NR | NR | NR |
| C18:1n9 | 9-Octadecenoic acid | TR | 1.8 | NR | TR | TR | TR | TR |
| C10:0 | Decanoic acid | TR | TR | 1.0 | 1.8 | TR | TR | TR |
| C17:0 | Heptadecanoic acid | TR | 1.5 | TR | TR | TR | TR | TR |
| C15:0 | Pentadecanoic acid | TR | NR | NR | NR | NR | NR | NR |
Strains : (1) O. massiliensis sp. nov., strain Marseille-P4747; (2) O. beijingensis GCS-AN-3T [data from Cao et al. (2014)]; (3) O. pentelensis RB3-7T (Felföldi et al. 2011); (4) O. thiooxydans DSM 14619T (Spring et al. 2004); (5) O. flava GY511T (Shi et al. 2019); (6) O. oryzae KADR8-3T (Heo et al. 2018); and (7) O. konkukae SK3863T (Yi et al. 2018).
TR = trace amounts < 1%; NR, not reported.
Genome properties
After assembly, strain Marseille-P4747 exhibited a genome size of 2830 447 bp, allocated in 19 scaffolds, with a 63.5 mol% G + C content (Fig. 2). Of the 2626 predicted genes, 2492 were protein-coding genes and 56 were RNAs (two 5S rRNA genes, two 16S rRNA genes, two 23S rRNA genes, 47 tRNA genes, and three noncoding RNAs). A total of 1855 genes were assigned to putative functions by COG or NR blast and 595 genes were annotated as hypothetical proteins. The distribution of genes into COG functional categories is presented in Figure S2 (Supporting Information). The whole-genome sequence of strain Marseille-P4747 was deposited in EMBL-EBI under accession number OEQA00000000.
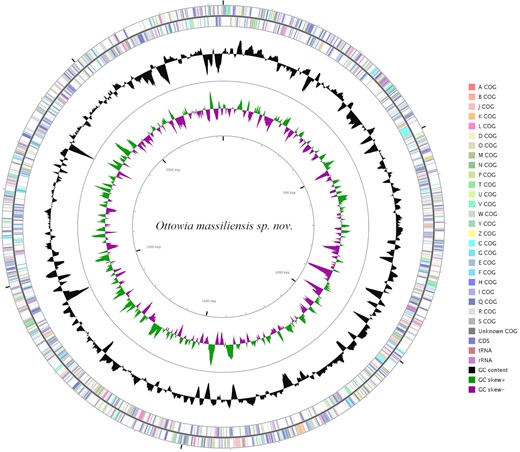
A graphical circular map of O. massiliensis sp. nov. genome colored by COG categories. The rRNAs operon (pink), tRNAs (brown), GC content plot (black), and GC skew (purple: negative values and olive: positive values).
Genome comparison
The genome size of strain Marseille-P4747 (2.83 Mbp) was bigger than Tibeticola sediminis (2.73 Mbp), but smaller than those of O. oryzae (3.9 Mbp), O. thiooxydans (7.05 Mbp), O. beijingensis (4.92 Mbp), and Zhizhongheella caldifontis (3.64 Mbp). The G + C content of strain Marseille-P4747 (63.5 mol%) was higher than that of O. thiooxydans (59 mol%), but lower than those of O. oryzae (66.8 mol%), O. beijingensis (67.6 mol%), T. sediminis (66.4 mol%), and Z. caldifontis (67.7 mol%). Strain Marseille-P4747 had the smallest number of protein-coding sequences (2492) among compared genomes.
The dDDH values of strain Marseille-P4747 ranged from 19.60% with strain Z. caldifontis to 21.50% with strain O. beijingensis (Table 3). These values were lower than the recommended threshold of 70% to delineate species (Auch et al. 2010), and thus suggest that strain Marseille-P4747 belonged to new species. In addition, OrthoANI values varied from 73.30% with Z. caldifontis to 77.03% with O. beijingensis, lower than the 95% threshold delimiting species (MeierKolthoff et al. 2013; Figure S3, Supporting Information).
| . | Om . | Ob . | Oo . | Ot . | Ts . | Zc . |
|---|---|---|---|---|---|---|
| Om | 21.5% ± 2.3 | 21.2% ± 2.3 | 20.4% ± 2.3 | 19.7% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Ob | 100% | 24.2% ± 2.3 | 2.7% ± 2.3 | 19.9% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Oo | 100% | 20.4% ± 2.3 | 19.6% ± 2.3 | 19.3% ± 2.3 | 19.3% ± 2.3 | |
| Ot | 100% | 18.9% ± 2.2 | 19.1% ± 2.3 | 19.1% ± 2.3 | ||
| Td | 100% | 19.2% ± 2.2 | 19.2%± 2.2 | |||
| Zc | 100% | 100% |
| . | Om . | Ob . | Oo . | Ot . | Ts . | Zc . |
|---|---|---|---|---|---|---|
| Om | 21.5% ± 2.3 | 21.2% ± 2.3 | 20.4% ± 2.3 | 19.7% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Ob | 100% | 24.2% ± 2.3 | 2.7% ± 2.3 | 19.9% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Oo | 100% | 20.4% ± 2.3 | 19.6% ± 2.3 | 19.3% ± 2.3 | 19.3% ± 2.3 | |
| Ot | 100% | 18.9% ± 2.2 | 19.1% ± 2.3 | 19.1% ± 2.3 | ||
| Td | 100% | 19.2% ± 2.2 | 19.2%± 2.2 | |||
| Zc | 100% | 100% |
dDDH: digital DNA–DNA hybridization. Om: Ottowia massiliensis; Ob: Ottowia beijingensis; Oo: Ottowia oryzae; Ot: Ottowia thiooxydans; Ts: Tibeticola sediminis; and Zc: Zhizhongheella caldifontis.
