-
PDF
- Split View
-
Views
-
Cite
Cite
Fajon Céline, Cauwet Gustave, Philippe Lebaron, Senka Terzic, Marijan Ahel, Alenka Malej, Patricija Mozetic, Valentina Turk, The accumulation and release of polysaccharides by planktonic cells and the subsequent bacterial response during a controlled experiment, FEMS Microbiology Ecology, Volume 29, Issue 4, August 1999, Pages 351–363, https://doi.org/10.1111/j.1574-6941.1999.tb00626.x
Close - Share Icon Share
Abstract
Seawater from the northern Adriatic, with low phosphorus (0.03 μM) and nitrogen (1.0 μM NO3 and 1.0 μM NH4) concentration, was incubated for 12 days in 20-l polycarbonate carboys. The addition of a nutrient mixture (0.6 μM PO4, 5.1 μM NO3, 1.8 μM NH4, 10.6 μM SiO2) induced a strong diatom bloom, reaching 25 μg l−1 Chl a. Primary and bacterial production were stimulated by the initial enrichment of nitrogen and phosphorus but ceased when N and P depletion occurred after 4 days. Inorganic N exhaustion resulted in a significant production (and accumulation) of dissolved and particulate carbohydrates. The initial accumulation of carbohydrates (CHO) in the particulate phase was followed 2 days later by a significant release of dissolved CHO. The bacterial response to this organic carbon source, as reflected by glucosidase activity, was probably inhibited by the severe P limitation following the phytoplankton bloom. In the exponential phase, when P concentration was sufficiently high to sustain a significant glucosidase activity, no increase in either dissolved organic carbon or dissolved total CHO was observed. We hypothesise that the periodic accumulation of dissolved organic carbon in the northern Adriatic is due to an excessive nitrogen enrichment followed by a concurrent N and P limitation.
1 Introduction
In most marine environments, dissolved organic carbon (DOC) is 20–50 times more abundant than the particulate organic carbon (POC) attributable to all the living organisms present there. This dissolved organic matter (DOM) originates from several sources: terrestrial organic matter brought by rivers, direct excretion by organisms or sub-products of biodegradation. On a broad scale, the mean concentration of DOM is stable with time. This has encouraged many authors to suggest that DOM in the sea is very stable and probably consists of the end-products of the various degradation processes [1]. The increasing interest in DOM which has occurred over the last decade, and the subsequent increase in data collected with more reliable techniques, has shown that some areas, such as coastal zones, often have higher DOC concentrations. This was frequently attributed to the contribution by rivers and to terrestrial inputs. More precise observations, undertaken as part of co-ordinated organic biogeochemical surveys, later suggested that the increased DOC concentration observed in coastal zones as well as in surface oceanic waters was strongly linked to primary production. More recently, Carlson et al. [2] and Williams [3] have shown that there is a seasonal accumulation of DOC due to a high excretion rate during the spring phytoplankton bloom and a lack of efficiency in the degradation of this DOM. Williams [3] concluded that DOC plays an important role in the export of organic carbon from the euphotic zone and that this carbon export is not related to the inorganic nitrogen import because of the weak biochemical coupling of C and N in DOM.
This observation, together with some mesocosm experiments [4], suggests that the release of DOM by marine organisms, especially by phytoplankton during blooms, is far from negligible and that this DOM does not undergo rapid biological degradation after production. DOC accumulation is observed seasonally in most areas, but has a higher amplitude (>100 μM) in the coastal zone. It seems that the higher the primary production, the more DOC is released and the greater is the accumulation. In extreme situations this DOC accumulation can lead to aggregation and enrich marine snow or form mucilaginous structures. This has for example been observed many times in the northern Adriatic where mucilage accumulates in such a way as to have dramatic ecological and economic consequences [5–7].
Most phytoplankton cells secrete surface-bound polysaccharides or muco-polysaccharides during all phases of their growth [8,9]. Interesting diel cycles of production and accumulation of carbohydrates have also been observed in subtropical areas [10]. This photosynthetic extracellular release (PER) can be influenced by the nutrient availability. Phosphorus depletion in the Mediterranean sea [11] and especially the high N:P ratio in the Adriatic Sea [12] have been frequently suggested to induce an accumulation of mucilage. Moreover, it has been suggested that P-limited phytoplankton release high molecular mass organic material that is more resistant to bacterial degradation [13], and thus, can easily accumulate.
The accumulation of mucilage results from a combination of complex chemical, biological and physical conditions yielding unbalanced conditions between production and degradation processes [14]. An excessive production of high molecular mass substances combined with slow degradation and low turbulent conditions of the water column facilitates the aggregation and accumulation of these polymers. Low degradability of the released polymers as well as the effect of P limitation on the metabolic activity of bacteria may be responsible for the relative inefficiency of bacterial degradation. Such low efficiency is generally not observed in the case of PER derived from N-limited conditions but only under P-limited conditions [14].
