-
PDF
- Split View
-
Views
-
Cite
Cite
Raeid M. M. Abed, Assad Al-Thukair, Dirk de Beer, Bacterial diversity of a cyanobacterial mat degrading petroleum compounds at elevated salinities and temperatures, FEMS Microbiology Ecology, Volume 57, Issue 2, August 2006, Pages 290–301, https://doi.org/10.1111/j.1574-6941.2006.00113.x
Close - Share Icon Share
Abstract
Cyanobacterial mats of the Arabian Gulf coast of Saudi Arabia experience extreme conditions of temperature and salinity. Because they are exposed to continuous oil pollution, they form ideal models for biodegradation under extreme conditions. We investigated the bacterial diversity of these mats using denaturing gradient gel electrophoresis and 16S rRNA cloning, and tested their potential to degrade petroleum compounds at various salinities (fresh water to 16%) and temperatures (5 to 50°C). Cloning revealed that c. 15% of the obtained sequences were related to unknown, possibly novel bacteria. Bacteria belonging to Beta-, Gamma- and Deltaproteobacteria, Cytophaga–Flavobacterium–Bacteroides group and Spirochetes, were detected. The biodegradation of petroleum compounds at different salinities by mat microorganisms showed that pristine and n-octadecane were optimally degraded at salinities between 5 and 12% (weight per volume NaCl) whereas the optimum degradation of phenanthrene and dibenzothiophene was at 3.5% salinity. The latter compounds were also degradable at 8% salinity. The same compounds were degraded at temperatures between 15 and 40°C but not at 5 and 50°C. The optimum temperature of degradation was 28–40°C for both aliphatics and aromatics. We conclude that the studied microbial mats from Saudi Arabia are rich in novel halotolerant and thermotolerant microorganisms with the potential to degrade petroleum compounds at elevated salinities and temperatures.
Introduction
Cyanobacterial mats develop well under extreme conditions, where the abundance and activity of grazing organisms is limited (Javor & Castenholz, 1984; Cohen, 1989; Farmer, 1992). The Arabian Gulf is a harsh environment, situated in a semi-arid region with high evaporation rates (200 cm year−1) (Khan & Al-Ajmi, 1998). The seawater salinity may reach up to 16% during warm seasons, when the temperature often exceeds 50°C. The coastal flats of the Gulf are covered by a variety of microbial mat systems (Al-Thukair & Al-Hinai, 1993) that are subjected to significant seasonal changes in temperature and salinity as well as to daily cycles of dehydration and rewetting by tides. Owing to the tidal regime, these mats experience highly variable environmental conditions that change quickly from moderate (3–4% salinity, 25°C) to extreme (16% salinity, 50°C) on a daily basis. These mats are of additional interest because of their frequent exposure to oil pollution from nearby terminals. Indeed, some of these mats became dominant only after the Gulf War in 1991, when more than 10.8 million barrels of crude oil was released into the Arabian Gulf (Sorkhoh, 1992; Fayad & Overton, 1995). In spite of the unique environmental settings of the mats, the diversity of their microbial communities has been poorly investigated. Earlier reports were based mainly on microscopy and cultivation techniques (Sorkhoh, 1993, 1995; Hoffmann, 1996; Höpner, 1996), where diversity is often underestimated.
Salinity and temperature are important key environmental parameters that influence the degradation process of petroleum compounds. These parameters influence the structure and physiology of existing microbial communities and change the physical and chemical properties of the pollutants (e.g. solubility and viscosity). The diversity and metabolic potential of degrading bacteria are considered to decrease as the environmental conditions become more extreme (Foght & McFarlane, 1999; Margesin & Schinner, 2001). Previous studies showed that rates of hydrocarbon biodegradation decreased with increasing salinity, and biodegradation could not be detected above 15% salinity (Ward & Brock, 1978; Rhykerd, 1995). Nevertheless, bacterial strains capable of performing pollutant degradation at high salt concentrations were isolated (Oren, 1992). The increase in temperature was shown to enhance biodegradation (Ward & Brock, 1976; Margesin & Schinner, 2001), although high temperatures are known to reduce the diversity of microorganisms. Saudi Arabia's contaminated mats experience these extreme parameters simultaneously, and thus the adaptation of bacterial communities and the chances for bioremediation are difficult to predict from present knowledge. Therefore, additional information on the capability of microbial communities within the mats to degrade oil components at variable salinities and temperatures is required.
