-
PDF
- Split View
-
Views
-
Cite
Cite
Cheikh Ibrahima Lo, Niokhor Dione, Aminata Mbaye, Patricia Fernández-Mellado Gómez, Issa Isaac Ngom, Camille Valles, Stéphane Alibar, Jean-Christophe Lagier, Florence Fenollar, Pierre-Edouard Fournier, Didier Raoult, Seydina M Diene, Limosilactobacillus caccae sp. nov., a new bacterial species isolated from the human gut microbiota, FEMS Microbiology Letters, Volume 368, Issue 18, September 2021, fnab128, https://doi.org/10.1093/femsle/fnab128
Close - Share Icon Share
ABSTRACT
Strain Marseille-P3519T isolated from the fecal flora of a 25-year-old healthy French woman was a Gram-positive anaerobic bacterium, non-motile and non-spore forming. The 16S rRNA gene sequence of Marseille-P3519 showed 97.73% of sequence similarity with Limosilactobacillus reuteri DSM 20016, the closest species, phylogenetically. Furthermore, the average nucleotide identity of strain Marseille-3519 with its closest related species was 75.8% that was very below the recommended threshold (>95–96%). Its genome had 2 237 367 bp with 45.42 mol% of G + C content. Major fatty acids were C16:0 (50.8%), C18:1n9 (18.0%), C18:2n6 (9.8%) and C19:1n9 (8.9%). It was catalase negative and fermented glycerol, glucose, fructose, D-maltose, lactose and mannose. These findings support that strain Marseille-P3519 ( = CSURP3519 = CECT 30110) is a new member of the genus Limosilactobacillus for which the name Limosilactobacillus caccae sp. nov., is proposed.
INTRODUCTION
The genus Lactobacillus (Beijerinck 1901), is the most diverse, extensive and important inside the Lactic acid bacteria group (Skerman, McGowan and Sneath 1980). Currently, the genus Lactobacillus is undergoing a major rework and 23 new genera have emerged from this rework (Zheng et al. 2020). Among them the genus Limosilactobacillus which is composed of 24 reclassified species (Parte 2018). The Lactobacilli members have still played a very important role in the food fermentation in humans because it can be present in the gastrointestinal tract in variable amounts depending on the host's age, the location in the digestive tract or involved species (Duar et al. 2017; Mu, Tavella and Luo 2018). In addition, some Lactobacilli are naturally associated with human milk as well as respiratory, digestive and genitourinary human microbiota (Fernández et al. 2013; Nicaise et al. 2019).
Here, a new bacterial species was described according to the concept of taxono-genomics previously reported (Fournier and Drancourt 2015; Bilen et al. 2019). Moreover, genomic comparison as well as phenotypic characteristics are provided for Limosilactobacillus caccae sp. nov., strain Marseille-P3519.
MATERIALS AND METHODS
Organism information
Stool specimen was provided by a healthy 25-year-old French patient. In 2016, the project on study of human microbiota by culturomics and metagenomics, had obtained favorable opinion from the ethical committee of the IFR48 (Marseille, France) under number 09–022 and the patient has given an informed consent. No taking of antibiotics by the patient during sampling, and the sample was freezed at −80°C as suggested previously (Dione et al. 2018).
Isolation and identification of strain
In November 2016, strain Marseille-P3519T was isolated following the same protocol used before (Afouda et al. 2017; Ndongo et al. 2020). The first identification was attempted with MALDI-TOF MS instrument (Bruker Daltonics, Leipzig, Germany), as previously described (Lo et al. 2015; Dione et al. 2016). After misidentification by MALDI-TOF MS, the 16S rRNA sequencing was performed as previously described (Morel et al. 2015).
Phenotypic characteristics
The capacity for growth at temperatures of 28, 37, 45 and 56°C was tested on Columbia agar enriched with 5% sheep blood by incubating under anaerobic conditions.
The spores forming, the motility and the production or not of catalase and oxidase by strain Marseille-P3519 were sought as is reported previously (Ngom et al. 2018). Using API ZYM and API 50 CH strips (bioMérieux), further biochemical characteristics of strain Marseille-P3519T were highlighted.
Antimicrobial susceptibility testing (AST) of strain Marseille-P3519 was performed using the disk diffusion method (Kirby–Bauer antibiotic testing) with amoxicillin, clindamycin, amoxicillin/clavulanic acid, imipenem, doxycycline, vancomycin, metronidazole, ciprofloxacin, trimethoprim/sulfamethoxazole, oxacillin, fosfomycin, erythromycin and rifampicin. The results were analyzed following to EUCAST 2018 recommendations (Matuschek et al. 2018). All genes involved in antibiotic resistance were identified from the genome sequences and confirmed by BLASTP against the described antibiotic resistance gene local database (Gupta et al. 2014).
Fatty acids detection of the strain Marseille-P3519 was done by Gas chromatography–mass spectrometry (GC/MS) as reported before (Sasser 2006; Dione et al. 2017). Electron micrographs scanning and Gram staining image were acquired with a Tecnai G20 Cryo (FEI, Hillsboro, USA)transmission electron microscope and an optic microscope DM1000 (Leica Microsystems, Nanterre Cedex, 92752 France), respectively.
Genome information
DNA extraction, sequencing and assembly
DNA extraction of strain Marseille-P3519 was performed on the EZ1 biorobot (Qiagen, Germantown, MD) with EZ1 DNA tissues kit. Obtained genomic DNA (gDNA) of Marseille-P3519 was sequenced using MiSeq instrument (Illumina, San Diego, CA) as previously described (Lo et al. 2016; Mbogning Fonkou et al. 2019). Global information of 7.2 Gb was recovered from a 765 K/mm2, a cluster density with a cluster passing quality control filters of 94.7% (14 162 000 passing filter paired reads). The index value of strain Marseille-P3519 was 7.18%. The 1 108 387 paired reads were trimmed and then assembled into contigs.
Annotation and comparison of genome
The genome was annotated using an approach as described previously (Lo et al. 2016). It is a set of software for highlighting the predicted protein sequences, tRNAs, rRNAs, Clusters of Orthologous Groups (COGs) or mobile genetic elements within the genome.
Artemis software (Rutherford et al. 2000) was required to manage genomic data. DNA Plotter software (Carver et al. 2009) has permitted to visualize the genomic features. Mauve alignment software (version 2.3.1) was used to align multiple genomic sequences (Darling et al. 2004).
RESULTS
Identification of the strain
MALDI-TOF MS instrument could not reliably identify our bacterial strain (Marseille-P3519) because its spectrum does not exist in our database. Therefore, after three unsuccessful identification attempts, we systematically proceeded to amplify and to sequence the 16S rRNA gene as described above (Morel et al. 2015).