Polysaccharides represent the main fraction of the extracellular exudates and in some cases are exclusively glucose polymers [15–17]. Bacteria play a key role in the degradation of these aggregates, due to their cell surface hydrolytic ectoenzymes which can hydrolyse polymers at the phytoplankton surface [18,19]. The main ectoenzymes involved in the degradation of polysaccharides are α- and β-glucosidases [20,21]. The synthesis of these enzymes depends on the physiological state of the bacterial cells and may be very sensitive to N and P availability. However, the full interaction of factors which control enzyme production remains unclear.
The experiment described here was designed to evaluate the influence of nutrient availability or deficiency on the production of DOM by a natural phytoplankton community and its utilisation by bacteria. It was performed with natural seawater samples collected from 1 m depth in the Gulf of Trieste (Adriatic Sea). The samples were enriched with nutrients in order to simulate a pulsed input such as might occur under natural conditions when heavy precipitation leads to increased nutrient input via rivers.
2 Materials and methods
2.1 Microcosm experiments
One large volume (100 litres) of natural seawater was collected at 1 m depth in the Gulf of Trieste (North Adriatic) in March 1996. After pre-filtration through a 200-μm screen, the initial volume was split and dispersed into three 20-l transparent polycarbonate bottles, which were incubated at 1 m depth. One bottle was sampled as a control without any nutrient addition (microcosm C), one had an addition of 0.6 μM phosphorus (microcosm P) and one had an addition of a mixture of nutrients (0.6 μM PO4, 5.1 μM NO3, 1.8 μM NH4, 10.6 μM SiO2; microcosm N+P+Si). In two previous experiments, it had been demonstrated that the addition of nitrogen alone in a phosphorus-limited sample did not initiate any bloom or any carbon release [22,23], thus these nutrient additions were not included in our experiment. The initial seawater contained 1.01 μM nitrate, 0.07 μM ammonium, 0.03 μM phosphate and 1.85 μM silicate. The microcosms were sampled every second day for all parameters studied. No duplicates were performed but another experiment conducted in parallel on a water sample collected from 16 m depth showed very similar patterns.
2.2 Phytoplankton biomass and primary production
Chlorophyll a was determined according to the method of Strickland and Parsons [24].
Phytoplankton production was measured using the 14C technique. A known concentration of 14C was added (6 μCi of NaH14CO3) to 75 ml of seawater in light and dark polycarbonate bottles. These sample bottles were incubated in duplicate at the same depth as the microcosm bottles (see Section 2.1) for 3 h. After incubation, cells were collected onto 0.2 μm pore size polycarbonate filters (Nuclepore). After acidification (5 M HCl), filters were placed into vials containing 5 ml of scintillation cocktail and the activity was measured on a Canberra TriCarb 2500 scintillation counter. The assimilation of carbon was calculated as described by Strickland and Parsons [24] and Gargas [25], assuming 5% isotope discrimination, and the total CO2 concentration was calculated from temperature, salinity and pH.
2.3 Bacterial production
Methyl-[3H]thymidine (85 Ci mmol 1−1, Amersham) was added to the samples at a final concentration of 12.2 nM. Sub-samples (5 ml) were incubated in triplicate at ambient seawater temperature in the dark. Two blanks were also processed, and immediately fixed with formaldehyde (4% final concentration). After incubation for 1 h the sub-samples were fixed with formaldehyde (4%, final concentration) and filtered onto 0.22 μm pore size cellulose ester membrane filters (Millipore GS, 25 mm filter diameter) and extracted three times with 5 ml ice-cold 5% TCA (Sigma) for 5 min. The filters were then placed in 4 ml of scintillation cocktail and radio-assayed.
Thymidine incorporation rates were converted to cell multiplication rates using a theoretical conversion factor of 1.7×1018 bacteria produced per mole of thymidine incorporated into TCA-insoluble fraction [26]. Cell multiplication rates were converted to carbon production by using a theoretical value of 20 fg C per cell [27].
2.4 Extracellular enzyme activities
Fluorogenic substrate analogues were used to estimate activities of α-glucosidase, β-glucosidase and leucine aminopeptidase at different sampling times in the microcosms [19]. 4-Methylumbelliferyl α-d-glucopyranoside (α-MUF), 4-methylumbelliferyl β-d-glucopyranoside (β-MUF) and l-leucine 7-amino-4-methylcoumarin (leu-MCA) were used to estimate potential hydrolysis rates of glucosidic and peptidic bonds, respectively [29,30].
All chemicals were purchased from Sigma and stock solutions stored at −20°C. Working solutions of α-MUF, β-MUF and leu-MCA were prepared with sterile distilled water and added to 4.5 ml of sample to obtain final concentrations of 2.5 μM, 2.5 μM and 25 μM, respectively. The samples were incubated in the dark at ambient seawater temperature for 1 h (the fluorescence signal increases linearly for at least 2 h). The fluorescence signal was recorded by comparison to a blank with a fluorescence spectrophotometer F-4500 (Hitachi) at the corresponding excitation and emission (ex/em) wavelengths. The wavelengths were 364/445 nm and 380/440 nm for α- and β-MUF and leu-MCA substrates, respectively. For quantitative assays, the amount of hydrolysed substrates was estimated with reference to standards based on known concentrations of 4-methylumbelliferone (for α-MUF and β-MUF) and 7-amino-4-methylcoumarin (for leu-MCA) (Sigma).
