-
PDF
- Split View
-
Views
-
Cite
Cite
Mossaab Maaloum, Pamela Afouda, Cheikh Ibrahima Lo, Gregory Dubourg, Thi Tien Nguyen, Anthony Levasseur, Rachid Saile, Didier Raoult, Pierre-Edouard Fournier, Prevotella merdae sp. nov., a new bacterial species isolated from human faeces, FEMS Microbiology Letters, Volume 369, Issue 1, 2022, fnac066, https://doi.org/10.1093/femsle/fnac066
Close - Share Icon Share
Abstract
Strain Marseille-P4119T was isolated from a faecal sample of a healthy 32-year-old faecal transplant donor. The bacterium was anaerobic, Gram-negative, rod-shaped, non-motile, and did not produce spores. We studied its phenotypic characteristics and sequenced its whole genome. The major fatty acids were C15:0anteiso and C15:0iso. The final genome assembly was 3912650 bp long with a 44.4 mol% G + C content, 3094 protein-coding genes and 74 RNA genes. Strain Marseille-P4119T exhibited a 97.10% 16S rRNA sequence identity and a 29.0% dDDH with Prevotella stercorea CB35T, OrthoANI values ranged from 68.5% with Prevotella enoeca to 77.4% with Prevotella stercorea, the phylogenetically closest bacterial species with standing in nomenclature. Based on the phylogenetic, phenotypic and genomic analyses, we propose the creation of the novel species Prevotella merdae sp. nov. The type strain is Marseille-P4119T ( = CSUR P4119T = CECT 9566T).
Abbreviations
- bp
base pairs
- CECT
Colección Española de Cultivos Tipo (Spanish Type Culture Collection)
- COG
Clusters of Orthologous Groups
- CSUR
Collection de Souches de l'Unité des Rickettsies (Unit collection of bacterial strains)
- dDDH
Digital DNA-DNA Hybridization
- FAME
Fatty Acid Methyl Ester
- GC/MS
Gas Chromatography/Mass Spectrometry
- gDNA
genomic DNA
- GGDC
Genome-to-Genome Distance Calculator
- MALDI-TOF MS
Matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry
- ORF
open reading frame
- RPS-BLAST
Reverse Position Specific BLAST
Introduction
In 1990, on the basis of phenotypic and phylogenetic specificities, Shah and Collins proposed the creation of the genus Prevotella (Shah and Collins 1990) to reclassify moderately saccharolytic Bacteroides species from the oral cavity. Prevotella melaninogenica, formerly named Bacteroides melaninogenicus was proposed as type species of the Prevotella genus.
Currently, members of the genus Prevotella are recognised as major components of the normal human microbiota. They colonise the human mouth, gut and vagina, and may be associated with various diseases, including breast (Glazunova et al. 2007), liver and spleen abscesses (Brook and Frazier 1998), dental infections, lung abscess (Kimberlin et al. 2015) and septicaemia (Berger et al. 2005). The rapid evolution of high-throughput sequencing technologies has enabled a better understanding of the human microbiota (Di Bella et al. 2013). Recent metagenomic studies demonstrated that a high abundance of Bacteroides and Prevotella species was associated with a healthy gut microbiota (Hollister et al. 2014). It was also observed that individuals with a diet rich in plant carbohydrates had a larger proportion of Prevotella species (Kovatcheva-Datchary et al. 2015).
In this paper, we report a novel bacterial strain, Marseille-P4119T that was isolated from a healthy human faecal sample. The strain was characterized using a combination of phenotypic, biochemical, and genomic properties. Strain Marseille-P4119T exhibited a 97.10% 16S rRNA sequence identity with Prevotella stercorea strain CB35T ( = DSM 18206T), the phylogenetically closest bacterial species with standing in nomenclature. Herein, we detail the characteristics of strain Marseille-P4119T and propose the creation of a new Prevotella species.
Materials and methods
Strain isolation and identification
Strain Marseille-P4119 was isolated from a feces specimen collected from a healthy 32-year-old male faecal transplant donor on 18 April 2017, at the Méditerranée Infection institute (Marseille, France) as part of the long term MEGAGUT culturomics project. The donor gave a written informed consent, and the study had been authorized by the IHU Méditerranée Infection ethics committee under number 2016–011.
The feces sample was preincubated in an anaerobic blood culture bottle in the presence of 5% sheep blood and 5% rumen fluid (bioMérieux) for 10 days, prior to being inoculated on 5% sheep blood-enriched Columbia agar (COS) plate (bioMérieux, Marcy l'Etoile, France) and incubated in anaerobic atmosphere at 37°C. Using a MALDI-TOF mass spectrometry (MALDI-TOF MS) Microflex LT spectrometer (Bruker Daltonics, Leipzig, Germany), we attempted identification of strain Marseille-P4119 as previously described (Seng et al. 2009). The obtained spectra (Fig. S1) were analyzed using the MALDI Biotyper 3.0 software (Bruker Daltonics) by standard pattern matching (with default parameter settings) against the spectra included in the database (Bruker database, constantly updated with the URMS database, (https://www.mediterranee-infection.com/acces-ressources/base-de-donnees/urms-data-base).
The reference spectrum of strain Marseille-P4119 was added to the URMS database (Fig. S1).
DNA was extracted from an individual colony using the EZ1 DNA Tissue Kit and an EZ1 Advanced XL BioRobot (Qiagen, Courtaboeuf, France). Genomic DNA (gDNA) was quantified by a Qubit assay using the high sensitivity kit (Life Technologies, Carlsbad, CA, USA). 16S rRNA amplification was performed using PCR and the universal primers fD1 and rP2 (Eurogentec, Angers, France) as previously described (Drancourt et al. 2000, Morel et al. 2015). Sequencing of the amplified products was performed using the Big Dye Terminator v1.1 Cycle Sequencing Kit and an ABI Prism 3130xl sequencer (Applied Biosystems). Subsequently, the 16S rRNA sequence was compared to the nr database (http://www.ncbi.nlm.nih.gov/genbank/). A 16S rRNA-based phylogenetic analysis of strain Marseille-P4119 and closely related species was conducted using the maximum likelihood method with the Tamura 3-parameter in the MEGA X software (Kumar et al. 2018).