The aim of this study was to explore the bacterial diversity of cyanobacterial mats along the coasts of Saudi Arabia using culture-independent approaches such as denaturing gradient gel electrophoresis (DGGE) and 16S rRNA gene cloning. The potential of the mat communities to degrade aliphatic (pristane and n-octadecane) and aromatic (phenanthrene and dibenzothiophene) compounds at different salinities and temperatures was also examined. The compounds were selected to represent major groups of oil constituents (branched alkane, straight-chain alkane, aromatic hydrocarbons and organosulphur compounds, respectively).
Materials and methods
Mat samples and environmental settings
Microbial mats were collected in November 2002 from Dawhat Al-Daffi in Saudi Arabia, north of Jubail. The site was selected because of its accessibility and location close to oil terminals. The mats developed directly after the 1991 oil spill and they are currently growing on top of sandy sediments that still contain oil residues. These mats are frequently exposed to occasional oil-spill incidents from nearby oil terminals. The air temperature at the sampling site is on average 17°C in winter, and may reach 50°C in a hot summer. At the time of sampling, the air temperature was 30°C and the salinity of the water was 5%. During high tide the entire site is flooded, whereas during low tide the seawater retreats and the area is exposed to air. High rates of evaporation during the low tide lead to salt precipitation and frequent desiccation of the mats (Fig. 1, left panel). The upper intertidal zone is usually drier than the lower intertidal zone. The continuous flushing of seawater by tides results in the formation of channels throughout the entire mat system (Fig. 1, right panel). These channels retain seawater during low tides, forming small lagoons that are surrounded by dry, slightly elevated sediments. Microbial mats from these channels were collected either frozen in liquid N2 for molecular analysis, or kept viable for biodegradation experiments.

The studied microbial mats from Saudi Arabia, showing a whitish top layer of salt resulting from high evaporation (left panel) and the channelling system within these mats (right panel).
Molecular analysis
Mat cores from low, middle and high intertidal zones (c. 300–500 mg each) were subjected to nucleic acid extraction, PCR and DGGE, as previously described (Abed, 2002b). PCR amplification of 16S rRNA genes was carried out using two sets of oligonucleotide primers. CYA359F (with a 40 nucleotide GC clamp at the 5′ end) and CYA781R were used as cyanobacteria-specific primers (Nübel, 1997), and GM5F with GC clamp in combination with the reverse primer 907R for the domain bacteria (Muyzer, 1995). Thermocycling was performed using a Mastercycler gradient cycler (Eppendorf, Hamburg, Germany). A hot-start program was performed for the cyanobacteria-specific primers as described by Nübel (1997). A hot-start touch-down program was used for the bacterial universal primers (GM5 and 907R) in order to minimize nonspecific amplification (Muyzer, 1995). DGGE was carried out using a Bio-Rad D-Code system and was run at 60°C and at a constant voltage of 200 V for 3.5 h. DGGE bands were excised manually, the DNA left to diffuse out in buffer overnight, and PCR reamplified. The amplification products were sequenced in both directions.
Phylogenetic analysis
Cyanobacterial phylogenetic trees were constructed based on long 16S rRNA gene sequences (more than 1300 bp) by applying a variety of methods integrated in the ARB software (Ludwig, 1998) such as maximum likelihood, maximum parsimony and neighbour joining (a maximum likelihood tree is presented in Figs 3 and 4). Partial sequences were not included in the calculation of trees. The 16S rRNA gene sequences of Escherichia coli and Bacillus subtilis were included in the calculations as outgroup sequences. Trees calculated using different methods were essentially equivalent, and maximum likelihood trees are presented here. The cyanobacterial forward and reverse complementary sequences obtained from DGGE bands were aligned against each other in order to obtain consensus sequences. These sequences were then aligned to the sequences in the ARB database using the alignment tool of the ARB software package and were inserted into the pre-established tree using the parsimony ARB tool and maintaining the overall tree topology without changes. Bacterial partial sequences were also inserted within a pre-established stable tree containing all bacterial sequences in the ARB database without allowing changes of the overall tree topology. The final tree was minimized for simplicity in presentation.