2.5 Nutrients and carbon measurements
Nutrient determinations were performed using standard colorimetric procedures according to Grasshoff et al. [31]. The atomic N/P ratio was calculated by dividing the sum of nitrate-N and ammonium-N concentrations (μM) by the phosphate-P concentration (μM). Dissolved organic nitrogen was calculated from total dissolved nitrogen after subtraction of the inorganic forms (NO3, NO2, NH4).
For particulate organic carbon and nitrogen, 200 ml of each sample was filtered onto precombusted Whatman GF/F filters (0.7 μm), using an all glass filtering system. The filtration was performed under reduced vacuum to avoid breakage of living cells. Three glass tubes of 10 ml of the filtrate were collected and poisoned with mercuric chloride (5 mg l−1). These were stored for DOC analysis. After each filtration, the filter holder and the filter were washed with a small volume of distilled water to eliminate all of the residual salt. The filters were then dried overnight in an oven at 50°C and stored in a desiccator until weighing and analysis of POC and PN.
Particulate and dissolved carbon and nitrogen were measured with an integrated system including a Shimadzu TOC 5000 carbon analyser, the corresponding suspended solid module (SSM) and a nitrogen analyser (Sievers NO analyser) in line with the carbon analyser. The oxidation of organic matter within the conditions of the apparatus (high temperature catalytic oxidation at 700°C for DOC and dry combustion at 900°C for POC) produces CO2 and NO. These combustion gases are carried by pure oxygen first to the non-dispersive infrared detector (NDIR) and then to the NO detector based on a chemiluminescence method.
Filters were first acidified with 5 N phosphoric acid to eliminate carbonates and dried at 200°C before being combusted in a furnace at 900°C. POC and PON are measured by comparison with calibration curves of standard solutions of acetanilide. The precision is 2 μg C for POC and 0.2 μg for PON, corresponding in this experiment to about 10 μg l−1 C and 1 μg l−1 N.
DOC and total dissolved nitrogen (TDN) are measured, after elimination of inorganic carbon by acidification and bubbling, by direct injection of 100 μl of the sample in a vertical furnace on a Pt/silica catalyst [32]. Calibration is made from potassium hydrogen phthalate (KHP) solutions (50–300 μM C) and from acetanilide solutions (2–20 μM N). The precision is 20 μM for DOC and 0.5 μM for TDN.
2.6 Carbohydrates
Samples of 100 ml were filtered onto Whatman GF/F filters in order to determine particulate and dissolved carbohydrates. Dissolved monosaccharides were determined directly, using a slightly modified MBTH method [33], while concentrations of total dissolved and particulate carbohydrates were determined after hydrolysis with 2 N HCl (100°C, 3 h) [34]. The concentration of polysaccharides was calculated by subtracting the monosaccharide concentration from the total carbohydrate concentration. All measurements were made in duplicate.
3 Results
3.1 Nutrient concentrations
In all microcosms, the dynamics of phosphate, nitrate and ammonium concentrations were followed for 12 days (Fig. 1). Phosphate concentration exhibited a significant decrease in both enriched microcosms. In the (N+P+Si)-enriched microcosm, phosphorus was consumed more extensively than under P enrichment and was exhausted after 4 days while P concentration stabilised at 0.2 μM after day 4 in the P-enriched microcosm (Fig. 1a). When N and P (and Si) were added, the ammonium concentration decreased rapidly to below 0.5 μM (Fig. 1c), followed after 2 days by a rapid decrease in nitrate concentration (Fig. 1b). On day 5, NO3 and PO4 concentrations fell to close to the detection limit while NH4 remained at a constant level (∼1 μM) in the control and P-enriched microcosms, 0.3 μM in (N+P+Si)-enriched microcosm). The NO3 concentrations were also close to the detection limit after day 2 in the other microcosms (C and P). In the N+P+Si microcosm, the N:P ratio increased during the first 4 days but was always in the 20–80 range. In the control microcosm, the N:P ratio increased at the end of the experiment while it remained low (<12) throughout the 10 days in the P microcosm.