Strain Marseille-P3519 exhibited 98.57% sequence similarity with Limosilactobacillus caviae MOZM2 (GenBank accession number NR157747) the phylogenetically closest bacterial species, which name was validly published (Fig. 1). Any bacterium with 16S rRNA gene sequence similarity of less than 98.65% is considered a new bacterial species according to previous authors (Stackebrandt 2011; Meier-Kolthoff et al. 2013). Thus, Marseille-P3519 is the type strain of L. caccae sp nov., which 16S rRNA gene sequence was registered in GenBank database with the accession number LT671596.
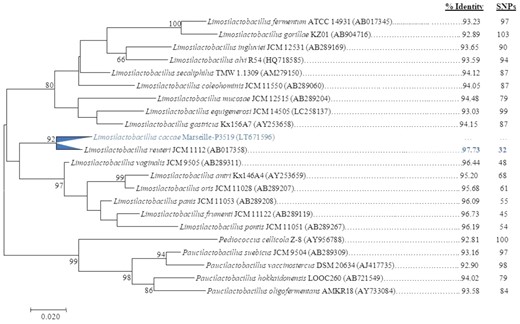
Phylogenetic tree highlighting the position of L. caccae strain Marseille-P3519T relative to other type strains within the Limosilactobacillus genus. Sequences were aligned using CLUSTALW, and phylogenetic inferences were obtained using the maximum-likelihood method within the MEGA software. Numbers at the nodes are percentages of bootstrap values obtained by repeating 500 times the analysis to generate a majority consensus tree.
Phenotypic characteristics
Strain Marseille-P3519 is anaerobic, Gram-positive, non-spore-forming and non-motile and rod-shaped bacterium. Although growth occurred between 28 and 45°C, its optimum was observed at 37°C after 24 h of incubation under anaerobic conditions. It is not able to grow at 56°C. Colonies of strain Marseille-P3519 are white, circular, opaque and smooth with a mean diameter of 0.4 mm on Columbia agar enriched with 5% sheep blood. Using electron microscopy, cells exhibit a diameter ranging from 0.8 to 1.0 μm (Figure S1, Supporting Information). Catalase and oxidase reactions were both negative.
In order to obtain a rapid and global vision of its metabolic capacities, we resort to bioMérieux API (analytical profile index) tests. Carbohydrate fermentation tests and enzymatic activities were determined using the API ZYM and 50 CH strips. Positive reactions were obtained for glycerol, erythritol, galactose, arabinose, glucose, D-ribose, fructose, xylose, mannose, methyl α-D-mannopyranoside, methyl α-D-glucopyranoside, N-acetylglucosamine, D-maltose, salicin, D-lactose, D-melibiose, D-saccharose, D-trehalose, D-raffinose, glycogen, gentiobiose, starch, D-turanose, esterase lipase, esterase, leucine arylamidase, cystine arylamidase, valine arylamidase, α-galactosidase, phosphatase acid and β- galactosidase. Phenotypic and biochemical characteristics of strain Marseille-P3519 were compared to those of other close representative strains in the Lactobacillaceae family (Table 1). The major fatty acids of strain Marseille-P3519 were Hexadecanoic acid (50%) and 9-Octadecenoic acid (18%). Minor amounts of unsaturated, branched and other saturated fatty acids were also detected (Table 2).
Differential characteristics of (1) Limosilactobacillus caccae strain Marseille-P3519T; (2) Limosilactobacillus antri strain DSM 16041(Roos, Engstrand and Jonsson 2005); (3) Limosilactobacillus pontis strain DSM 8475 (Vogel et al. 1994); (4) Limosilactobacillus vaginalis strain DSM 5837 (Zheng etal. 2020); (5) Limosilactobacillus gastricus strain DSM 16045 (Roos, Engstrand and Jonsson 2005); (6) Limosilactobacillus oris strain DSM 4864 (Zheng et al. 2020); (7) Limosilactobacillus frumenti strain DSM 13145 (Müller, Ehrmann and Vogel 2000); (8) Limosilactobacillus coleohominis strain DSM 14060 (Nikolaitchouk et al. 2001); (9) Limosilactobacillus panis strain DSM 6035 (Zheng et al. 2020) and (10) Limosilactobacillus reuteri strain DSM 20016 (Kandler, Stetter and Köhl 1980).
| Characteristics . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.8–1.0 | 1.2–2 | 0.3–0.6 | 0.5–0.8 | 0.9–1.2 | 0.8–1.0 | 0.3 | ND | 0.7–0.9 | 0.7–1.0 |
| Oxygen requirement | Ana | Ana | Ana | FA | Ana | FA | FA | FA | Ana | Ana |
| Gram strain | + | + | + | + | + | + | + | + | + | + |
| Optimal growth temperature | 37°C | 25–45°C | 15–45°C | 30–45°C | 37°C | 30–40°C | 45°C | 45°C | 30–45°C | 35–38°C |
| Acid production from: | ||||||||||
| Arabinose | + | + | - | - | - | w | w | - | w | w |
| Ribose | + | + | + | - | - | + | + | + | + | + |
| Xylose | + | + | - | - | - | w | - | ND | + | w |
| Galactose | + | + | - | + | + | + | + | + | + | + |
| Fructose | + | + | + | ND | + | + | + | ND | + | + |
| Mannose | + | - | - | ND | + | + | + | ND | + | - |
| Mannitol | - | w | - | - | - | - | + | - | - | - |
| Sorbitol | - | - | - | - | - | - | + | - | ND | - |
| N-acetylglucosamine | + | - | - | - | w | - | + | ND | - | - |
| Amygdalin | - | - | - | ND | + | + | + | ND | - | - |
| Arbutin | - | - | - | - | + | + | + | ND | - | - |
| Esculin | - | w | - | - | + | - | + | - | + | - |
| Salicin | + | - | - | - | + | - | + | ND | + | - |
| Cellobiose | - | - | - | - | + | + | + | ND | - | - |
| Lactose | + | - | - | + | + | + | + | - | + | + |
| Melibiose | + | + | - | + | + | + | + | - | + | + |
| Trehalose | + | - | - | - | + | + | + | - | - | - |
| Melezitose | - | - | - | - | - | - | + | ND | - | - |
| Raffinose | + | + | - | + | + | + | + | - | + | + |
| % of G + C content | 45.4 | 51.1 | 53.4 | 40.4 | 41.6 | 49.9 | 42.5 | 40.8 | 48.0 | 38.4 |
| Source | Human gut | Human gut | Sourdough | Human vagina | Human gut | Human mouth | Sourdough | Human vagina | Sourdough | Faeces |
| Characteristics . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.8–1.0 | 1.2–2 | 0.3–0.6 | 0.5–0.8 | 0.9–1.2 | 0.8–1.0 | 0.3 | ND | 0.7–0.9 | 0.7–1.0 |
| Oxygen requirement | Ana | Ana | Ana | FA | Ana | FA | FA | FA | Ana | Ana |
| Gram strain | + | + | + | + | + | + | + | + | + | + |
| Optimal growth temperature | 37°C | 25–45°C | 15–45°C | 30–45°C | 37°C | 30–40°C | 45°C | 45°C | 30–45°C | 35–38°C |
| Acid production from: | ||||||||||