Phylogenetic tree (maximum likelihood) for cyanobacteria based on publicly available, almost complete 16S rRNA genes from members of the cyanobacterial line. Escherichia coli and Bacillus subtilis were used as outgroups. The partial 16S rRNA gene sequences from our excised denaturing gradient gel electrophoresis bands were placed phylogenetically using parsimony criteria without changing the topology of the pre-established tree. The bar indicates 10% sequence divergence. Bootstrap values from 1000 trees are included and indicated as % at relevant nodes.
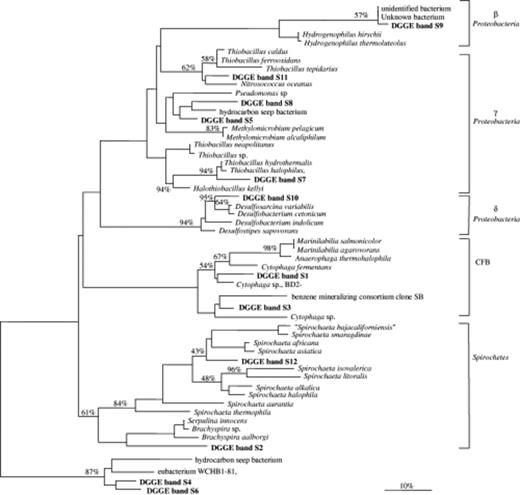
Unrooted phylogenetic tree showing affiliations based on partial bacterial 16S rRNA genes and selected sequences from members of various bacterial clusters, including Beta, Gamma and Delta subclasses of the Proteobacteria, the Cytophaga–Flavobacterium–Bacteroides group (CFB) and Spirochetes. The tree was simplified for clarity by omitting all sequences between clusters. The scale bar indicates 10% estimated sequence divergence. Bootstrap values from 1000 trees are included and indicated as % at relevant nodes.
Construction of a clone library
To expand the results from DGGE analysis, a clone library from the low intertidal mat was constructed. PCR was performed on the DNA extracted from this mat using the GM3 and GM4 primers (Muyzer, 1995). PCR products were purified using the QIAquick PCR purification kit (Diagen, Düsseldorf, Germany) and were cloned by applying a pGEM-T plasmid vector system and competent E. coli JM 109 cells (Promega, Heidelberg, Germany). The clones obtained were screened for the presence of inserts. Plasmids were prepared from positive clones using the Wizard Plus Minipreps Kit (Promega). The plasmid inserts were sequenced with an ABI PRISM 3100 genetic analyser (Applied Biosystems, Foster City, CA).
Preparation of organo-clay complexes
n-Octadecane, pristane, phenanthrene, and dibenzothiophene were selected as model compounds for petroleum constituents. Hydrophobic clay was used as a carrier substance for the petroleum model compounds (Grötzschel, 2002). To a 2% aqueous suspension of montmorillonite KSF (Aldrich, Steinheim, Germany), 10 mM trimethylammmonium (BTMA) chloride was added slowly to obtain 0.8 mmol BTMA g−1 clay [modified after (El-Nahhal, 2000)]. The mixture was stirred for 24 h, washed three times to remove excess BTMA chloride, and then freeze-dried. To adsorb the model compounds, the hydrophobic clay (BTMA-montmorillonite) was suspended in n-hexane. The mixture of the model compounds (in total 20 mg per 100 mg of hydrophobic clay dissolved in n-hexane) was slowly added under continuous stirring. The slurry was dried in a vacuum rotary evaporator, which yielded a homogeneous powder of organo-clay complex loaded with 16.67 wt% of petroleum model compounds (hereafter designated as OCC). The adsorbed amount of model compounds was re-extracted with dichloromethane (DCM) and analysed by gas chromatography.