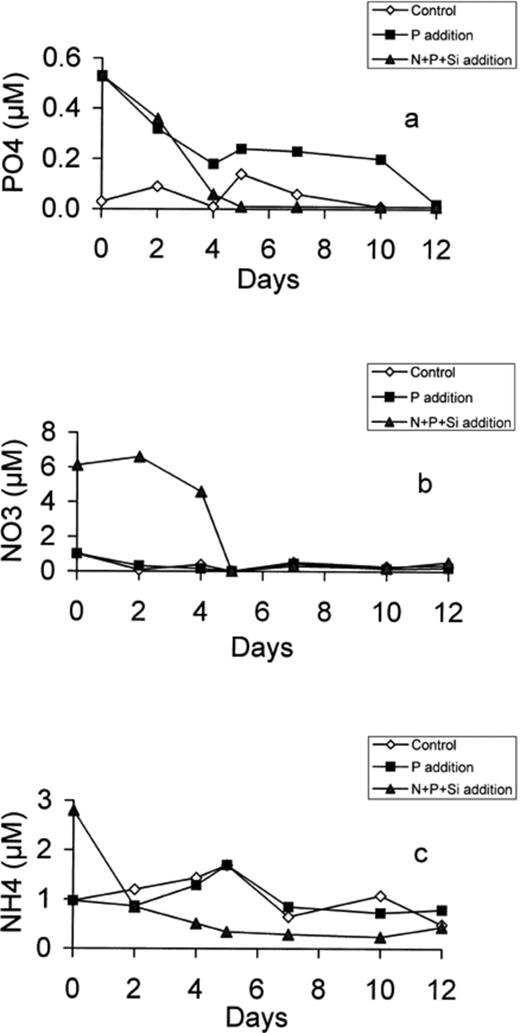
Development of PO4 (a), NO3 (b) and NH4 (c) concentration in the control, P-enriched and (N+P+Si)-enriched microcosms.
3.2 Biomass estimation
Phytoplankton biomass was estimated on the basis of chlorophyll measurements. While there was almost no increase in Chl a in microcosm C (initial value 0.94 μg l−1), we observed a slight increase in microcosm P (2.13 μg l−1 on day 5) and a significant increase in microcosm N+P+Si, where Chl a reached 22 μg l−1 on day 5 (Fig. 2). Addition of nutrients to the previously nutrient-limited sample provoked a strong diatom bloom, as demonstrated by the high concentration of fucoxanthin (Terzic, personal communication). The low production in microcosm P was due to the limitation in nitrogen, leaving some phosphorus not utilised (Fig. 1a).
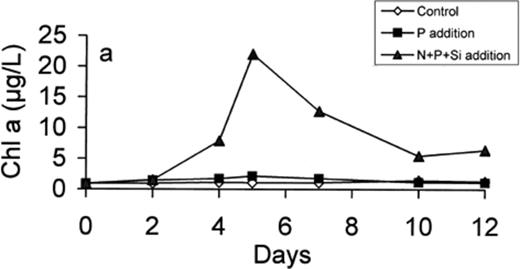
Development of chlorophyll a concentration in the control, P-enriched and (N+P+Si)-enriched microcosms.
3.3 Bacteria and heterotrophic nanoflagellate (HNAN) abundance
The total bacterial counts increased slowly in both enriched microcosms during the first 2 days (Fig. 3a). This increase was maintained for both enriched conditions (P and N+P+Si) and reached maximum values on day 4, while the total abundance decreased in the control microcosm. The total bacterial counts decreased and stabilised between days 5 and 7, probably in connection with the increase in HNAN numbers. The abundance of HNAN increased similarly in both enriched microcosms during the first 5 days and then stabilised in the P microcosm and increased sharply under (N+P+Si)-enriched conditions (Fig. 3b).
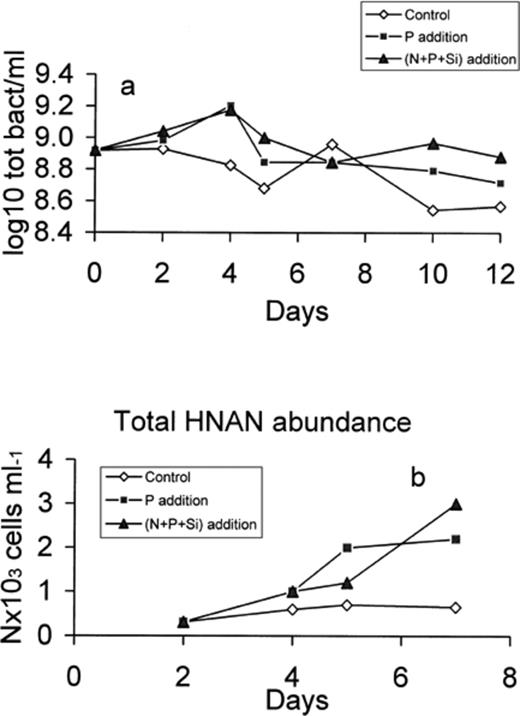
Total bacteria (a) and HNAN (b) abundance in the control, P-enriched and (N+P+Si)-enriched microcosms.