Bacterial morphology and growth conditions
The growth of strain Marseille-P4119 was attempted at different temperatures (28°C, 37°C, 45°C) and atmospheres (anaerobic, microaerophilic and aerobic). The anaerobic and microaerophilic atmospheres were obtained using the GasPak™ EZ Anaerobe and Campy container systems, respectively (Becton Dickinson, Le Pont de Claix, France), according to the manufacturer's instructions. Tolerance to salt (5 g l−1, 10 g l−1, 15 g l−1) and pH (5, 5.5, 6, 6.5, 7, 7.5, and 8) were also evaluated in anaerobic atmosphere.
To determine the bacterial morphology, cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for at least one hour at 4°C. A drop of cell suspension was deposited for approximately five minutes on a glow-discharged formvar carbon film on 400 mesh nickel grids (FCF400-Ni, EMS). The grids were dried on blotting paper and cells were negatively stained for 10 s with 1% ammonium molybdate solution in filtered water at room temperature. Electron micrographs were acquired using a Tecnai G20 (FEI company) at an operating voltage of 60 kV.
Biochemical properties
The biochemical characteristics of strain Marseille-P4119 were tested using API ZYM, API 50 CH, and API Rapid ID 32A strips (bioMérieux), following the manufacturer's instructions. To study sporulation, bacterial cells were subjected to a thermal shock at 80°C for 20 minutes and were then inoculated on Columbia blood agar and incubated for 48 hours at 37°C in anaerobic atmosphere. Antibiotic susceptibility was evaluated using the E-test gradient method (bioMérieux) to determine the minimal inhibitory concentration (MIC) of each tested antibiotic. To do so, a 0.5 McFarland turbidity standard of the bacterial inoculum was prepared by suspending the culture in a sterile saline solution (NaCl 0.85%) of MH-F as recommended by EUCAST (Matuschek et al. 2014). MICs were measured at the intersection of the E-test strips with the elliptical zones of inhibition (Citron et al. 1991).
Cellular fatty acid methyl ester (FAME) analysis of strain Marseille-P4119 was performed by GC/MS. Two samples were prepared with approximately 50 mg of bacterial biomass per tube harvested from several culture plates. FAMEs were prepared as described by Sasser (2006). GC/MS analyses were carried out as described by Dione et al. (2016). Briefly, FAMEs were separated using an Elite 5-MS column and monitored by mass spectrometry (Clarus 500–SQ 8 S, Perkin Elmer, Courtaboeuf, France). A spectral database search was performed using MS Search 2.0 operated with the Standard Reference Database 1A (NIST, Gaithersburg, USA) and the FAME mass spectral database (Wiley, Chichester, UK).
Genome sequencing and analysis
The gDNA of strain Marseille-P4119 was sequenced using a MiSeq sequencer (Illumina, San Diego, CA, USA) and the mate-pair and paired-end strategies. The gDNA was barcoded in order to be mixed with 11 other projects with the Nextera Mate-Pair sample prep kit (Illumina), and with 16 other projects with the Nextera XT DNA sample prep kit (Illumina). The gDNA was quantified by a Qubit assay using a high sensitivity kit (Life Technologies, Carlsbad, CA, USA) to 82.2 ng µl−1.
To prepare the paired-end library, the gDNA was diluted to 1 ng of each genome as input. The tagmentation step fragmented and tagged the DNA. Then limited cycle PCR amplification (12 cycles) completed the tag adapters and introduced dual-index barcodes. The library profile was validated on an Agilent 2100 BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) with a DNA High sensitivity labchip and the fragment size was estimated to 1.5 kb. After purification on AMPure XP beads (Beckman Coulter Inc, Fullerton, CA, USA), the libraries were then normalized on specific beads according to the Nextera XT protocol (Illumina). Normalized libraries were pooled for sequencing on the MiSeq. Automated cluster generation and paired-end sequencing with dual index reads were performed in a single 39-hour run in a 2 × 250-bp format.
The mate-pair library was prepared with 1.41 µg of gDNA using the Nextera mate-pair Illumina guide. The gDNA sample was simultaneously fragmented and tagged with a mate-pair junction adapter. The fragmentation pattern was validated on an Agilent 2100 BioAnalyzer (Agilent Technologies) with a DNA 7500 labchip. DNA fragments ranged in size from 1.5 kb up to 11 kb with an optimal size at 7.49 kb. No size selection was performed and 326.7 ng of tagmented fragments were circularized. The circularized DNA was mechanically sheared to small fragments with an optimal size of 650 bp on the Covaris device S2 in T6 tubes (Covaris, Woburn, MA, USA). The library profile was visualized on a High Sensitivity Bioanalyzer LabChip (Agilent Technologies) and the final concentration library was measured at 24.82 nmol l−1. The libraries were normalized at 2 nM and pooled. After a denaturation step and dilution at 18 pM, the pool of libraries was loaded onto the reagent cartridge and then onto the instrument along with the flow cell. Automated cluster generation and a sequencing run were performed in a single 39-hour run in a 2 × 151-bp format.
Total information of 6 Gb was obtained from a 623 K/mm2 cluster density with a cluster passing quality control filters of 97.5% (11904000 passing filter paired reads). Within this run, the index representation for strain Marseille-P4119 was determined to 8.46%. The 1007519 paired read quality was examined using FastQC v0.11.9 (Brown et al. 2017). Low quality reads were depleted, and then assembly was obtained using SPAdes (version 3.14.1) with default parameters (Bankevich et al. 2012). The sequence coverage was 128x.
Sequences were annotated using the Prokaryotic Genome Annotation Pipeline (PGAP) (Tatusova et al.2016). Predicted bacterial protein-coding sequences were searched against the Clusters of Orthologous Groups (COG) databases from the NCBI Conserved Domain Database (CDD), using NCBI's Reverse Position Specific BLAST (RPS-BLAST) (Yang et al. 2020) algorithm.
Signal peptides and transmembrane helices were predicted using SignalP (Dyrløv Bendtsen et al. 2004) and TMHMM (Krogh et al. 2001), respectively. Artemis (Rutherford et al. 2000) and DNA Plotter (Carver et al. 2009) were used for data management and visualization of genomic features, respectively. The PHAge Search Tool (PHAST) was used to detect prophage sequences (Zhou et al.2011).