| Arabinose | + | + | - | - | - | w | w | - | w | w |
| Ribose | + | + | + | - | - | + | + | + | + | + |
| Xylose | + | + | - | - | - | w | - | ND | + | w |
| Galactose | + | + | - | + | + | + | + | + | + | + |
| Fructose | + | + | + | ND | + | + | + | ND | + | + |
| Mannose | + | - | - | ND | + | + | + | ND | + | - |
| Mannitol | - | w | - | - | - | - | + | - | - | - |
| Sorbitol | - | - | - | - | - | - | + | - | ND | - |
| N-acetylglucosamine | + | - | - | - | w | - | + | ND | - | - |
| Amygdalin | - | - | - | ND | + | + | + | ND | - | - |
| Arbutin | - | - | - | - | + | + | + | ND | - | - |
| Esculin | - | w | - | - | + | - | + | - | + | - |
| Salicin | + | - | - | - | + | - | + | ND | + | - |
| Cellobiose | - | - | - | - | + | + | + | ND | - | - |
| Lactose | + | - | - | + | + | + | + | - | + | + |
| Melibiose | + | + | - | + | + | + | + | - | + | + |
| Trehalose | + | - | - | - | + | + | + | - | - | - |
| Melezitose | - | - | - | - | - | - | + | ND | - | - |
| Raffinose | + | + | - | + | + | + | + | - | + | + |
| % of G + C content | 45.4 | 51.1 | 53.4 | 40.4 | 41.6 | 49.9 | 42.5 | 40.8 | 48.0 | 38.4 |
| Source | Human gut | Human gut | Sourdough | Human vagina | Human gut | Human mouth | Sourdough | Human vagina | Sourdough | Faeces |
-, Negative reaction; +, positive reaction; Ana, Anaerobic; FA, facultative anaerobic; ND not determined and w, weakly positive reaction.
Differential characteristics of (1) Limosilactobacillus caccae strain Marseille-P3519T; (2) Limosilactobacillus antri strain DSM 16041(Roos, Engstrand and Jonsson 2005); (3) Limosilactobacillus pontis strain DSM 8475 (Vogel et al. 1994); (4) Limosilactobacillus vaginalis strain DSM 5837 (Zheng etal. 2020); (5) Limosilactobacillus gastricus strain DSM 16045 (Roos, Engstrand and Jonsson 2005); (6) Limosilactobacillus oris strain DSM 4864 (Zheng et al. 2020); (7) Limosilactobacillus frumenti strain DSM 13145 (Müller, Ehrmann and Vogel 2000); (8) Limosilactobacillus coleohominis strain DSM 14060 (Nikolaitchouk et al. 2001); (9) Limosilactobacillus panis strain DSM 6035 (Zheng et al. 2020) and (10) Limosilactobacillus reuteri strain DSM 20016 (Kandler, Stetter and Köhl 1980).
| Characteristics . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.8–1.0 | 1.2–2 | 0.3–0.6 | 0.5–0.8 | 0.9–1.2 | 0.8–1.0 | 0.3 | ND | 0.7–0.9 | 0.7–1.0 |
| Oxygen requirement | Ana | Ana | Ana | FA | Ana | FA | FA | FA | Ana | Ana |
| Gram strain | + | + | + | + | + | + | + | + | + | + |
| Optimal growth temperature | 37°C | 25–45°C | 15–45°C | 30–45°C | 37°C | 30–40°C | 45°C | 45°C | 30–45°C | 35–38°C |
| Acid production from: | ||||||||||
| Arabinose | + | + | - | - | - | w | w | - | w | w |
| Ribose | + | + | + | - | - | + | + | + | + | + |
| Xylose | + | + | - | - | - | w | - | ND | + | w |
| Galactose | + | + | - | + | + | + | + | + | + | + |
| Fructose | + | + | + | ND | + | + | + | ND | + | + |
| Mannose | + | - | - | ND | + | + | + | ND | + | - |
| Mannitol | - | w | - | - | - | - | + | - | - | - |
| Sorbitol | - | - | - | - | - | - | + | - | ND | - |
| N-acetylglucosamine | + | - | - | - | w | - | + | ND | - | - |
| Amygdalin | - | - | - | ND | + | + | + | ND | - | - |
| Arbutin | - | - | - | - | + | + | + | ND | - | - |
| Esculin | - | w | - | - | + | - | + | - | + | - |
| Salicin | + | - | - | - | + | - | + | ND | + | - |
| Cellobiose | - | - | - | - | + | + | + | ND | - | - |
| Lactose | + | - | - | + | + | + | + | - | + | + |
| Melibiose | + | + | - | + | + | + | + | - | + | + |
| Trehalose | + | - | - | - | + | + | + | - | - | - |
| Melezitose | - | - | - | - | - | - | + | ND | - | - |
| Raffinose | + | + | - | + | + | + | + | - | + | + |
| % of G + C content | 45.4 | 51.1 | 53.4 | 40.4 | 41.6 | 49.9 | 42.5 | 40.8 | 48.0 | 38.4 |
| Source | Human gut | Human gut | Sourdough | Human vagina | Human gut | Human mouth | Sourdough | Human vagina | Sourdough | Faeces |
| Characteristics . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell diameter (μm) | 0.8–1.0 | 1.2–2 | 0.3–0.6 | 0.5–0.8 | 0.9–1.2 | 0.8–1.0 | 0.3 | ND | 0.7–0.9 | 0.7–1.0 |
| Oxygen requirement | Ana | Ana | Ana | FA | Ana | FA | FA | FA | Ana | Ana |
| Gram strain | + | + | + | + | + | + | + | + | + | + |
| Optimal growth temperature | 37°C | 25–45°C | 15–45°C | 30–45°C | 37°C | 30–40°C | 45°C | 45°C | 30–45°C | 35–38°C |
| Acid production from: | ||||||||||
| Arabinose | + | + | - | - | - | w | w | - | w | w |
| Ribose | + | + | + | - | - | + | + | + | + | + |
| Xylose | + | + | - | - | - | w | - | ND | + | w |
| Galactose | + | + | - | + | + | + | + | + | + | + |
| Fructose | + | + | + | ND | + | + | + | ND | + | + |
| Mannose | + | - | - | ND | + | + | + | ND | + | - |
| Mannitol | - | w | - | - | - | - | + | - | - | - |
| Sorbitol | - | - | - | - | - | - | + | - | ND | - |
| N-acetylglucosamine | + | - | - | - | w | - | + | ND | - | - |
| Amygdalin | - | - | - | ND | + | + | + | ND | - | - |
| Arbutin | - | - | - | - | + | + | + | ND | - | - |
| Esculin | - | w | - | - | + | - | + | - | + | - |
| Salicin | + | - | - | - | + | - | + | ND | + | - |
| Cellobiose | - | - | - | - | + | + | + | ND | - | - |
| Lactose | + | - | - | + | + | + | + | - | + | + |
| Melibiose | + | + | - | + | + | + | + | - | + | + |
| Trehalose | + | - | - | - | + | + | + | - | - | - |
| Melezitose | - | - | - | - | - | - | + | ND | - | - |
| Raffinose | + | + | - | + | + | + | + | - | + | + |
| % of G + C content | 45.4 | 51.1 | 53.4 | 40.4 | 41.6 | 49.9 | 42.5 | 40.8 | 48.0 | 38.4 |
| Source | Human gut | Human gut | Sourdough | Human vagina | Human gut | Human mouth | Sourdough | Human vagina | Sourdough | Faeces |
-, Negative reaction; +, positive reaction; Ana, Anaerobic; FA, facultative anaerobic; ND not determined and w, weakly positive reaction.