3.4 Phytoplankton and bacterioplankton production
After enrichment, primary production increased in the (N+P+Si)-enriched microcosm (Fig. 4a). Despite the P enrichment, the production was low in this microcosm due to the rapid exhaustion of nitrogen (see Fig. 1). In the P-enriched microcosm, the maximum rate of production was 1.39 μg C l−1 h−1 (day 2) whereas it reached 6.56 μg C l−1 h−1 under (N+P+Si)-enriched conditions (day 5), decreasing after day 5 and stabilising at less than 2 μg C l−1 h−1 (days 10–12). The high production observed in the N+P+Si microcosm was due to the production of large phytoplankton cells (>10 μm) as determined by size-fractionated primary production (data not shown).

Primary production (a), primary production exudates (b) and bacterial production (c) in the control, P-enriched and (N+P+Si)-enriched microcosms.
A fraction of the 14C fixed by photosynthesis is rapidly released by planktonic cells (exudation). In microcosms C and P, excretion remained low (respectively 0.4 and 0.7 μg C l−1 h−1), but it increased to 1.9 μg C l−1h−1 in microcosm N+P+Si on day 5 (Fig. 4b). These exudates represented respectively 80%, 50% (P) and 29% (N+P+Si) of the carbon fixed by primary production.
Bacterial production increased faster than primary production and similar patterns appeared under P- and (N+P+Si)-enriched conditions (Fig. 4c). The production rates exhibited two peaks with maximum values on days 2 and 5. After day 7, the production increased again in the N+P+Si microcosm and fell close to the detection limit in microcosm P. No significant increase of bacterial production was detected in the control.
3.5 Extracellular enzyme activity
Extracellular glucosidases activities remained constant and close to the detection limit in both the control and P-enriched microcosms, except for a small increase in β-glucosidase activity on day 10 in the P microcosm (Fig. 5b). Addition of the nutrient mixture yielded a major peak in α- and β- glucosidase activities by days 4 and 5, respectively (Fig. 5a,b). Then both activities rapidly declined and became undetectable 1 day after the peak. The aminopeptidase activity increased significantly in both enriched microcosms during the first 2 days and then declined and stabilised at a low level in the P microcosm, while it was constant up to day 5 in the (N+P+Si) microcosm and then increased again to reach a high and constant activity after 10 days (Fig. 5c). The activity increased slowly in the control and stabilised at a low level after day 4. There was no direct correlation between hydrolase activity, bacterial production and the concentration of organic nutrients for any of the substrates tested.
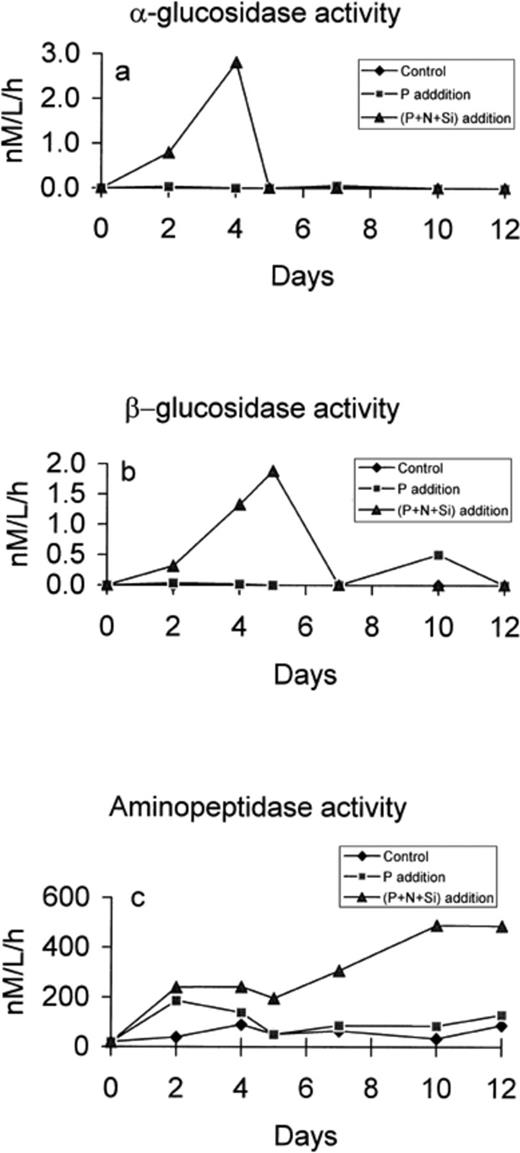
Concentration of α-glucosidase (a), β-glucosidase (b) and aminopeptidase (c) in the control, P-enriched and (N+P+Si)-enriched microcosms.
3.6 Dissolved and particulate organic matter
Particulate organic carbon (POC) and nitrogen (PON) increased in all microcosms, but to the greatest extent in the P+N+Si microcosm where they reached 1000 μg C l−1 and 105 μg N l−1, respectively, after 10 days (Fig. 6a,b). The C/N ratio of the particulate matter increased slightly in all microcosms (from 5.5 to 7 in microcosm P and up to 9 in microcosms C and N+P+Si; Fig. 6c). The POC/Chl a ratio (Fig. 6d) shows some remarkable differences. The sharp increase of this ratio at the end of the experiment, in microcosm N+P+Si, is mainly due to the accumulation of particulate polysaccharides. If we subtract the carbohydrate-C from POC, then the ratio is much lower, remaining below 100 (Fig. 6e).