Genomic similarities between strain Marseille-P4119 and closely-related species were calculated using the digital DNA-DNA hybridization (dDDH) evaluated with the Genome-to-Genome Distance Calculator (GGDC 2.0) (Meier-Kolthoff et al. 2013) and OrthoANI software (Lee et al. 2016). These included the genomes from Prevotella brevis strain ATCC 19188T, Prevotella buccae strain ATCC 33574T, Prevotella dentalis strain DSM 3688T, Prevotella enoeca strain 12259T, Prevotella melaninogenica strain ATCC 25845T, Prevotella pleuritidis strain JCM 14110T, Prevotella shahii strain DSM 15611T and Prevotella stercorea strain DSM 18206T.
Results and discussion
Phylogenetic analysis
Strain Marseille-P4119 exhibited a 97.10% 16S rRNA gene sequence identity with Prevotella stercorea strain CB35T (GenBank accession number NR_041364.1), the phylogenetically closest bacterial species with a validly published name (Fig. 1a). As this value was lower than the 98.65% threshold defined by Kim et al. (Kim et al. 2014) for defining a new species, we classified strain Marseille-P4119 as a representative of a putative new species in the genus Prevotella. The 16S rRNA sequence was deposited in GenBank under accession number LT984637. A Core genome alignment obtained using the Roary pipeline (Page et al. 2015) with default parameters displayed a similar topology, with strain Marseille-P4119 grouping with with Prevotella stercorea strain CB35T (Fig. 1b).
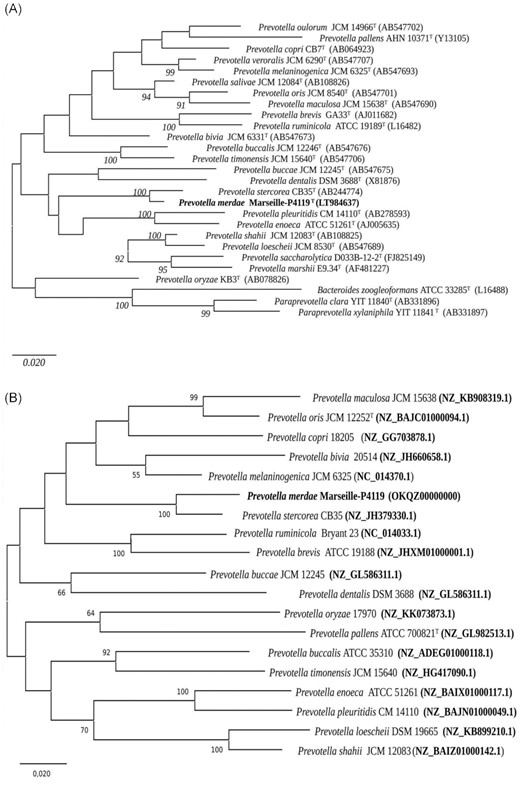
(A) 16S rRNA-bases phylogenetic analysis of Prevotella merdae sp. nov. strain Marseille-P4119T relative to closely related bacterial species with standing in nomenclature. Phylogenetic inferences were obtained using the neighbour-joining method and the Tamura-3 parameter. The analysis involved 27 nucleotide sequences. There were a total of 1549 positions in the final dataset. The respective GenBank accession numbers for 16S rRNA sequences are indicated in parentheses. Only bootstrap values >90% were retained. The scale bar indicates a 2% nucleotide sequence divergence. (B) Phylogenetic tree built using an alignment of core genome sequences and the maximum-likelihood method. Only bootstrap values >50% were retained. The scale bar indicates a 2% nucleotide sequence divergence.
Phenotypic and biochemical features
Growth of strain Marseille-P4119 was only observed in anaerobic atmosphere, at a temperature growth range of 32°C–37°C (optimal growth at 37°C). The bacterium was able to grow in NaCl concentrations lower than 5 g.l−1, and at a pH ranging from 6.5 to 7.5 (optimal growth at 7.5). Cells were Gram-negative (Fig. S2a), not motile, rod-shaped and did not produce spores. Their mean length and width were 4.15 µm and 0.77 µm, respectively (Fig. S2b). Colonies were convex, asymmetric and yellowish with a diameter of 0.5–1.5 mm on 5% sheep blood-enriched Columbia agar (bioMérieux). Catalase and oxidase activities were negative. Biochemical properties of strain Marseille-P4119T were evaluated using API ZYM, API 50 CH and API Rapid 32A strips, (Table S1). These characteristics were compared to those of closely related species (Table 1).
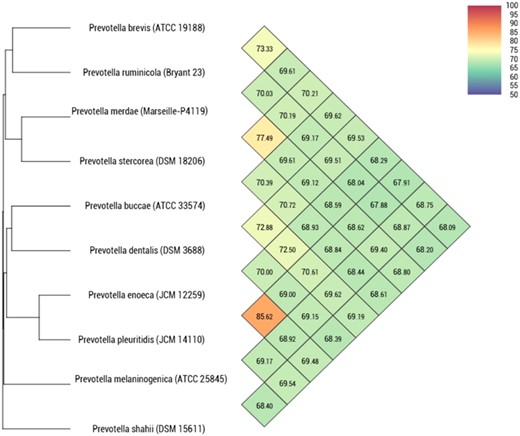
Heatmap generated with OrthoANI values calculated using the OAT software between strain Marseille-P4119T and closely related species with standing in nomenclature.
Differential characteristics of 1, Prevotella merdae Marseille-P4119T, 2, Prevotella stercorea CB35T(Hayashi et al. 2007); 3, Prevotella buccae ATCC 33574T (Johnson et al.1985); 4, Prevotella dentalis DSM 3688T (Haapasalo et al. 1986); 5, Prevotella enoeca 12259T(Moore et al. 1994)); 6, Prevotella melaninogenica ATCC 25845T (Shah and Collins 1990; Wu et al. 1992); 7, Prevotella pleuritidis JCM 14110T (Sakamoto et al. 2007); 8, Prevotella shahii DSM 15611T (Sakamoto et al. 2004).