Biodegradation experiments
Two sets of 250 mL Erlenmeyer flasks were prepared: one to test degradation under different salinities and the other to test degradation under different temperatures. Hypersaline seawater media of 5%, 8%, 12% and 16% weight per volume (w/v) final total salinity were prepared by adding appropriate amounts of NaCl to natural seawater. Nitrogen (3.75 mM NH4Cl) and phosphorus (1.1 mM KH2PO4) sources were added, and the media were then autoclaved. All flasks received 100 mL of medium, 1 g of mat material, and 100 mg OCC. The following controls were used: (i) medium with OCC without mat material (abiotic control); and (ii) medium with OCC and autoclaved mat core. The latter control was used to correct for the adsorption of OCC to mat material. Degradation of the compounds was tested at salinities of 3.5%, 5%, 8%, 12%, and 16% (w/v NaCl) and incubation at 28°C. Degradation was also tested at 3.5% salinity and at temperatures of 5, 15, 28, 40 and 50°C. All flasks were incubated on a rotary shaker at 100 rpm with a light regime of 12 h light/12 h dark. The light intensity was 100 μmol photons m−2 s−1 (photosynthetically available radiation). Flasks were sampled at days 2, 6, 12, 18 and 26. Samples (2 mL) were collected after vigorous shaking of the flasks in order to suspend the solid OCC and to obtain homogenous samples. These samples were stored in 20 mL glass tubes sealed with Teflon-coated screw caps at −20°C prior to extraction.
Chemical analyses
The samples were extracted with 3.5 volumes of dichloromethane (7 mL) by vertical shaking for 3 h at room temperature. The organic phase was collected and immediately analysed with a Perkin-Elmer AutoSystem gas chromatograph (Perkin-Elmer GmbH) equipped with a CTC A200S Auto-sampler (CTC Analytics), a flame ionization detector (FID) and an Optima-5 column (50 m × 0.32 mm; film thickness 0.25 μm). The oven was operated for 2 min at 60°C, heated at a rate of 20°C min−1 to 150°C and at a rate of 5°C min−1 to 310°C, and then kept at 310°C for 10 min. The injection port and FID were operated at 250 and 350°C respectively. The model hydrocarbons were quantified by integration of the FID signals and comparison with standards. The calculated initial amount of each model compound in 2 mL sample was 66.67 μg. Data on the abundance of the model compounds are presented relative to this value.
Results
Microbial diversity within the studied mats
Denaturing gradient gel electrophoresis analysis of the cyanobacterial populations from samples collected at different distances from the tidal water (less than 50 m apart from each other) revealed differences in the community composition (Fig. 2, left panel). The DGGE profile from the lower intertidal mat displayed two major bands (SA1C1 and SA1C2) that had sequences affiliated to sequences of Arthrospira, Lyngbya, Phormidium and Oscillatoria species (Fig. 3). The middle tidal mat showed three major bands, with sequences closely related to the newly described genus Halomicronema (Abed, 2002a) with 94% sequence similarity (band SA2C1), to Phormidium minutum D5 with 95% sequence similarity (band SA2C2), and to Microcoleus chthonoplastes with 98% sequence similarity (band SA2C3). From the upper intertidal mat, the sequences of DGGE bands SA3C1 and SA3C4 were phylogenetically affiliated to Phormidium minutum D5 (92.5% similarity) and Microcoleus chthonoplastes (94.5% similarity), respectively. The closest relative to band SA3C2 and band SA3C3 sequences was the sequence of Oscillatoria neglecta M-82.