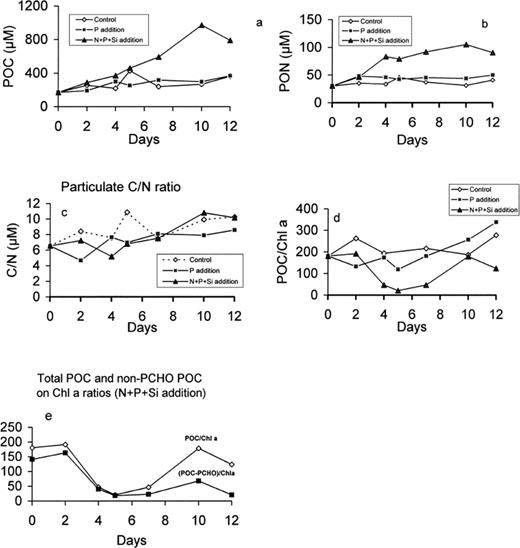
Particulate organic carbon (a) and nitrogen (b) concentration, C/N ratio (c), POC/chlorophyll ratio (d) in the control, P-enriched and (N+P+Si)-enriched microcosms and non-CHO POC vs chlorophyll ratio (e) in (N+P+Si)-enriched microcosms.
DOC was relatively constant in microcosms C and P (∼110 μM) but increased up to 170 μM in microcosm N+P+Si. This increase only occurred after day 5 (Fig. 7). Total dissolved nitrogen remained relatively constant after an initial decrease due to the utilisation of inorganic nitrogen. Dissolved organic nitrogen (DON), calculated as the difference between TDN and NO3+NH4, exhibited a slight increase between day 5 and day 12 (from about 7 to 9 μM).
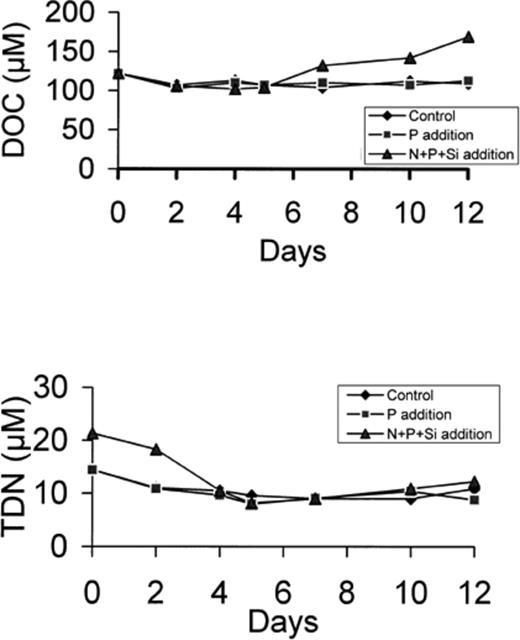
DOC (a) and TDN (b) concentration in the control, P-enriched and (N+P+Si)-enriched microcosms.
The concentration of particulate carbohydrates (PTCHO) increased 15-fold after day 5 in the N+P+Si microcosm (Fig. 8a). The concentration of both dissolved monosaccharides and polysaccharides also increased, but only after day 7. This increase was comparable to that in the microcosm filled with 16 m depth seawater (data not shown). This accumulation of carbohydrates was not observed in either the control or P-enriched microcosms. During the exponential phase (days 0–5), POC increased gradually while PTCHO remained constant. In contrast, during the stationary phase, POC and PTCHO increase at approximately the same rate, suggesting that almost all newly formed POC was particulate carbohydrate (Fig. 8b).
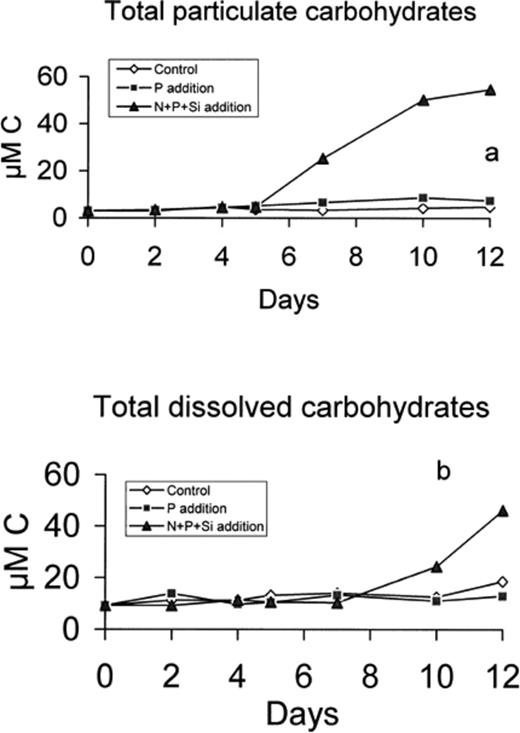
Total particulate (a) and dissolved (b) carbohydrate concentration in control, P-enriched and (N+P+Si)-enriched microcosms.