Cellular fatty acid composition (%) of the strain Marseille-P3519T compared to other Limosilactobacillus speciesa.
| . | . | L. caccae sp. nov. . | L. alvi . | L. reuteri . | L. vaginalis . | L. fastidiosus . |
|---|---|---|---|---|---|---|
| Fatty acids . | Names . | Marseille-P3519T . | R54T . | DSM 20016T . | DSM 5837T . | WF-MO7-1T . |
| C16:0 | Hexadecanoic acid | 50.8 | 20.0 | 37.4 | 23.7 | 33.4 |
| C18:1n9 | 9-Octadecenoic acid | 18.0 | 64.9 | 6.7 | 33.3 | 17.2 |
| C18:2n6 | 9,12-Octadecadienoic acid | 9.8 | 0 | 0 | 0 | 0 |
| C19:1n9 | 10-Nonadecenoic acid | 8.9 | 1.0 | 5.4 | 15.7 | 0 |
| C18:0 | Octadecanoic acid | 6.1 | 2.7 | 7.1 | 0 | 1.7 |
| C18:1n7 | 11-Octadecenoic acid | 3.8 | 5.2 | 13.9 | 15.5 | 0 |
| C14:0 | Tetradecanoic acid | 0 | 4.3 | 2.3 | 7.4 | 7.6 |
| . | . | L. caccae sp. nov. . | L. alvi . | L. reuteri . | L. vaginalis . | L. fastidiosus . |
|---|---|---|---|---|---|---|
| Fatty acids . | Names . | Marseille-P3519T . | R54T . | DSM 20016T . | DSM 5837T . | WF-MO7-1T . |
| C16:0 | Hexadecanoic acid | 50.8 | 20.0 | 37.4 | 23.7 | 33.4 |
| C18:1n9 | 9-Octadecenoic acid | 18.0 | 64.9 | 6.7 | 33.3 | 17.2 |
| C18:2n6 | 9,12-Octadecadienoic acid | 9.8 | 0 | 0 | 0 | 0 |
| C19:1n9 | 10-Nonadecenoic acid | 8.9 | 1.0 | 5.4 | 15.7 | 0 |
| C18:0 | Octadecanoic acid | 6.1 | 2.7 | 7.1 | 0 | 1.7 |
| C18:1n7 | 11-Octadecenoic acid | 3.8 | 5.2 | 13.9 | 15.5 | 0 |
| C14:0 | Tetradecanoic acid | 0 | 4.3 | 2.3 | 7.4 | 7.6 |
Data of L. reuteri DSM 20016T and L. vaginalis DSM 5837T were collected from the BacDive website (https://bacdive.dsmz.de/) and those of L. fastidiosus were retrieved from its original publication (Li et al. 2021).
Cellular fatty acid composition (%) of the strain Marseille-P3519T compared to other Limosilactobacillus speciesa.
| . | . | L. caccae sp. nov. . | L. alvi . | L. reuteri . | L. vaginalis . | L. fastidiosus . |
|---|---|---|---|---|---|---|
| Fatty acids . | Names . | Marseille-P3519T . | R54T . | DSM 20016T . | DSM 5837T . | WF-MO7-1T . |
| C16:0 | Hexadecanoic acid | 50.8 | 20.0 | 37.4 | 23.7 | 33.4 |
| C18:1n9 | 9-Octadecenoic acid | 18.0 | 64.9 | 6.7 | 33.3 | 17.2 |
| C18:2n6 | 9,12-Octadecadienoic acid | 9.8 | 0 | 0 | 0 | 0 |
| C19:1n9 | 10-Nonadecenoic acid | 8.9 | 1.0 | 5.4 | 15.7 | 0 |
| C18:0 | Octadecanoic acid | 6.1 | 2.7 | 7.1 | 0 | 1.7 |
| C18:1n7 | 11-Octadecenoic acid | 3.8 | 5.2 | 13.9 | 15.5 | 0 |
| C14:0 | Tetradecanoic acid | 0 | 4.3 | 2.3 | 7.4 | 7.6 |
| . | . | L. caccae sp. nov. . | L. alvi . | L. reuteri . | L. vaginalis . | L. fastidiosus . |
|---|---|---|---|---|---|---|
| Fatty acids . | Names . | Marseille-P3519T . | R54T . | DSM 20016T . | DSM 5837T . | WF-MO7-1T . |
| C16:0 | Hexadecanoic acid | 50.8 | 20.0 | 37.4 | 23.7 | 33.4 |
| C18:1n9 | 9-Octadecenoic acid | 18.0 | 64.9 | 6.7 | 33.3 | 17.2 |
| C18:2n6 | 9,12-Octadecadienoic acid | 9.8 | 0 | 0 | 0 | 0 |
| C19:1n9 | 10-Nonadecenoic acid | 8.9 | 1.0 | 5.4 | 15.7 | 0 |
| C18:0 | Octadecanoic acid | 6.1 | 2.7 | 7.1 | 0 | 1.7 |
| C18:1n7 | 11-Octadecenoic acid | 3.8 | 5.2 | 13.9 | 15.5 | 0 |
| C14:0 | Tetradecanoic acid | 0 | 4.3 | 2.3 | 7.4 | 7.6 |
Data of L. reuteri DSM 20016T and L. vaginalis DSM 5837T were collected from the BacDive website (https://bacdive.dsmz.de/) and those of L. fastidiosus were retrieved from its original publication (Li et al. 2021).