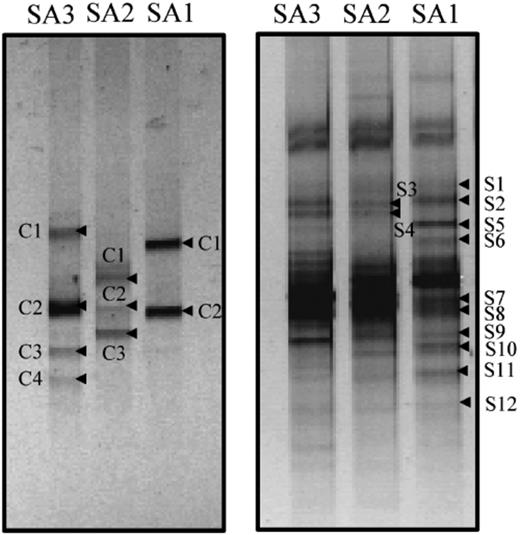
Denaturing gradient gel electrophoresis fingerprints of PCR-amplified 16S rRNA gene fragments obtained from microbial mats present at low (SA1), middle (SA2) and high (SA3) intertide, using cyanobacteria-specific (left panel) and universal bacterial (right panel) primers. The indicated bands were excised, reamplified and sequenced.
The DGGE profile generated using the universal bacterial primers is presented in Fig. 2, right panel. Twelve bacterial sequences, excluding those of the cyanobacteria that were also amplified by the same primers, were obtained. The phylogenetic reconstruction of the sequences showed affiliation to Beta-, Gamma- and Deltaproteobacteria, the Cytophaga–Flavobacterium–Bacteroides (CFB) group, and Spirochetes (Fig. 4). Most DGGE fragments (DGGE bands S5, 7, 8 and 11) were derived from Gammaproteobacteria, and some of those sequences were related to sulphur-oxidizing bacterial species. The sequence of DGGE band S10 was closely related to the sulphate-reducing bacterium Desulfosarcina variabilis. DGGE bands S5 and S8 had sequences that were related to an environmental clone from a hydrocarbon seep environment, whereas the sequence of band S9 was related to an unknown species. In the CFB group, the sequences of DGGE bands S1 and S3 were related to the known Cytophaga fermentans.
Cloning provided a more detailed picture of the bacterial diversity within the low intertidal mat. A total of 96 clones were randomly picked and sequenced. Most clones belonged to the Gamma- and Deltaproteobacteria (20 and 18 clones, respectively) and to the CFB group (19 clones). The remaining clones were distributed among cyanobacteria (8 clones), Holophaga/Acidobacteria/Geothrix (3 clones), Verrumicrobia (3 clones), Chloroflexus (4 clones), Alphaproteobacteria (2 clones), Spirochetes (3 clones) and Planctomycetes (5 clones). Approximately 15% of the clones obtained were not related to known microorganisms in the database. Most of the Gammaproteobacteria were related to sulphur-oxidizing bacteria, and 70% of them had more than 96% sequence similarity to the sequence of the chemolithotrophic bacterium Thiobacillus hydrothermalis (accession number M90662). Two clones belonged to the phototrophic purple sulphur bacteria; one was affiliated to Thiobaca trueperi and the other to a Halochromatium species. Only three clones of the Gammaproteobacteria were related to the genera Pseudomonas and Alcanivorax, which are known to include oil degraders. The Deltaproteobacteria included mainly sequences from sulphate-reducing bacteria belonging to the genera Desulfococcus, Desulfonema, Desulfosarcina, Desulfomonile, and Desulfocapsa. The cyanobacterial clones were related mainly to filamentous cyanobacteria from the genera Phormidium and Microcoleus. A group of clones was related to species of the genus Deinococcus, which compromises coccoid bacteria that are resistant to UV and solar radiation (Rainey, 1997).