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.7 | 0.2–0.4 | 0.3–0.6 | 0.7–1 | 0.5–2 | NR | 0.8–1.7 | 0.5–0.8 |
| Gelatin digestion | − | − | + | − | + | + | + | − |
| Acid produced from: | ||||||||
| D-mannose | + | + | − | + | − | − | + | + |
| Salicin | − | − | + | − | − | − | − | + |
| D-cellobiose | + | − | + | + | − | − | − | + |
| D-raffinose | + | + | − | + | − | + | − | + |
| API RAPID ID 32 A : | ||||||||
| Arginine dihydrolase | + | − | − | − | − | − | − | − |
| α-arabinosidase | − | + | + | + | − | − | − | NR |
| Raffinose | + | + | − | − | − | + | − | + |
| Glutamic acid decarboxylase | + | − | − | − | − | − | + | NR |
| API ZYM: | ||||||||
| α-galactosidase | + | + | + | − | − | + | − | w |
| β-glucosidase | − | + | + | − | − | − | − | − |
| N-acetyl-β-glucosaminidase | + | − | − | + | + | + | + | + |
| α-fucosidase | + | − | − | − | + | + | + | + |
| Major Fatty acids | Iso-C15:0, anteiso-C15:0 | Iso- C15:0, anteiso- C15:0 | Anteiso-C15:0 | Iso-C14:0; C16:0 | Anteiso-C15:0; C16:0 | C18:1ω9c, anteiso-C15:0 | Anteiso-C15:0, C16:0, C18:1ω9c | C18:1ω9c, C16:0, C16:03-OH |
| Source of isolation | Human | Human | Human | Human | Human | Human | Human | Human |
| G + C content (mol%) | 44 | 48 | 51 | 56 | 47 | 41 | 45 | 44 |
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.7 | 0.2–0.4 | 0.3–0.6 | 0.7–1 | 0.5–2 | NR | 0.8–1.7 | 0.5–0.8 |
| Gelatin digestion | − | − | + | − | + | + | + | − |
| Acid produced from: | ||||||||
| D-mannose | + | + | − | + | − | − | + | + |
| Salicin | − | − | + | − | − | − | − | + |
| D-cellobiose | + | − | + | + | − | − | − | + |
| D-raffinose | + | + | − | + | − | + | − | + |
| API RAPID ID 32 A : | ||||||||
| Arginine dihydrolase | + | − | − | − | − | − | − | − |
| α-arabinosidase | − | + | + | + | − | − | − | NR |
| Raffinose | + | + | − | − | − | + | − | + |
| Glutamic acid decarboxylase | + | − | − | − | − | − | + | NR |
| API ZYM: | ||||||||
| α-galactosidase | + | + | + | − | − | + | − | w |
| β-glucosidase | − | + | + | − | − | − | − | − |
| N-acetyl-β-glucosaminidase | + | − | − | + | + | + | + | + |
| α-fucosidase | + | − | − | − | + | + | + | + |
| Major Fatty acids | Iso-C15:0, anteiso-C15:0 | Iso- C15:0, anteiso- C15:0 | Anteiso-C15:0 | Iso-C14:0; C16:0 | Anteiso-C15:0; C16:0 | C18:1ω9c, anteiso-C15:0 | Anteiso-C15:0, C16:0, C18:1ω9c | C18:1ω9c, C16:0, C16:03-OH |
| Source of isolation | Human | Human | Human | Human | Human | Human | Human | Human |
| G + C content (mol%) | 44 | 48 | 51 | 56 | 47 | 41 | 45 | 44 |
All strains are Gram-negative, non-spore-forming, non-motile, and anaerobic. They are also negative for catalase, nitrate reductase, and urease. All strains used D-glucose, D-maltose, D-lactose, α-glucosidase, leucyl glycine arylamidase, alanine arylamidase, and alkaline phosphatase, while did not used L-arabinose, L-rhamnose, D-mannitol, D-sorbitol, D-melezitose, β-glucuronidase, L-tryptophane, proline arylamidase, phenylalanine arylamidase, tyrosine arylamidase, glycine arylamidase, histidine arylamidase, and serine arylamidase. +, positive; -, negative; w, weakly; NR, Not reported.
Differential characteristics of 1, Prevotella merdae Marseille-P4119T, 2, Prevotella stercorea CB35T(Hayashi et al. 2007); 3, Prevotella buccae ATCC 33574T (Johnson et al.1985); 4, Prevotella dentalis DSM 3688T (Haapasalo et al. 1986); 5, Prevotella enoeca 12259T(Moore et al. 1994)); 6, Prevotella melaninogenica ATCC 25845T (Shah and Collins 1990; Wu et al. 1992); 7, Prevotella pleuritidis JCM 14110T (Sakamoto et al. 2007); 8, Prevotella shahii DSM 15611T (Sakamoto et al. 2004).