It is known that the Lactobacilli species develop a natural resistance to vancomycin (Salminen et al. 2006; Goldstein, Tyrrell and Citron 2015). However, different strains or species could have variable profile face to glycopeptides (Goldstein et al. 1995; Salminen et al. 2006). Currently, the best characterized intrinsic resistance in Lactic Acid Bacteria is surely the vancomycin-resistant phenotype (Goldstein, Tyrrell and Citron 2015). Indeed, several Lactobacilli species have terminal D-alanine residue replaced by D-lactate or D-serine at the muramyl-pentapeptide to prevent binding of vancomycin, which makes these species resistant to this antibiotic (Delcour et al. 1999). In addition, chromosomal mutations related to antibiotic resistance have also been described for lactobacilli. Antibiotic resistance determinants found in lactobacilli include hydrolysis, acetylation, enzymatic modification, efflux, ribosomal methylation and ribosomal protection (Gueimonde et al. 2013). Furthermore, resistance to teicoplanin has been observed for strain Marseille-P3519, as previously described in another members of Lactobacilli (Phillips and Golledge 1992). Intrinsic resistance was observed also for Colistin and Metronidazole, as previously described for Lacticaseibacillus rhamnosus and Lacticaseibacillus paracasei (Verdenelli et al. 2009; Gajdács, Spengler and Urbán 2017) and for another Lactobacilli species (Mändar et al. 2001).
Regarding strain Marseille-P3519, as shown in Table 3, the performed antibiotic susceptibility testing, using disc diffusion method according to EUCAST 2018 recommendations (Matuschek et al. 2018), reveals that Marseille-P3519T strain was susceptible to fusidic acid (10 µg/disc), imipenem (10), rifampicin (30), erythromycin (15), doxycycline (30), gentamicin (15 and 500), cefoxitin (30), penicillin G (10), linezolid (30), doripenem (10), nitrofurantoin (300) and tobramycin (10), but was resistant to four antibiotics tested, including vancomycin (30), teicoplanin (30), colistin (50) and metronidazole (4), all detected inside its genome. These are the resistance genes to vancomycin and metronidazole (Table 4).
| Antibiotics . | Codes . | Diameter (mm) . | Results . |
|---|---|---|---|
| Penicillin G | P 10 | 41.9 | S |
| Cefoxitin | FOX 30 | 43.3 | S |
| Doripenem | DOR 10 | 39.1 | S |
| Imipenem | IPM 10 | 38.5 | S |
| Tobramycin | TOB 10 | 33.7 | S |
| Gentamicin | CN 15 | 40.9 | S |
| Doxycycline | DO 30 | 34.3 | S |
| Vancomycin | VA 30 | 7.25 | R |
| Teicoplanin | TEC 30 | 7.25 | R |
| Colistin | CT 50 | 16.9 | R |
| Metronidazole | MET 4 | 7.25 | R |
| Rifampicin | RA 30 | 36.9 | S |
| Erythromycin | E 15 | 37.8 | S |
| Linezolid | LNZ 30 | 40.4 | S |
| Fusidic Acid | FA 10 | 25.4 | S |
| Nitrofurantoin | F 300 | 34.3 | S |
| Antibiotics . | Codes . | Diameter (mm) . | Results . |
|---|---|---|---|
| Penicillin G | P 10 | 41.9 | S |
| Cefoxitin | FOX 30 | 43.3 | S |
| Doripenem | DOR 10 | 39.1 | S |
| Imipenem | IPM 10 | 38.5 | S |
| Tobramycin | TOB 10 | 33.7 | S |
| Gentamicin | CN 15 | 40.9 | S |
| Doxycycline | DO 30 | 34.3 | S |
| Vancomycin | VA 30 | 7.25 | R |
| Teicoplanin | TEC 30 | 7.25 | R |
| Colistin | CT 50 | 16.9 | R |
| Metronidazole | MET 4 | 7.25 | R |
| Rifampicin | RA 30 | 36.9 | S |
| Erythromycin | E 15 | 37.8 | S |
| Linezolid | LNZ 30 | 40.4 | S |
| Fusidic Acid | FA 10 | 25.4 | S |
| Nitrofurantoin | F 300 | 34.3 | S |
S, Susceptible and R, Resistant
| Antibiotics . | Codes . | Diameter (mm) . | Results . |
|---|---|---|---|
| Penicillin G | P 10 | 41.9 | S |
| Cefoxitin | FOX 30 | 43.3 | S |
| Doripenem | DOR 10 | 39.1 | S |
| Imipenem | IPM 10 | 38.5 | S |
| Tobramycin | TOB 10 | 33.7 | S |
| Gentamicin | CN 15 | 40.9 | S |
| Doxycycline | DO 30 | 34.3 | S |
| Vancomycin | VA 30 | 7.25 | R |
| Teicoplanin | TEC 30 | 7.25 | R |
| Colistin | CT 50 | 16.9 | R |
| Metronidazole | MET 4 | 7.25 | R |
| Rifampicin | RA 30 | 36.9 | S |
| Erythromycin | E 15 | 37.8 | S |
| Linezolid | LNZ 30 | 40.4 | S |
| Fusidic Acid | FA 10 | 25.4 | S |
| Nitrofurantoin | F 300 | 34.3 | S |
| Antibiotics . | Codes . | Diameter (mm) . | Results . |
|---|---|---|---|
| Penicillin G | P 10 | 41.9 | S |
| Cefoxitin | FOX 30 | 43.3 | S |
| Doripenem | DOR 10 | 39.1 | S |
| Imipenem | IPM 10 | 38.5 | S |
| Tobramycin | TOB 10 | 33.7 | S |
| Gentamicin | CN 15 | 40.9 | S |
| Doxycycline | DO 30 | 34.3 | S |
| Vancomycin | VA 30 | 7.25 | R |
| Teicoplanin | TEC 30 | 7.25 | R |
| Colistin | CT 50 | 16.9 | R |
| Metronidazole | MET 4 | 7.25 | R |
| Rifampicin | RA 30 | 36.9 | S |
| Erythromycin | E 15 | 37.8 | S |
| Linezolid | LNZ 30 | 40.4 | S |
| Fusidic Acid | FA 10 | 25.4 | S |
| Nitrofurantoin | F 300 | 34.3 | S |
S, Susceptible and R, Resistant
Antibiotic resistance genes in L. caccae strain Marseille-P3519T with a threshold > 30% of identity.