4 Discussion
From the reaction of both the phytoplankton and bacterial populations to the stimulation effects induced by a change in nutrient concentration, we can try to determine the possible mechanisms occurring. In the initial sample, nitrogen (1 μM NO3 and 1 μM NH4) and phosphorus (0.03 μM) were at low concentrations. This explains the very low primary as well as bacterial production observed in the control experiment. The addition of phosphorus (P microcosm) allowed a slight increase in primary production in the first 2 days, which rapidly ceased due to the complete exhaustion of nitrate. In contrast, bacterial production increased almost as much as in the N+P+Si microcosm, showing the strong dependence of bacterial growth on phosphorus concentration and the quasi-independence from nitrogen [35]. The persistence of some ammonium (∼1 μM) (Fig. 1) seems to allow bacteria to grow but has almost no effect on phytoplankton production. The situation is completely different when phosphorus (as PO4) and nitrogen (as NO3 and NH4) are added (microcosm N+P+Si). This mixture stimulates both primary and bacterial production. At an initial concentration of 6 μM, nitrate was completely exhausted after 5 days, as were ammonium and phosphorus. Obviously, the addition of phosphorus is of great benefit to bacteria, even when the nitrogen concentration is low. This influence is more likely to be direct than indirect; the addition of P alone gave rise to a comparable bacterial production to that observed in the N+P+Si microcosm, while primary production was much lower. This is in agreement with previous observations concerning phosphorus limitation in Mediterranean waters [10,36,37]. On day 5, phosphorus is exhausted and bacterial production drops dramatically (microcosm N+P+Si) while POC continues to increase from 200 to 1000 μg C l−1 (Fig. 6). During the same 4 days of exponential growth, PON increases rapidly (from 30 to 80 μg l−1). This PON increase slows down during the rest of the experiment (from 80 to 105 μg l−1) representing the stationary phase. These two phases correspond respectively to an increase in protein biomass (number of cells) and to an accumulation of polysaccharides. The C/N ratio was almost stable (∼5–6) for the first 4 days, increasing to 9.5 on day 10 (Fig. 7). This relates well to the particulate carbohydrate concentration, which remains stable (below 50 μg C l−1) for the first 5 days, and then increases sharply.
The dissolved fraction is also affected by the biological mechanisms. While DOC is relatively constant in the C and P experiments (100–120 μM) it increases after day 5 to 170 μM in the N+P+Si microcosm (Fig. 8). This increase, representing about 55–60 μM, is comparable to the increase in total dissolved carbohydrates (about 50 μM−C). The change in nutrient concentration and in DOM composition should affect the bacterial activity. The maxima observed in bacterial production (at days 2 and 5) correspond to different situations. On day 2, nutrients are present in sufficient concentration to allow bacteria to grow on the DOM pool. Effectively, DOC slightly decreases during this period (by about 15 μM). If we assume that bacterial production has a yield of about 30% of BCD, at day 2 the BCD should be about 2.5 μg C l−1 h−1, and it cannot be explained by the primary production exudates (about 0.77 μg C l−1 h−1). Bacteria are thus also using the pre-existing DOC. A decrease of about 15μM represents 180 μg carbon utilised in 24 h (3.6 μg l−1 h−1). It is more than enough to explain the bacterial production measured.
The concentration in dissolved carbohydrates is stable during this period, but we cannot differentiate whether this is due to a lack of production and utilisation or if there is an equilibrium between these two fluxes. More probably, as was suggested in a previous experiment [38], the protein DOM is mainly affected by this bacterial activity. The low values measured for glucosidase, compared to protease activity, seem to confirm this hypothesis. On day 5, the situation is very different. This coincides with a total depletion in nutrients and the beginning of the stationary phase for phytoplankton. This also coincides with the maximum β-glucosidase production (absent at day 7), the end of α-glucosidase production and a further increase in protease activity. At this point, exudates issued from primary production are maximum (1.90 μg C l−1 h−1). Bacterial production (0.72 μg C l−1 h−1) represents an approximate BCD of 2.2 μg C l−1 h−1 that needs a consumption of 0.3 μg C l−1 h−1 (21.6 μg in 3 days) of existing DOC to be explained. It would represent a DOC decrease of less than 2 μM, impossible to appreciate.