Degradation of petroleum compounds at different salinities
Recovery of the four compounds was, in most cases, more than 70% of the initially added amounts (Fig. 5). A slight decrease in the concentration of the four compounds was observed in the abiotic controls throughout the experiment, mainly as a result of experimental and analytical errors. Degradation of the four studied compounds was not possible in fresh-water conditions. The degradation of pristane and n-octadecane was observed at salinities between 3.5% and 16%. At 3.5–12% salinities and after 12 days of incubation, the concentrations of pristane and n-octadecane decreased to c. 25% and 14% of the initially added amount, respectively, and these amounts remained unchanged until the end of the experiment. Pristane was almost nondegradable at 16% salinity, whereas n-octadecane was considerably degraded at this salinity. Unlike the aliphatic compounds, which were degradable at different salinities, the degradation of aromatics was only observed at 3.5% salinity and to a lesser extent at 8% salinity. Phenanthrene and dibenzothiophene showed a similar pattern of degradation. At 3.5% salinity and after incubation for 12 days, both compounds were completely degraded. Whereas the degradation of phenanthrene and dibenzothiophene was not significant at 5% salinity, a further increase to 8% resulted in a measurable degradation to 25% of the initially added concentrations. Above this salinity, no significant degradation of either compound was detected.

Biodegradation of petroleum model compounds by microbial mats from Saudi Arabia at various salinities. The data are expressed as percentages relative to the initially added amounts (i.e. 3.33 mg of each model compound adsorbed on 100 mg of organo-clay complex particles). Dotted bars, pristane; cross-hatched bars, n-octadecane; black bars, phenanthrene; white bars, dibenzothiophene.
Degradation of petroleum compounds at different temperatures
The concentration of the four compounds in the controls did not change dramatically during the incubation period (Fig. 6). Pristane and n-octadecane showed similar degradation behaviour at different temperatures. Neither compound was degradable at 5 and 50°C, but both exhibited a significant decrease in their amounts at temperatures between 15 and 40°C. At 15°C, the concentrations of n-octadecane and pristane decreased after incubation for 26 days to 6% and 16% of the initial amounts, respectively. After incubation for 12 days at 28 and 40°C, c. 75% of pristane was degraded, whereas 88% of n-octadecane was degraded after just 6 days. The residues of pristane and n-octadecane remained undegraded even after incubation for 26 days. Phenanthrene and dibenzothiophene also exhibited a similar pattern of degradation, but one that was different from that of the aliphatics. In this case, degradation of the compounds was negligible or not at all possible at temperatures of 5, 15 and 50°C, but in contrast they were completely degraded at 28 and 40°C. The disappearance of the compounds from the medium was noticeable after incubation for only 12 and 18 days at 28 and 40°C, respectively. Prior to that, a lag phase was observed during which the amounts of added compounds did not change dramatically. This lag phase was longer at 40°C than at 28°C. The optimum temperature for the degradation of aromatics was 28°C.
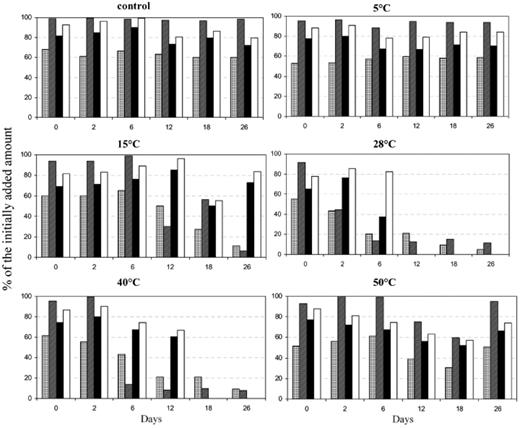
Biodegradation of petroleum model compounds by microbial mats from Saudi Arabia at various temperatures. The data are expressed as percentages relative to the initially added amounts (i.e. 3.33 mg of each model compound adsorbed on 100 mg of organo-clay complex particles). Dotted bars, pristane; cross-hatched bars, n-octadecane; black bars, phenanthrene; white bars, dibenzothiophene.
Discussion
The gradients in environmental conditions in the studied site exert selective stresses on the microorganisms that consequently determine their diversity and distribution. For example, the differences in the DGGE fingerprints at different tidal positions indicate an influence of the extent of wetting and desiccation on the bacterial composition of the mats. Bacteria that are more tolerant to desiccation live higher up in the intertidal zone, where they escape competition from those dominating the low intertidal zone. Studies related to the effects of environmental stress on ecosystems have concluded that microbial diversity decreases as environmental extremity increases, and that this is often accompanied by shifts in community composition (Ward, 1998; Benlloch, 2002; Rothrock & Garcia-Pichel, 2005). Our results are in agreement with this conclusion.