| . | . | . | Functions . | ARGANNOT . | |
|---|---|---|---|---|---|
| Antibiotic class . | ORFs . | Size (aa) . | . | % Identity . | E-value . |
| Glycopeptide : Vancomycin | 205 | 188 | VanZ like family protein | 33.93 | 1.60e-07 |
| 273 | 228 | Response regulator ArlR | 40.09 | 1.04e-48 | |
| 407 | 231 | Response regulator ArlR | 37.28 | 8.00e-40 | |
| 715 | 231 | Response regulator MprA | 37.40 | 3.72e-22 | |
| 734 | 330 | D-lactate dehydrogenase | 34.96 | 9.66e-40 | |
| 835 | 363 | D-lactate dehydrogenase | 37.81 | 1.01e-51 | |
| 937 | 226 | Response regulator protein GraR | 32.14 | 3.53e-32 | |
| 1019 | 236 | Sensor transduction protein regX3 | 47.30 | 4.24e-17 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 46.64 | 2.88e-69 | |
| 1107 | 329 | D-lactate dehydrogenase | 35.03 | 1.10e-40 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 48.44 | 7.44e-71 | |
| 1183 | 376 | Alkaline phosphatase synthesis sensor protein PhoR | 36.80 | 2.04e-44 | |
| 1435 | 239 | Transcriptional regulatory protein SrrA | 38.56 | 3.67e-47 | |
| 1489 | 376 | D-alanine–D-alanine ligase vanA | 37.05 | 3.33e-61 | |
| 2030 | 330 | D-lactate dehydrogenase | 34.81 | 2.32e-58 | |
| Best BLAST hit in NCBI | |||||
| Nitroimidazole: Metronidazole | 207 | 151 | Peroxide-responsive repressor PerR | 31 | 2.46e-21 |
| 407 | 231 | Response regulator ArlR | 31 | 7.07e-11 | |
| 429 | 405 | Acyltransferase PapA5 | 41 | 0.19 | |
| 446 | 501 | Altronate dehydratase | 39 | 2.29e-11 | |
| 695 | 426 | Homoserine dehydrogenase | 44 | 5.19e-83 | |
| 705 | 207 | Transcriptional regulatory protein LiaR | 30 | 1.09e-08 | |
| 715 | 231 | Response regulator MprA | 31 | 1.53e-11 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 30 | 9.70e-11 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 37 | 3.08e-13 | |
| 1248 | 184 | NADH pyrophosphatase | 41 | 0.94 | |
| 1263 | 233 | HTH-type transcriptional repressor YvoA | 34 | 3.25e-09 | |
| 1373 | 338 | Glycerol-3-phosphate dehydrogenase (NAD(P)+) | 45 | 8.39e-103 | |
| 1536 | 359 | Recombinase A | 57 | 5.08e-142 | |
| 1846 | 707 | DNA topoisomerase 1 | 54 | 0.00 | |
| . | . | . | Functions . | ARGANNOT . | |
|---|---|---|---|---|---|
| Antibiotic class . | ORFs . | Size (aa) . | . | % Identity . | E-value . |
| Glycopeptide : Vancomycin | 205 | 188 | VanZ like family protein | 33.93 | 1.60e-07 |
| 273 | 228 | Response regulator ArlR | 40.09 | 1.04e-48 | |
| 407 | 231 | Response regulator ArlR | 37.28 | 8.00e-40 | |
| 715 | 231 | Response regulator MprA | 37.40 | 3.72e-22 | |
| 734 | 330 | D-lactate dehydrogenase | 34.96 | 9.66e-40 | |
| 835 | 363 | D-lactate dehydrogenase | 37.81 | 1.01e-51 | |
| 937 | 226 | Response regulator protein GraR | 32.14 | 3.53e-32 | |
| 1019 | 236 | Sensor transduction protein regX3 | 47.30 | 4.24e-17 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 46.64 | 2.88e-69 | |
| 1107 | 329 | D-lactate dehydrogenase | 35.03 | 1.10e-40 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 48.44 | 7.44e-71 | |
| 1183 | 376 | Alkaline phosphatase synthesis sensor protein PhoR | 36.80 | 2.04e-44 | |
| 1435 | 239 | Transcriptional regulatory protein SrrA | 38.56 | 3.67e-47 | |
| 1489 | 376 | D-alanine–D-alanine ligase vanA | 37.05 | 3.33e-61 | |
| 2030 | 330 | D-lactate dehydrogenase | 34.81 | 2.32e-58 | |
| Best BLAST hit in NCBI | |||||
| Nitroimidazole: Metronidazole | 207 | 151 | Peroxide-responsive repressor PerR | 31 | 2.46e-21 |
| 407 | 231 | Response regulator ArlR | 31 | 7.07e-11 | |
| 429 | 405 | Acyltransferase PapA5 | 41 | 0.19 | |
| 446 | 501 | Altronate dehydratase | 39 | 2.29e-11 | |
| 695 | 426 | Homoserine dehydrogenase | 44 | 5.19e-83 | |
| 705 | 207 | Transcriptional regulatory protein LiaR | 30 | 1.09e-08 | |
| 715 | 231 | Response regulator MprA | 31 | 1.53e-11 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 30 | 9.70e-11 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 37 | 3.08e-13 | |
| 1248 | 184 | NADH pyrophosphatase | 41 | 0.94 | |
| 1263 | 233 | HTH-type transcriptional repressor YvoA | 34 | 3.25e-09 | |
| 1373 | 338 | Glycerol-3-phosphate dehydrogenase (NAD(P)+) | 45 | 8.39e-103 | |
| 1536 | 359 | Recombinase A | 57 | 5.08e-142 | |
| 1846 | 707 | DNA topoisomerase 1 | 54 | 0.00 | |
Antibiotic resistance genes in L. caccae strain Marseille-P3519T with a threshold > 30% of identity.