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.7 | 0.2–0.4 | 0.3–0.6 | 0.7–1 | 0.5–2 | NR | 0.8–1.7 | 0.5–0.8 |
| Gelatin digestion | − | − | + | − | + | + | + | − |
| Acid produced from: | ||||||||
| D-mannose | + | + | − | + | − | − | + | + |
| Salicin | − | − | + | − | − | − | − | + |
| D-cellobiose | + | − | + | + | − | − | − | + |
| D-raffinose | + | + | − | + | − | + | − | + |
| API RAPID ID 32 A : | ||||||||
| Arginine dihydrolase | + | − | − | − | − | − | − | − |
| α-arabinosidase | − | + | + | + | − | − | − | NR |
| Raffinose | + | + | − | − | − | + | − | + |
| Glutamic acid decarboxylase | + | − | − | − | − | − | + | NR |
| API ZYM: | ||||||||
| α-galactosidase | + | + | + | − | − | + | − | w |
| β-glucosidase | − | + | + | − | − | − | − | − |
| N-acetyl-β-glucosaminidase | + | − | − | + | + | + | + | + |
| α-fucosidase | + | − | − | − | + | + | + | + |
| Major Fatty acids | Iso-C15:0, anteiso-C15:0 | Iso- C15:0, anteiso- C15:0 | Anteiso-C15:0 | Iso-C14:0; C16:0 | Anteiso-C15:0; C16:0 | C18:1ω9c, anteiso-C15:0 | Anteiso-C15:0, C16:0, C18:1ω9c | C18:1ω9c, C16:0, C16:03-OH |
| Source of isolation | Human | Human | Human | Human | Human | Human | Human | Human |
| G + C content (mol%) | 44 | 48 | 51 | 56 | 47 | 41 | 45 | 44 |
| Properties . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . |
|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.7 | 0.2–0.4 | 0.3–0.6 | 0.7–1 | 0.5–2 | NR | 0.8–1.7 | 0.5–0.8 |
| Gelatin digestion | − | − | + | − | + | + | + | − |
| Acid produced from: | ||||||||
| D-mannose | + | + | − | + | − | − | + | + |
| Salicin | − | − | + | − | − | − | − | + |
| D-cellobiose | + | − | + | + | − | − | − | + |
| D-raffinose | + | + | − | + | − | + | − | + |
| API RAPID ID 32 A : | ||||||||
| Arginine dihydrolase | + | − | − | − | − | − | − | − |
| α-arabinosidase | − | + | + | + | − | − | − | NR |
| Raffinose | + | + | − | − | − | + | − | + |
| Glutamic acid decarboxylase | + | − | − | − | − | − | + | NR |
| API ZYM: | ||||||||
| α-galactosidase | + | + | + | − | − | + | − | w |
| β-glucosidase | − | + | + | − | − | − | − | − |
| N-acetyl-β-glucosaminidase | + | − | − | + | + | + | + | + |
| α-fucosidase | + | − | − | − | + | + | + | + |
| Major Fatty acids | Iso-C15:0, anteiso-C15:0 | Iso- C15:0, anteiso- C15:0 | Anteiso-C15:0 | Iso-C14:0; C16:0 | Anteiso-C15:0; C16:0 | C18:1ω9c, anteiso-C15:0 | Anteiso-C15:0, C16:0, C18:1ω9c | C18:1ω9c, C16:0, C16:03-OH |
| Source of isolation | Human | Human | Human | Human | Human | Human | Human | Human |
| G + C content (mol%) | 44 | 48 | 51 | 56 | 47 | 41 | 45 | 44 |
All strains are Gram-negative, non-spore-forming, non-motile, and anaerobic. They are also negative for catalase, nitrate reductase, and urease. All strains used D-glucose, D-maltose, D-lactose, α-glucosidase, leucyl glycine arylamidase, alanine arylamidase, and alkaline phosphatase, while did not used L-arabinose, L-rhamnose, D-mannitol, D-sorbitol, D-melezitose, β-glucuronidase, L-tryptophane, proline arylamidase, phenylalanine arylamidase, tyrosine arylamidase, glycine arylamidase, histidine arylamidase, and serine arylamidase. +, positive; -, negative; w, weakly; NR, Not reported.
The major fatty acids identified for strain Marseille-P4119 were branched structures: 12-methyl-tetradecanoic acid (C15:0 anteiso) and 13-methyl-tetradecanoic acid (C15:0 iso). Specific 3-hydroxy fatty acids were described with low abundance. Minor amounts of unsaturated and other saturated structures were also detected (Table 2).
Cellular fatty acid composition of strain Marseille-P4119T and closely related type strains of Prevotella spp. Strains: 1, Prevotella merdae Marseille-P4119T; 2, Prevotella stercorea CB35T (Hayashi et al. 2007); 3, Prevotella buccae ATCC 33574T (Brondz and Olsen 1991); 4, Prevotella enoeca 12259T (Sakamoto et al. 2007); 5, Prevotella melaninogenica ATCC 25845T (Hayashi et al. 2007); 6, Prevotella pleuritidis JCM 14110T (Sakamoto et al. 2007); 7, Prevotella shahii DSM 15611T (Hayashi et al. 2007).
| Fatty acids . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| C13:0 iso | TR | 1.2 | 0.7 | NA | 0.6 | NA | NA |
| C14:0 | 1.7 | 0.8 | 1.5 | 3.3 | 1.2 | 3.2 | 10.9 |
| C14:0 iso | 4.5 | 2.7 | 2.4 | 1.1 | 3.4 | 2.3 | 4.4 |
| C15:0 | TR | TR | TR | TR | TR | 1 | 1.0 |
| C15:0 anteiso | 40.9 | 26.2 | 51 | 7.8 | 29.7 | 23 | 6.8 |
| C15:0 iso | 23.7 | 23.7 | 6.0 | 1.4 | 9.6 | 5.6 | 3.4 |
| C16:0 | 12.3 | 3.8 | 12.2 | 5.8 | 8.2 | 1.9 | 16.9 |
| C16:0 iso | 1.8 | 2.7 | 2.3 | 1 | 2.9 | 1.8 | 1 |
| C16:0 3-OH | 1.0 | 1 | 4.1 | TR | 4 | TR | 16.3 |
| C16:0 3-OH iso | TR | 1.2 | TR | 7.5 | 1.2 | 9.7 | 1.9 |
| C17:0 3-OH iso | 4.9 | 6.4 | TR | 2.9 | 9.5 | 5.5 | 1.3 |
| C17:0 anteiso | 1.1 | 1.3 | 4.0 | 1.1 | 2.1 | 2.7 | NA |
| C17:0 iso | TR | TR | 5.4 | NA | 1.7 | TR | NA |
| C18:0 | 1.0 | 0.8 | TR | 5,8 | 0.7 | 1,9 | 2.8 |
| C18:1n9 | 2.6 | 14.7 | TR | 20,4 | 12.6 | 17,9 | 18.7 |
| C18:2n6 | TR | 2.2 | TR | 5,1 | NA | 1,5 | 8.9 |
| Fatty acids . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| C13:0 iso | TR | 1.2 | 0.7 | NA | 0.6 | NA | NA |
| C14:0 | 1.7 | 0.8 | 1.5 | 3.3 | 1.2 | 3.2 | 10.9 |
| C14:0 iso | 4.5 | 2.7 | 2.4 | 1.1 | 3.4 | 2.3 | 4.4 |
| C15:0 | TR | TR | TR | TR | TR | 1 | 1.0 |
| C15:0 anteiso | 40.9 | 26.2 | 51 | 7.8 | 29.7 | 23 | 6.8 |
| C15:0 iso | 23.7 | 23.7 | 6.0 | 1.4 | 9.6 | 5.6 | 3.4 |
| C16:0 | 12.3 | 3.8 | 12.2 | 5.8 | 8.2 | 1.9 | 16.9 |
| C16:0 iso | 1.8 | 2.7 | 2.3 | 1 | 2.9 | 1.8 | 1 |
| C16:0 3-OH | 1.0 | 1 | 4.1 | TR | 4 | TR | 16.3 |
| C16:0 3-OH iso | TR | 1.2 | TR | 7.5 | 1.2 | 9.7 | 1.9 |
| C17:0 3-OH iso | 4.9 | 6.4 | TR | 2.9 | 9.5 | 5.5 | 1.3 |
| C17:0 anteiso | 1.1 | 1.3 | 4.0 | 1.1 | 2.1 | 2.7 | NA |
| C17:0 iso | TR | TR | 5.4 | NA | 1.7 | TR | NA |
| C18:0 | 1.0 | 0.8 | TR | 5,8 | 0.7 | 1,9 | 2.8 |
| C18:1n9 | 2.6 | 14.7 | TR | 20,4 | 12.6 | 17,9 | 18.7 |
| C18:2n6 | TR | 2.2 | TR | 5,1 | NA | 1,5 | 8.9 |
Symbol: TR, Trace amount; NA, not available. Values are expressed as percentages of fatty acids.