The studied microbial mats seem to be rich in novel species, because more than 15% of the obtained sequences were related to unknown bacteria. The uniqueness of these bacteria may be a result of the unusual environmental setting. The cyanobacterium Microcoleus chthonoplastes found in these mats has been detected in many hypersaline environments (Prufert-Bebout & Garcia-Pichel, 1994; Garcia-Pichel, 1996) and has been shown to grow in cultures at salinities of up to 12% (Karsten, 1996). The detection of Deinococcus-related sequences indicates that these mats contain bacterial populations that are resistant to UV and solar radiation (Rainey, 1997), which is unusually high in the region. Most of the acquired sequences from Gamma- and Deltaproteobacteria were related to sulphur-oxidizing and sulphate-reducing bacteria, respectively, indicating an active sulphur cycle in these mats. The detection of phototrophic purple sulphur bacteria further supports this assumption. Oil pollution is well known to stimulate the sulphur cycle significantly (Lovely, 1997; Kleikemper, 2002), because strains of sulphate-reducing bacteria are able to degrade petroleum compounds (Widdel & Rabus, 2001). The detection of sequences related to oil-degrading sulphate-reducing bacteria in our mat (e.g. DGGE band S10) suggests a possible role of these microorganisms in anaerobic oil degradation. Although these mats showed a strong potential in degrading hydrocarbons under aerobic conditions, few sequences related to known oil-degrading aerobic bacteria, such as Pseudomonas and Alcanivorax, were detected. Therefore, it is presumed that degradation might be performed by, in addition to these bacteria, other yet-uncultured novel bacterial populations. The identification of these novel bacteria is underway.
Effect of salinity on oil biodegradation
The studied mat was able to degrade the given compounds efficiently in hypersaline conditions, and the degradation patterns suggest its richness in halophilic oil-degrading bacteria. The inability to degrade these compounds in fresh-water conditions supports the notion that these bacteria are strictly marine. Degradation of the aromatic compounds, unlike the alkanes, was restricted to a narrow range of salinity, mainly because of their toxicity and limited solubility. Degradation of the aromatics at 3.5% and 8% and not at 5% salinity suggests the presence of two distinct aromatic-degrading bacterial populations with growth optima at these salinities. Degradation of alkanes was partial in all cases, probably as a result of the limited accessibility of the compounds by bacteria, a phenomenon that has been observed in previous experiments (Abed, 2002b). It is probable that these compounds were trapped by strong binding in the clay mineral interlayers, decreasing bioavailability (Abed, 2002b). In spite of these limitations, montmorillonite clay was found to serve as a good carrier system for low-soluble petroleum compounds (Abed, 2002b; Grötzschel, 2002).
Our results also suggest that rates of hydrocarbon degradation decrease significantly at very high salinities, in agreement with previous reports on other hypersaline environments (Ward & Brock, 1978; Rhykerd, 1995). This decrease can only be attributed to salinity and not to other factors such as oxygen or nutrient limitations, because the slurries were continuously shaken and the medium contained N and P. Salinity influences biodegradation rates either by reducing bacterial activity (Walker & Calwell, 1975) or by limiting the solubility of hydrocarbons. This inhibitory effect of salinity was shown to be more pronounced for aromatic than for aliphatic compounds (Mille, 1991).
Several halophilic bacteria have been shown to degrade hydrocarbons (Oren, 1992; Margesin & Schinner, 2001). Marinobacter hydrocarbonoclasticus grew at salinities of up to 20% and degraded various aliphatic and aromatic hydrocarbons (Gauthier, 1992). A Marinobacter-dominant halophilic culture isolated from an oil production facility in Oklahoma degraded benzene, toluene, ethylbenzene, and xylenes (BTEX) completely at 14% salinity within 1–2 weeks (Nicholson & Fathepure, 2004). Indeed, isolation of oil-degrading bacteria from our mats revealed a dominance of Marinobacter species that degraded various alkanes at salinities of up to 8% (data not shown).