| . | . | . | Functions . | ARGANNOT . | |
|---|---|---|---|---|---|
| Antibiotic class . | ORFs . | Size (aa) . | . | % Identity . | E-value . |
| Glycopeptide : Vancomycin | 205 | 188 | VanZ like family protein | 33.93 | 1.60e-07 |
| 273 | 228 | Response regulator ArlR | 40.09 | 1.04e-48 | |
| 407 | 231 | Response regulator ArlR | 37.28 | 8.00e-40 | |
| 715 | 231 | Response regulator MprA | 37.40 | 3.72e-22 | |
| 734 | 330 | D-lactate dehydrogenase | 34.96 | 9.66e-40 | |
| 835 | 363 | D-lactate dehydrogenase | 37.81 | 1.01e-51 | |
| 937 | 226 | Response regulator protein GraR | 32.14 | 3.53e-32 | |
| 1019 | 236 | Sensor transduction protein regX3 | 47.30 | 4.24e-17 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 46.64 | 2.88e-69 | |
| 1107 | 329 | D-lactate dehydrogenase | 35.03 | 1.10e-40 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 48.44 | 7.44e-71 | |
| 1183 | 376 | Alkaline phosphatase synthesis sensor protein PhoR | 36.80 | 2.04e-44 | |
| 1435 | 239 | Transcriptional regulatory protein SrrA | 38.56 | 3.67e-47 | |
| 1489 | 376 | D-alanine–D-alanine ligase vanA | 37.05 | 3.33e-61 | |
| 2030 | 330 | D-lactate dehydrogenase | 34.81 | 2.32e-58 | |
| Best BLAST hit in NCBI | |||||
| Nitroimidazole: Metronidazole | 207 | 151 | Peroxide-responsive repressor PerR | 31 | 2.46e-21 |
| 407 | 231 | Response regulator ArlR | 31 | 7.07e-11 | |
| 429 | 405 | Acyltransferase PapA5 | 41 | 0.19 | |
| 446 | 501 | Altronate dehydratase | 39 | 2.29e-11 | |
| 695 | 426 | Homoserine dehydrogenase | 44 | 5.19e-83 | |
| 705 | 207 | Transcriptional regulatory protein LiaR | 30 | 1.09e-08 | |
| 715 | 231 | Response regulator MprA | 31 | 1.53e-11 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 30 | 9.70e-11 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 37 | 3.08e-13 | |
| 1248 | 184 | NADH pyrophosphatase | 41 | 0.94 | |
| 1263 | 233 | HTH-type transcriptional repressor YvoA | 34 | 3.25e-09 | |
| 1373 | 338 | Glycerol-3-phosphate dehydrogenase (NAD(P)+) | 45 | 8.39e-103 | |
| 1536 | 359 | Recombinase A | 57 | 5.08e-142 | |
| 1846 | 707 | DNA topoisomerase 1 | 54 | 0.00 | |
| . | . | . | Functions . | ARGANNOT . | |
|---|---|---|---|---|---|
| Antibiotic class . | ORFs . | Size (aa) . | . | % Identity . | E-value . |
| Glycopeptide : Vancomycin | 205 | 188 | VanZ like family protein | 33.93 | 1.60e-07 |
| 273 | 228 | Response regulator ArlR | 40.09 | 1.04e-48 | |
| 407 | 231 | Response regulator ArlR | 37.28 | 8.00e-40 | |
| 715 | 231 | Response regulator MprA | 37.40 | 3.72e-22 | |
| 734 | 330 | D-lactate dehydrogenase | 34.96 | 9.66e-40 | |
| 835 | 363 | D-lactate dehydrogenase | 37.81 | 1.01e-51 | |
| 937 | 226 | Response regulator protein GraR | 32.14 | 3.53e-32 | |
| 1019 | 236 | Sensor transduction protein regX3 | 47.30 | 4.24e-17 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 46.64 | 2.88e-69 | |
| 1107 | 329 | D-lactate dehydrogenase | 35.03 | 1.10e-40 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 48.44 | 7.44e-71 | |
| 1183 | 376 | Alkaline phosphatase synthesis sensor protein PhoR | 36.80 | 2.04e-44 | |
| 1435 | 239 | Transcriptional regulatory protein SrrA | 38.56 | 3.67e-47 | |
| 1489 | 376 | D-alanine–D-alanine ligase vanA | 37.05 | 3.33e-61 | |
| 2030 | 330 | D-lactate dehydrogenase | 34.81 | 2.32e-58 | |
| Best BLAST hit in NCBI | |||||
| Nitroimidazole: Metronidazole | 207 | 151 | Peroxide-responsive repressor PerR | 31 | 2.46e-21 |
| 407 | 231 | Response regulator ArlR | 31 | 7.07e-11 | |
| 429 | 405 | Acyltransferase PapA5 | 41 | 0.19 | |
| 446 | 501 | Altronate dehydratase | 39 | 2.29e-11 | |
| 695 | 426 | Homoserine dehydrogenase | 44 | 5.19e-83 | |
| 705 | 207 | Transcriptional regulatory protein LiaR | 30 | 1.09e-08 | |
| 715 | 231 | Response regulator MprA | 31 | 1.53e-11 | |
| 1087 | 235 | Transcriptional regulatory protein YycF | 30 | 9.70e-11 | |
| 1182 | 229 | Alkaline phosphatase synthesis transcriptional regulatory protein PhoP | 37 | 3.08e-13 | |
| 1248 | 184 | NADH pyrophosphatase | 41 | 0.94 | |
| 1263 | 233 | HTH-type transcriptional repressor YvoA | 34 | 3.25e-09 | |
| 1373 | 338 | Glycerol-3-phosphate dehydrogenase (NAD(P)+) | 45 | 8.39e-103 | |
| 1536 | 359 | Recombinase A | 57 | 5.08e-142 | |
| 1846 | 707 | DNA topoisomerase 1 | 54 | 0.00 | |
Genome properties
The genome is 2 237 367 bp long with 45.42 mol% G + C content. It is composed of 11 contigs with a size ranging from 1160 to 909 365 bp. Of the 2107 predicted genes, 2047 were protein-coding genes and 60 were RNAs (four genes are 5S rRNA, one gene is 16S rRNA, two genes are 23S rRNA and 53 genes are tRNA genes). A total of 1603 genes (78.31%) were assigned a putative function (by cogs or by NR blast). A total of 63 genes were identified as ORFans (3.08%). The remaining genes were annotated as hypothetical proteins (297 genes = > 14.51%). Table S1 (Supporting Information) summarizes the properties and statistics of the genome. The GenBank accession number for whole genome shotgun project of strain Marseille-P3519T is FTOY00000000.
Genome comparison
Limosilactobacillus caccae sp. nov. strain Marseille-P3519 was compared with the closest Limosilactobacillus species, particularly in terms of genome size, G + C content and proteome similarity (Table S2, Supporting Information). The draft genome sequence of strain Marseille-P3519T (2.23 Mbp) is smaller than those of Limosilactobacillus antri (2.24 Mbp), but larger than those of Limosilactobacillus pontis (1.66 Mbp), Limosilactobacillus vaginalis (1.78 Mbp), Limosilactobacillus gastricus (1.84 Mb), Limosilactobacillus oris (2.03 Mbp), Limosilactobacillus frumenti (1.73 Mpb), Limosilactobacillus coleohominis (1.71 Mbp), Limosilactobacillus panis (2.00 Mbp) and L. reuteri (2.03 Mbp). The G + C content of strain Marseille-P3519T (45.42 mol%) is smaller than those of L. antri (51.11 mol%), L. pontis (53.45 mol%), L. oris (49.98 mol%) and L. panis (48.08 mol%), but larger than those of L. vaginalis (40.46 mol%), L. gastricus (41.64 mol%), L. frumenti (42.55 mol%), L. coleohominis (40.81 mol%) and L. reuteri (38.45 mol%). When comparing the number of genes in our strain with those of Limosilactobacillus sp., we note that strain Marseille-P3519T (n = 2047) has more genes than those of L. antri (n = 2035), L. pontis (n = 1585), L. vaginalis (n = 1688), L. gastricus (n = 1773), L. oris (n = 1862), L. frumenti (n = 1641), L. coleohominis (n = 1715), L. panis (n = 1852) and L. reuteri (n = 1730). As shown in Fig. 2 and Table 6, comparison of the Marseille-P3519 genome with those of the most closely related species reveals several specific genome contents, such as a complete and intact integrated prophage (phiRv2) with a size of 46.55 kb and 36.75 mol% G + C and a CRISPR-Cas cluster of 14.08 kb and 36.7 mol% G + C. BlastN analysis of this prophage sequence demonstrates its presence in L. reuteri genomes only (data not shown).