Cellular fatty acid composition of strain Marseille-P4119T and closely related type strains of Prevotella spp. Strains: 1, Prevotella merdae Marseille-P4119T; 2, Prevotella stercorea CB35T (Hayashi et al. 2007); 3, Prevotella buccae ATCC 33574T (Brondz and Olsen 1991); 4, Prevotella enoeca 12259T (Sakamoto et al. 2007); 5, Prevotella melaninogenica ATCC 25845T (Hayashi et al. 2007); 6, Prevotella pleuritidis JCM 14110T (Sakamoto et al. 2007); 7, Prevotella shahii DSM 15611T (Hayashi et al. 2007).
| Fatty acids . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| C13:0 iso | TR | 1.2 | 0.7 | NA | 0.6 | NA | NA |
| C14:0 | 1.7 | 0.8 | 1.5 | 3.3 | 1.2 | 3.2 | 10.9 |
| C14:0 iso | 4.5 | 2.7 | 2.4 | 1.1 | 3.4 | 2.3 | 4.4 |
| C15:0 | TR | TR | TR | TR | TR | 1 | 1.0 |
| C15:0 anteiso | 40.9 | 26.2 | 51 | 7.8 | 29.7 | 23 | 6.8 |
| C15:0 iso | 23.7 | 23.7 | 6.0 | 1.4 | 9.6 | 5.6 | 3.4 |
| C16:0 | 12.3 | 3.8 | 12.2 | 5.8 | 8.2 | 1.9 | 16.9 |
| C16:0 iso | 1.8 | 2.7 | 2.3 | 1 | 2.9 | 1.8 | 1 |
| C16:0 3-OH | 1.0 | 1 | 4.1 | TR | 4 | TR | 16.3 |
| C16:0 3-OH iso | TR | 1.2 | TR | 7.5 | 1.2 | 9.7 | 1.9 |
| C17:0 3-OH iso | 4.9 | 6.4 | TR | 2.9 | 9.5 | 5.5 | 1.3 |
| C17:0 anteiso | 1.1 | 1.3 | 4.0 | 1.1 | 2.1 | 2.7 | NA |
| C17:0 iso | TR | TR | 5.4 | NA | 1.7 | TR | NA |
| C18:0 | 1.0 | 0.8 | TR | 5,8 | 0.7 | 1,9 | 2.8 |
| C18:1n9 | 2.6 | 14.7 | TR | 20,4 | 12.6 | 17,9 | 18.7 |
| C18:2n6 | TR | 2.2 | TR | 5,1 | NA | 1,5 | 8.9 |
| Fatty acids . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . |
|---|---|---|---|---|---|---|---|
| C13:0 iso | TR | 1.2 | 0.7 | NA | 0.6 | NA | NA |
| C14:0 | 1.7 | 0.8 | 1.5 | 3.3 | 1.2 | 3.2 | 10.9 |
| C14:0 iso | 4.5 | 2.7 | 2.4 | 1.1 | 3.4 | 2.3 | 4.4 |
| C15:0 | TR | TR | TR | TR | TR | 1 | 1.0 |
| C15:0 anteiso | 40.9 | 26.2 | 51 | 7.8 | 29.7 | 23 | 6.8 |
| C15:0 iso | 23.7 | 23.7 | 6.0 | 1.4 | 9.6 | 5.6 | 3.4 |
| C16:0 | 12.3 | 3.8 | 12.2 | 5.8 | 8.2 | 1.9 | 16.9 |
| C16:0 iso | 1.8 | 2.7 | 2.3 | 1 | 2.9 | 1.8 | 1 |
| C16:0 3-OH | 1.0 | 1 | 4.1 | TR | 4 | TR | 16.3 |
| C16:0 3-OH iso | TR | 1.2 | TR | 7.5 | 1.2 | 9.7 | 1.9 |
| C17:0 3-OH iso | 4.9 | 6.4 | TR | 2.9 | 9.5 | 5.5 | 1.3 |
| C17:0 anteiso | 1.1 | 1.3 | 4.0 | 1.1 | 2.1 | 2.7 | NA |
| C17:0 iso | TR | TR | 5.4 | NA | 1.7 | TR | NA |
| C18:0 | 1.0 | 0.8 | TR | 5,8 | 0.7 | 1,9 | 2.8 |
| C18:1n9 | 2.6 | 14.7 | TR | 20,4 | 12.6 | 17,9 | 18.7 |
| C18:2n6 | TR | 2.2 | TR | 5,1 | NA | 1,5 | 8.9 |
Symbol: TR, Trace amount; NA, not available. Values are expressed as percentages of fatty acids.
The strain was susceptible to all antibiotics tested, with the exception of amikacin (MIC 256 μg.ml−1). MICs were as follows: amoxicillin (0.125 μg.ml−1), benzylpenicillin (0.032 μg.ml−1), cefotaxime (0.75 μg.ml−1), ceftriaxone (0.50 μg.ml−1), imipenem (0.125 μg.ml−1), vancomycin (2 μg.ml−1), doxycycline (3 μg.ml−1), and metronidazole (0.064 μg.ml−1).