| . | Om . | Ob . | Oo . | Ot . | Ts . | Zc . |
|---|---|---|---|---|---|---|
| Om | 21.5% ± 2.3 | 21.2% ± 2.3 | 20.4% ± 2.3 | 19.7% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Ob | 100% | 24.2% ± 2.3 | 2.7% ± 2.3 | 19.9% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Oo | 100% | 20.4% ± 2.3 | 19.6% ± 2.3 | 19.3% ± 2.3 | 19.3% ± 2.3 | |
| Ot | 100% | 18.9% ± 2.2 | 19.1% ± 2.3 | 19.1% ± 2.3 | ||
| Td | 100% | 19.2% ± 2.2 | 19.2%± 2.2 | |||
| Zc | 100% | 100% |
| . | Om . | Ob . | Oo . | Ot . | Ts . | Zc . |
|---|---|---|---|---|---|---|
| Om | 21.5% ± 2.3 | 21.2% ± 2.3 | 20.4% ± 2.3 | 19.7% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Ob | 100% | 24.2% ± 2.3 | 2.7% ± 2.3 | 19.9% ± 2.3 | 19.6% ± 2.3 | 19.6% ± 2.3 |
| Oo | 100% | 20.4% ± 2.3 | 19.6% ± 2.3 | 19.3% ± 2.3 | 19.3% ± 2.3 | |
| Ot | 100% | 18.9% ± 2.2 | 19.1% ± 2.3 | 19.1% ± 2.3 | ||
| Td | 100% | 19.2% ± 2.2 | 19.2%± 2.2 | |||
| Zc | 100% | 100% |
dDDH: digital DNA–DNA hybridization. Om: Ottowia massiliensis; Ob: Ottowia beijingensis; Oo: Ottowia oryzae; Ot: Ottowia thiooxydans; Ts: Tibeticola sediminis; and Zc: Zhizhongheella caldifontis.
Conclusion
In summary, the phenotypic, biochemical, and genomic studies carried out on strain Marseille-P4747 were consistent in confirming its novelty. In addition, 16S rRNA and genome sequence analysis justified its classification into a new Ottowia species, for which we formally propose the name O. massiliensis sp. nov.
Description of O. massiliensis sp. nov.
Ottowia massiliensis (mas.si.li.en’ sis, L. fem. adj. massiliensis, from Massilia, the Latin name for Marseille, where strain Marseille-P4747T was isolated). Growth is observed after 3 days of incubation on 5% sheep blood-enriched Columbia agar at 37°C under aerobic conditions. Colonies are smooth and beige, with a diameter ranging from 0.2 to 1.0 mm. Cells are Gram-stain-negative rods, with a length ranging from 1.68 to 2.3 µm and a width of 0.58 to 0.78 µm. It is nonmotile. Catalase and oxidase activities are negative. Positive reactions are observed for esterase (C4), esterase lipase (C8), leucine arylamidase, trypsin, and acid phosphatase. Urease is not produced. Nitrate is not reduced but gelatin is hydrolyzed. The major fatty acids are hexadecanoic acid (C16:0), 11-octadecenoic acid (C18:1n7), and tetradecanoic acid (C14:0).
The genome size is 2830 447 bp, with a 63.5 mol% G + C content. The genome and the 16S rRNA sequences of strain Marseille-P4747T are deposited in GenBank under accession numbers OEQA00000000 and LT960589, respectively.
The type strain Marseille-P4747T ( = CSUR P4747 = CECT 30348) was isolated from the fecal microbiome of a healthy donor.
Authors’ contributions
Conceptualization, P.E.F. and D.R.; methodology, P.E.F., C.I.L., and R.S.; validation, D.R. and P.E.F.; formal analysis, M.M., C.I.L., S.N., and M.M.M.; investigation, M.M.; strain culture, S.N., M.M.M., and S.A.; writing—original draft preparation, M.M.; writing—review and editing, C.I.L; supervision, P.E.F.; and funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors thank Takashi Irie, Kyoko Imai, Shigeki Matsubara, Taku Sakazume, Yusuke Ominami, Hisada Akiko, and the Hitachi team of Japan (Hitachi High-Technologies Corporation, Science and Medical Systems Business Group 24–14, Nishi-shimbashi 1-chome, Minato-ku, Tokyo 105–8717, Japan) for the collaborative study conducted together with the IHU Méditerranée Infection, and for the installation of a SU5000 microscope at the IHU Méditerranée Infection. Authors also thank Ludivine Brechard for sequencing the genome and Amael Fadlane for culturing the type strain from CSUR collection, and Sara Bellali, for taking the scanning electron microscope photographs.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency under the program “Investissements d'avenir,” reference ANR-10-IAHU-03, the Région Provence Alpes Côte d'Azur, and the European funding FEDER PRIMI.