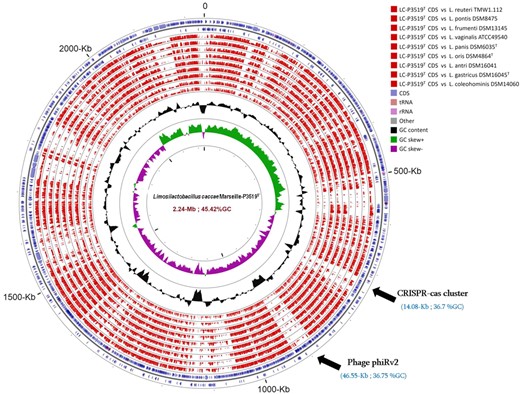
Graphical circular map of the genome of L. caccae P3519T compared to those of the most closely related species. From outside to the center, ring 1 and 2: genes on the forward and reverse strand (blue circles); from ring 3 to 11: genomes of the most closely related species (red circles); ring 12: GC skew; ring 13: % G + C content. Details of species names, genome sizes, genome %GC content, number of proteins and genes are provided in Table 6.
OrthoANI analysis showed that L. oris and L. antri shared the highest genomic similarity value (85.76%) while L. gastricus and L. panis had the lowest value found (68.21%). When L. caccae is compared to other Limosilactobacillus spp., we find that it shared 75.83% with L. reuteri and 68.77% with L. gastricus as being respectively the highest and lowest values in terms of ANI (Figure S2, Supporting Information). These values are below recommended cut-off values for delineating the species barrier in prokaryotic organisms.
DISCUSSION
Recent emerging approaches in microbiology have made it possible to characterize new, previously unknown bacteria. Culturomics is the pioneering method in the exploration of gut microbiota and provides a better understanding of microbial diversity. Therefore, strain Marseille-P3519 was isolated from a French healthy woman using a culturomic approach (Lagier et al. 2015). Based on its 16S rRNA of 98.57% of sequence similarity with L. caviae strain MOZM2, strain Marseille-P3519 was considered a new candidate bacterial species in the genus Limosilactobacillus on the basis that the cut-off value for sequence similarity for species delineation was determined to be 98.65% (Kim et al. 2019). Strain Marseille-P3519 is resistant to vancomycin, colistin and metronidazole. Similar resistance to this group of antibiotics has also been observed in other Lactobacilli previously studied (Mändar et al. 2001). For this reason, it was not surprising to observe resistance genes following its genome analysis.
The genus Limosilactobacillus contains 24 species, including six subspecies, among which L. caccae sp. nov., newly described here. The type strain of L. caccae sp. nov., was strain Marseille-P3519T, which is a rod-shaped, non-spore-forming and non-motile bacterium, as most of Limosilactobacillus criteria. A complete description including many genomic and phenotypic details is provided in this report.
Finally, based on the results obtained with the phenotypic, phylogenetic, biochemical and genomic evidences presented here, we formally propose the creation of L. caccae sp. nov., containing the strain Marseille-P3519T. This bacterial strain was first isolated from the fecal flora of a 25-year-old healthy French woman as part of the culturomics study of the gut microbiota.
Description of L. caccae sp. nov.
Limosilactobacillus caccae (cac'cae. Gr. fem. n. kakke, faeces; N.L. gen. n. caccae of faeces, to refer to the stool sample from which this bacterium was isolated). Cells were Gram-positive, rod-shaped, non-spore-forming, non-motile and anaerobic. The strain Marseille-P3519T grows on 5% sheep blood-enriched Columbia agar at 37°C in anaerobic atmosphere after 48 h of incubation. Colonies appear white, opaque, circular and smooth on blood agar with a mean diameter of 0.4 mm in diameter. Optimal growth was observed at 37°C under the same conditions. The major fatty acid found for this strain was Hexadecanoic acid (50%) and 9-Octadecenoic acid (18%). Negative reactions were obtained for catalase and oxidase tests. Strain Marseille-P3519 T is resistant to antibiotics, such as Vancomycin (30 µg), Metronidazole (4 µg), Colistin (50 µg) and Teicoplanin (30 µg), but it was susceptible to fusidic acid, imipenem, rifampicin, erythromycin, doxycycline, gentamicin, cefoxitin, penicillin G, linezolid, doripenem, nitrofurantoin and tobramycin. Using API ZYM and 50CH strips, positive reactions were obtained with glycerol, esterase lipase, arabinose, D-ribose, xylose, galactose, glucose, fructose, mannose, cystine arylamidase, phosphatase acid, methyl α-D-mannopyranoside, salicin, starch, glycogen, gentiobiose, esterase, leucine arylamidase, valine arylamidase, α-galactosidase, β- galactosidase, D-maltose, D-lactose, D-melibiose, sucrose, D-trehalose and D-raffinose. The genome size of L. caccae strain Marseille-P3519T is about 2.23 Mbp long with 45.42 mol% G + C content. The Genbank Accession number for the 16S rRNA gene sequence of strain Marseille-P3519T is LT671596 and the one referring to the whole genome shotgun project is FTOY00000000. It was isolated from the ileum of a sick French woman. The type strain Marseille-P3519T ( = CSUR P3519 = CECT 30110) was first isolated from the fecal flora of a 25-year-old healthy French woman.
CONTRIBUTION OF AUTHORS
ND and CIL contributed to data acquisition, analysis and interpretation and wrote the original draft of the manuscript. AM and PFMG participated in data analysis and revised the manuscript. VM, IIN and CV contributed to data acquisition and analysis. NA contributed to the fatty acids profiling. FF, PEF and JCL helped to interpret data and revised the manuscript. DR contributed to the conception of the study. SMD designed and coordinated the study and revised the manuscript.
ACKNOWLEDGEMENTS
We are grateful to the European funding FEDER PRIMI, the Région Provence-Alpes-Côte d'Azur, Institut Hospitalo-Universitaire (IHU) Méditerranée Infection and the National Research Agency under the program « Investissements d'avenir », reference ANR-10-IAHU-03.
Conflicts of interest
None declared.
REFERENCES
Author notes
Cheikh Ibrahima Lo and Niokhor Dione contributed equally