Genomic features
The final genome assembly of strain Marseille-P4119 was 3912 650-bp-long, with 27 scaffolds and a 44.4 mol% G + C content. We identified 3176 predicted genes, including 3094 protein-coding genes and 74 RNAs (four 5S rRNAs, five 16S rRNAs, three 23S rRNAs, three non-coding RNAs and 59 tRNAs) (Fig. 2). A total of 2237 genes (68.2%) were assigned a putative function, peptide signals were identified in 704 genes (21.5%), transmembrane helices in 641 (19.5%), and 108 ORFs (3.2%) were classified as pseudogenes. Two incomplete prophage regions of 8.5Kb and 10.4Kb were also identified.
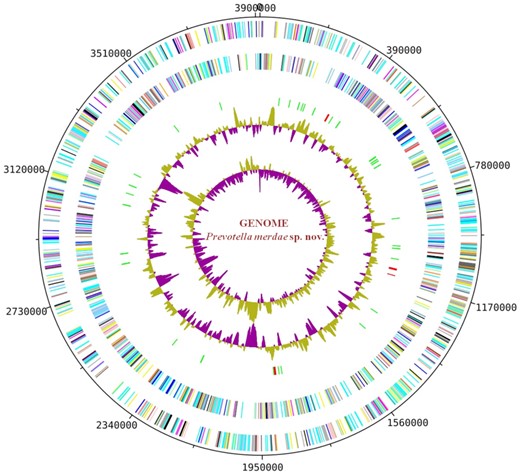
Graphic circular map of the chromosome. From the outside in, open reading frames oriented in the forward (coloured by COG categories) direction, open reading frames oriented in the reverse (coloured by COG categories) direction, RNA operon (red), and tRNAs (green), GC content plot, and GC skew (purple: negative values, olive: positive values).
Strain Marseille-P4119 exhibited a larger genome (3.9 Mb) than those of all other compared species. Its G + C content (44.4%) was higher than those of P. melaninogenica (41.1%) and P. shahii (44.3%), but lower than P. buccae, P. dentalis, P. enoeca, P. stercorea, and P. pleuritidis (50–51%, 56–60%, 47%, 48.2%, and 45.4%, respectively). With 3094 protein-coding genes, strain Marseille-P4119 possessed more genes than all other compared strains (Table S2). The distribution of genes into COG categories was similar in all compared genomes (Fig. S3), with the exception of category X (Mobilome) that was over-represented in strain Marseille-P4119.
When compared to other Prevotella species, strain Marseille-P4119 exhibited dDDH values ranging from 20.40% with P. buccae to 29.0% with P. stercorea (dDDH) (Table 3), and OrthoANI values ranging from 68.5% with P. enoeca to 77.4% with P. stercorea (Fig. 3). These values (< 70% and <95% for dDDH and OrthoANI, respectively) confirm that strain Marseille-P4119 may be considered as a representative of a new species in the Prevotella genus.
| . | Pmer . | Prum . | Pbuc . | Pden . | Peno . | Pmel . | Pple . | Psha . | Pste . |
|---|---|---|---|---|---|---|---|---|---|
| Pmer | 100% | 22.7% ±2.3 | 20.4% ±2.3 | 23.5% ±2.4 | 24.8% ±2.4 | 23.0% ±2.3 | 24.7% ±2.4 | 28.1% ±2.4 | 29.0% ±2.4 |
| Prum | 100% | 19.4% ±2.3 | 21.0% ±2.3 | 21.9% ±2.3 | 23.1% ±2.3 | 22.5% ±2.3 | 21.6% ±2.3 | 20.6% ±2.3 | |
| Pbuc | 100% | 23.1% ±2.4 | 35.1% ±2.4 | 26.7% ±2.4 | 26.6% ±2.4 | 20.7% ±2.3 | 19.7% ±2.3 | ||
| Pden | 100% | 26.4% ±2.4 | 25.0% ±2.4 | 25.0% ±2.4 | 22.1% ±2.3 | 21.0% ±2.3 | |||
| Peno | 100% | 25.9% ±2.4 | 34.6% ±2.4 | 21.3% ±2.4 | 22.7% ±2.3 | ||||
| Pmel | 100% | 25.5% ±2.4 | 26.2% ±2.4 | 21.7% ±2.3 | |||||
| Pple | 100% | 26.2% ±2.4 | 24.0% ±2.4 | ||||||
| Psha | 100% | 21.5% ±2.3 | |||||||
| Pste | 100% |
| . | Pmer . | Prum . | Pbuc . | Pden . | Peno . | Pmel . | Pple . | Psha . | Pste . |
|---|---|---|---|---|---|---|---|---|---|
| Pmer | 100% | 22.7% ±2.3 | 20.4% ±2.3 | 23.5% ±2.4 | 24.8% ±2.4 | 23.0% ±2.3 | 24.7% ±2.4 | 28.1% ±2.4 | 29.0% ±2.4 |
| Prum | 100% | 19.4% ±2.3 | 21.0% ±2.3 | 21.9% ±2.3 | 23.1% ±2.3 | 22.5% ±2.3 | 21.6% ±2.3 | 20.6% ±2.3 | |
| Pbuc | 100% | 23.1% ±2.4 | 35.1% ±2.4 | 26.7% ±2.4 | 26.6% ±2.4 | 20.7% ±2.3 | 19.7% ±2.3 | ||
| Pden | 100% | 26.4% ±2.4 | 25.0% ±2.4 | 25.0% ±2.4 | 22.1% ±2.3 | 21.0% ±2.3 | |||
| Peno | 100% | 25.9% ±2.4 | 34.6% ±2.4 | 21.3% ±2.4 | 22.7% ±2.3 | ||||
| Pmel | 100% | 25.5% ±2.4 | 26.2% ±2.4 | 21.7% ±2.3 | |||||
| Pple | 100% | 26.2% ±2.4 | 24.0% ±2.4 | ||||||
| Psha | 100% | 21.5% ±2.3 | |||||||
| Pste | 100% |
Prevotella merdae (Pmer); Prevotella ruminicola (Prum); Prevotella buccae (Pbuc); Prevotella dentalis (Pden); Prevotella enoeca (Peno); Prevotella melaninogenica (Pmel); Prevotella pleuritidis (Pple); Prevotella shahii (Psha) and Prevotella stercorea (Pste).