Effect of temperature on oil biodegradation
Our experiments demonstrated a pronounced influence of temperature on the degradation of the studied compounds. The optimum temperature for degradation was 28°C, which is close to the ambient temperature at the time of sampling. Degradation was possible at 40°C but not at 50°C nor below 15°C, indicating that these mats are inhabited by moderately thermophilic oil-degrading bacteria. Other studies on mats from the same region revealed a dominance of thermophilic oil-utilizing bacteria (Sorkhoh, 1993) with the ability to degrade crude oil at 40–45°C (Al-Maghrabi, 1999).
Several investigations into the effect of temperature on degradation rates in cold and hot environments were performed (Backman & Jansson, 2005; Coulon, 2005; Leven & Schnurer, 2005; Polymenakou & Stephanou, 2005). Most studies concluded that degradation is preferably performed at higher temperatures, probably by stimulating the enzymes involved in the degradation process (Kohring, 1989; Wu, 1996). The metabolites of polycyclic aromatic hydrocarbon degradation under thermophilic and mesophilic conditions were shown to be different as a result of the influence of temperature on enzyme activity (Müller, 1998; Annweiler, 2000). Higher temperatures also reduce the viscosity of crude oil and thus increases its diffusion through sediments, a process that renders oil components accessible to bacteria.
Extreme conditions and their implications for bioremediation
Extreme environmental conditions may represent a natural barrier to hydrocarbon degradation, rendering bioremediation in these environments problematic. Two main strategies have been developed to bioremediate high-salt environments: one is reducing the salinity (Rhykerd, 1995); and the other is making the bacteria to function in the presence of high salt concentrations (Oren, 1992). While the former approach involved irrigation of contaminated fields with fresh water or diluted seawater, the latter approach involved producing genetically engineered halophilic oil-degrading bacteria (Kapley, 1999), bioaugmentation with artificial consortia, or stimulating the activity of indigenous bacteria (Al-Hadhrami, 1996). The irrigation of polluted sediments in Kuwait facilitated hydrocarbon biodegradation (Radwan, 1995; Al-Daher, 1998; Balba, 1998) but induced changes in the community composition. Inoculation of contaminated sites with genetically modified microorganisms or consortia isolated from another environment was in most cases unsuccessful (Radwan, 1995; Vogel, 1996; Lee & Merlin, 1999). Our results show that hypersaline polluted sites can be rich in oil-degrading bacteria and that the addition of new bacteria is not needed. Therefore, attempts at the bioremediation of such sites should consider ways of stimulating existing bacteria to degrade oil components, rather than introducing new strains.
Temperature is also a key factor in determining the need and type of the most suitable bioremediation approach. Bacterial activity and biodegradation rates can change seasonally as a function of temperature. Ward & Brock (1976) demonstrated a clear correlation between seasonal changes in temperature and oxidation of hexadecane, with optimum degradation rates at 20–25°C in the summer. Similarly, the mineralization of crude oil by soil samples from Louisiana salt marshes changed depending on the season (Jackson & Pardue, 1997). As a consequence, bioremediation might be needed in certain seasons and not in others. In hot environments, the volatile hydrocarbon fraction of oil evaporates quickly, leaving longer-chain aliphatic and aromatic components, which are more difficult to degrade and may persist in nature for a long time. In the Gulf region, this process resulted in the formation of a layer of tar that settled over large areas of the coast. This tar layer is resistant to biodegradation, and bioremediation in such cases is irrelevant. Therefore other strategies such as the mechanical collection of tar layers should be considered.
Acknowledgements
We would like to thank King Fahd University for Petroleum and Minerals (KFUPM) for their support, and Mr Saji and Mr Saeed for their assistance on the field trip. We also would like to thank Florin Musat for his introduction to gas chromatography and Jürgen Köster for preparing the organo-clay complexes. This research was financially supported by the Deutsche Forschungsgemeinschaft (grant BE 2167/4) and by the Max Planck Society.
References