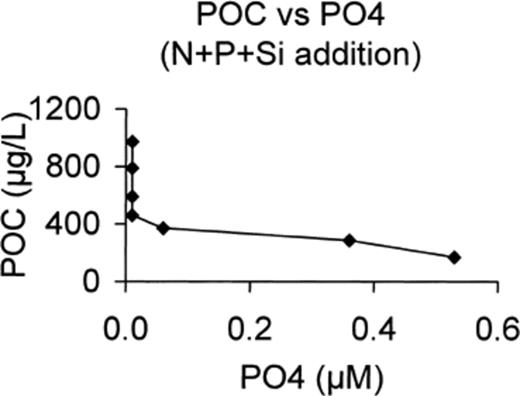
It also coincides with the production of particulate polysaccharides in the N+P+Si microcosm. If we plot the POC concentration vs phosphate concentration (Fig. 9), we can see that it increases slowly with decreasing phosphate and increases much more as soon as phosphorus concentration reaches 0.01 μM. It seems that when the nutrients are all consumed, the phytoplankton and bacterial communities enter stationary phase, which has several direct consequences such as the production of polysaccharides and an increase in protease activity. Phytoplankton cell lysis follows, as seen by the decrease in Chl a content, and this is probably responsible for a progressive release of dissolved polysaccharides and some monosaccharides. However, the glucosidic activities seem to be rapidly limited by the lack of nutrients, probably more by P than N. This explains the accumulation of dissolved polysaccharides at the same rate they are released from cells. The increasing bacterial production at day 12 is mainly supported by protein matter issued from cell lysis.
The observations made during this experiment allow us to draw some conclusions based on the co-variability of several parameters.
The enrichment in nutrients, phosphorus alone or phosphorus with nitrogen, enhances the biological production. Bacterial production (and activity) is very dependent on phosphorus concentration but almost independent of nitrogen concentration. An enrichment in phosphorus, at low nitrogen content, allows rapid growth and activity of the bacterial population. A small fraction of DOC (15 μM C) is utilised during this period, together with fresh exudates, probably in relation to the high protease activity. The C/N ratio of DOM seems also to decrease slightly.
The addition of nitrogen does not appreciably change these parameters. When all the phosphorus is consumed, the bacterial production, as well as the glucosidase activity, decreases rapidly, indicating the dependence of bacteria on P.
In contrast, phytoplankton need quite high nitrogen and phosphorus concentrations in order to bloom. Although there is still a discussion about the limiting role of P and N in the development and organisation of coastal planktonic communities in the Mediterranean [37,39–41], the exponential growth phase occurs as long as nutrients are not exhausted. When N and P concentrations become very low, a stationary phase occurs with decreasing values for primary production. During this second step, several other parameters change markedly. The chlorophyll a concentration increases during the exponential phase but decreases rapidly during the stationary phase, suggesting a decay of the phytoplankton population. This corresponds to an increase in protease activity, showing that this activity is not directly inhibited by the lack of nutrients. This activity seems directed towards the particulate organic matter becoming available by the ageing of the planktonic community. During this period, while Chl a and living cells are decreasing, POC is still increasing but PON is almost stable, causing an increase in the C/N ratio, from about 6 to more than 9. This carbon enrichment is due to a high production of polysaccharides momentarily stored in (or at the surface of) the phytoplankton cells. Two days later, some of these polysaccharides are released from the cells, increasing the DOC concentration. The results we obtained in several experiments showed that the carbon stored as polysaccharides in the cells or in the dissolved form can represent up to 80% of the total carbon. As observed here, when phosphorus is lacking, the ability of the bacteria to use this carbon-rich substrate is limited, leading to an accumulation of DOC and DCHO. During a mesocosm experiment designed to study the formation of aggregates [42], it was demonstrated that the high carbohydrate concentration can be responsible for the aggregation process but a high ecto-enzymatic activity (glucosidases), especially if developed by bacteria attached to diatoms, can inhibit aggregation [43,44].
Under specific conditions (stable and stationary water) the accumulated DOC can coagulate and form aggregates of increasing size and stability. The role of carbohydrates in coagulating processes was already stressed by several authors [45]. At high concentration and on large scales these aggregates can form mucilaginous structures as observed in the northern Adriatic. From this study, and some previous observations, we can conclude that the production of mucilage can occur due to sudden nutrient inputs into a stable water body. In such a situation a strong bloom develops, producing high carbon concentrations. As nutrients subsequently become depleted, high concentrations of carbohydrates are produced. The lack of phosphorus makes bacteria almost unable to degrade this substrate, thus it can accumulate to further produce large aggregates.
Acknowledgements
This investigation is part of the project PALOMA (Production and Accumulation of Labile Organic Matter in the Adriatic) supported by the Environment and Climate programme of the European Community (Contract EV5V-CT94-0420), and by the PECO programme (contract CIPD-CT94-0106) for the Slovenian partner. The participation of scientists from the Rudjer Boskovic Institute of Zagreb was possible only with the support of the French and Croatian Governments through a French-Croatian co-operation programme. We are grateful to all members of the Marine Station in Piran for their permanent help during the experiment. We also thank Carol Robinson for kindly reviewing the manuscript and improving the English and the two anonymous reviewers for their helpful and constructive comments and suggestions.
References