| . | Pmer . | Prum . | Pbuc . | Pden . | Peno . | Pmel . | Pple . | Psha . | Pste . |
|---|---|---|---|---|---|---|---|---|---|
| Pmer | 100% | 22.7% ±2.3 | 20.4% ±2.3 | 23.5% ±2.4 | 24.8% ±2.4 | 23.0% ±2.3 | 24.7% ±2.4 | 28.1% ±2.4 | 29.0% ±2.4 |
| Prum | 100% | 19.4% ±2.3 | 21.0% ±2.3 | 21.9% ±2.3 | 23.1% ±2.3 | 22.5% ±2.3 | 21.6% ±2.3 | 20.6% ±2.3 | |
| Pbuc | 100% | 23.1% ±2.4 | 35.1% ±2.4 | 26.7% ±2.4 | 26.6% ±2.4 | 20.7% ±2.3 | 19.7% ±2.3 | ||
| Pden | 100% | 26.4% ±2.4 | 25.0% ±2.4 | 25.0% ±2.4 | 22.1% ±2.3 | 21.0% ±2.3 | |||
| Peno | 100% | 25.9% ±2.4 | 34.6% ±2.4 | 21.3% ±2.4 | 22.7% ±2.3 | ||||
| Pmel | 100% | 25.5% ±2.4 | 26.2% ±2.4 | 21.7% ±2.3 | |||||
| Pple | 100% | 26.2% ±2.4 | 24.0% ±2.4 | ||||||
| Psha | 100% | 21.5% ±2.3 | |||||||
| Pste | 100% |
| . | Pmer . | Prum . | Pbuc . | Pden . | Peno . | Pmel . | Pple . | Psha . | Pste . |
|---|---|---|---|---|---|---|---|---|---|
| Pmer | 100% | 22.7% ±2.3 | 20.4% ±2.3 | 23.5% ±2.4 | 24.8% ±2.4 | 23.0% ±2.3 | 24.7% ±2.4 | 28.1% ±2.4 | 29.0% ±2.4 |
| Prum | 100% | 19.4% ±2.3 | 21.0% ±2.3 | 21.9% ±2.3 | 23.1% ±2.3 | 22.5% ±2.3 | 21.6% ±2.3 | 20.6% ±2.3 | |
| Pbuc | 100% | 23.1% ±2.4 | 35.1% ±2.4 | 26.7% ±2.4 | 26.6% ±2.4 | 20.7% ±2.3 | 19.7% ±2.3 | ||
| Pden | 100% | 26.4% ±2.4 | 25.0% ±2.4 | 25.0% ±2.4 | 22.1% ±2.3 | 21.0% ±2.3 | |||
| Peno | 100% | 25.9% ±2.4 | 34.6% ±2.4 | 21.3% ±2.4 | 22.7% ±2.3 | ||||
| Pmel | 100% | 25.5% ±2.4 | 26.2% ±2.4 | 21.7% ±2.3 | |||||
| Pple | 100% | 26.2% ±2.4 | 24.0% ±2.4 | ||||||
| Psha | 100% | 21.5% ±2.3 | |||||||
| Pste | 100% |
Prevotella merdae (Pmer); Prevotella ruminicola (Prum); Prevotella buccae (Pbuc); Prevotella dentalis (Pden); Prevotella enoeca (Peno); Prevotella melaninogenica (Pmel); Prevotella pleuritidis (Pple); Prevotella shahii (Psha) and Prevotella stercorea (Pste).
Conclusion
In this study, on the basis of phenotypic and genomic characteristics, we formally propose the creation of the species Prevotella merdae sp. nov., which contains strain Marseille-P4119T as type strain.
Description of Prevotella merdae sp. nov
Prevotella merdae (mer'dae. L. gen. fem. n. merdae of faeces, referring to the source of the type strain). Colonies are convex, asymmetric and yellowish with a diameter of 0.5–1.5 mm on 5% sheep blood-enriched Columbia agar. Cells are anaerobic and Gram-negative rods. They do not produce oxidase and catalase, are not motile and do not produce spores. Cells measure a mean 4.15 μm in length and 0.77 μm in width. Using API strips (API 50 CH,API Rapid 32A and API ZYM) (bioMérieux), positive reactions are observed for D-glucose, D-Mannose, D-cellobiose, D-maltose, D-lactose, saccharose, D-raffinose, L-arginine, mannose, raffinose, Glutamic acid decarboxylase, arylamidase alkaline phosphatase, leucyl glycine arylamidase and alanine arylamidase, alkaline phosphatase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α-galactosidase, β-galactosidase, α-glucosidase, N-acetyl-β-glucosaminidase and α-fucosidase. The most abundant fatty acids are C15:0 anteiso and C15:0 iso.
The genome size of strain Marseille-P4119T is 3912650–bp-long with a 44.4 mol% G + C content. The 16S rRNA and genome sequences are deposited in EMBL/EBI under accession numbers LT984637 and OKQZ00000000, respectively.
The type strain Marseille-P4119T ( = CSUR P4119 = CECT 9566) was isolated from the feces of a healthy 32-year-old faecal transplant donor.
Author disclosure statement
The authors declare no conflict of interest in relation to this research.
Funding information
This study was supported by the Mediterranee Infection foundation and by the French Government under the ‘Investissements d'avenir’ (Investments for the Future) programme managed by the Agence Nationale de la Recherche (National Agency for Research) under reference: Méditerranée Infection 10-IAHU-03. This work was also supported by Région Provence Alpes Côte d'Azur and the European Regional Development Fund ERDF PRIMI funding.
Author contribution
Conceptualisation, PEF and DR; methodology, PEF, GD, CIL, and RS; validation, DR, GD and PEF; formal analysis, MM, PA, TTN and AL; investigation, MM, PA, TTN, and AL; Strain culture, MM, PA; writing-original draft preparation, MM, PA, and CIL; writing-review and editing, MM, PA, GD, and CIL; supervision, PEF; funding acquisition, DR. All authors have read and agreed to the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors thank Claudia Andrieu for corrections, and Nicholas Armstrong and Philippe Decloquement for the analysis of fatty acids.
Conflicts of interests
None declared.
References
Author notes
Mossaab Maaloum and Pamela Afouda have contributed equally to the work.



