-
PDF
- Split View
-
Views
-
Cite
Cite
Massimo Stefano Silvetti, Fabrizio Drago, Antonella De Santis, Giorgia Grutter, Lucilla Ravà, Lidia Monti, Rodolfo Fruhwirth, Single-centre experience on endocardial and epicardial pacemaker system function in neonates and infants, EP Europace, Volume 9, Issue 6, June 2007, Pages 426–431, https://doi.org/10.1093/europace/eum043
Close - Share Icon Share
Abstract
Endocardial (ENDO) or epicardial (EPI) pacing systems are implanted in infants but it remains unclear which system should be preferred.
We evaluated the results of children ≤1 year who underwent pacemaker (PM) implantation at our centre with a retrospective analysis. Between 1992 and 2004, 56 patients, 37 of whom had other congenital heart defects (CHDs), received a PM at 4.4 ± 3.8 months of age for atrioventricular block (n = 52) and sinus node dysfunction. Rate-responsive ventricular demand pacing (VVIR) PMs were implanted in 25 patients (19 ENDO), dual-chamber demand pacing (DDD) in 29, and rate-responsive atrial demand pacing (AAIR) in 2 (all EPI). Follow-up (FU) was 4.5 ± 3.5 (range 0.3–13) years: 15 pacing system failures occurred among the 56 patients (26%) after 4.5 ± 3.2 years, with a significantly reduced success rate for EPI (21-fold increase of the risk of failure) and complex CHD. Also in patients without surgery for CHD, EPI showed a worse outcome. Among the 91 leads implanted, failures occurred more significantly in EPI (18% of atrial, 24% of ventricular leads) than in ENDO (5% of ventricular leads). No venous occlusion was found at FU.
Single-lead, VVIR ENDO pacing had higher efficiency and safety than EPI, and it might be the best choice for PM implantation in infants. However, because of small patient numbers and lack of longer FU, these findings should be treated with caution.
Introduction
Factors that limit the longevity of pacemaker (PM) systems implanted in children include somatic growth, the child's active lifestyle, susceptibility to infections, and the intrinsic life of the PM battery and leads. These problems are further compounded in infants and neonates. Even in the era of steroid-eluting leads, it remains unclear whether the epicardial1–5 or the endocardial5–8 pacing system is best for neonates and infants. We report our experience with PM implantation in neonates and infants, seeking to understand which pacing system is most suitable in this difficult population.
Methods
Since 1982–2004,8 345 patients have undergone permanent PM implantation and are followed in our institution. We reviewed the records of these patients and identified all those who underwent PM implantation at ≤12 months of age.
The techniques used for the initial implantation were recorded. We recorded any complications during the follow-up, the change of the pacing system or the pacing mode and the clinical status at the most recent follow-up. Patients were grouped according to pacing modality, and the outcome of patients with an epicardial pacing system was compared with that of patients with the transvenous system. Furthermore, pacing thresholds, sensing values, and impedances in patients with transvenous pacing were compared with those in patients with steroid-eluting epicardial leads.
The study complies with the Declaration of Helsinki. Informed consent of the parents of the subjects has been obtained at the moment of the procedure.
Definitions
Pacing system implantation was defined as the placement of a new PM generator and one or more new leads. PM replacement was defined as the placement of only the PM generator without the insertion of new leads. Complications recorded were divided into two categories: early (occurring in the first 3 months after implantation) and late (>3 months).8 Lead malfunction requiring new pacing system implantation was defined as: (i) exit block, (ii) abnormal threshold increase with the need of high output values causing early battery depletion and/or partial loss of capture, and (iii) lead fracture.
Implantation procedure
Epicardial pacing
The PM generator was placed in the abdominal wall in a subcutaneous or in a submuscular (generally in neonates) pocket. The leads were inserted by standard surgical techniques either through sternotomy and lateral thoracotomy or by using a subxiphoid approach.8 Epicardial leads were regularly placed on the right atrium and ventricle and rarely on the left atrium.
Transvenous pacing
The technique utilized for endocardial pacing has been described previously.8,9 In brief, at initial PM implant, the endocardial pacing lead was inserted by transcutaneous puncture of the subclavian vein and fixed to the subcutaneous tissue with a slowly absorbable ligature. Ventricular leads were positioned in the non-systemic ventricular apex. Pacemaker generators were placed in prepectoral pockets.
An atrial loop7 was added to the slowly absorbable ligature in the last two patients to increase longevity with somatic growth. All the procedures were performed under general anaesthesia. Antibiotic prophylaxis was routinely given perioperatively to all patients. Patients were kept in the hospital at bed rest for at least 48 h.
Acute and chronic pacing thresholds (measured with a pulse width of 0.40 or 0.50 ms at implantation), impedances, and sensing of spontaneous atrial or ventricular electrograms were evaluated during the implantation procedure with a Medtronic Pacing System Analyzer Model 5311 B or a Medtronic 8090 Analyzer (Medtronic, Inc., Minneapolis, MN, USA) and during follow-up telemetric interrogation with appropriate analysers.
Follow-up
The follow-up schedule was as described already.8,9 In brief, patients were generally followed up at 1, 3, 6, and then every 6 months or as needed. They underwent clinical examination, echocardiography, telemetric PM interrogation, and standard electrocardiogram at every follow-up visit; Holter monitoring, exercise testing (after 6 years of age) yearly; chest X-ray every 1–2 years. At the last follow-up visit, a Doppler echo examination of the subclavian vein was performed in patients with transvenous leads to determine the venous patency.
Statistical analysis
Statistical analysis was carried out using the Stata Package, version 8.0 (StataCorp. 2003, Stata Corporation, College Station, TX, USA). Data are reported as a mean ± standard deviation or as a median and range when appropriate. The Kaplan–Meier method and log-rank test were used to study the outcome of the pacing systems implanted in infants. Failures included lead malfunction, device infection, pocket erosion. Complications that did not require a new pacing system implantation (i.e. infections treated with drugs, early postoperative lead dislodgement that required only the repositioning of the lead, atrial undersensing requiring DDD PM downgrading to VVIR) were recorded as complications but not included in the analysis of the outcome of the first pacing system implanted, as this was not replaced. Death unrelated to pacing was considered as ‘censored’ or lost to follow up. A Cox multivariate proportional hazard model was used to explore factors associated with the longevity of systems implanted in neonates. Variables included in the model as predictors were those that were statistically significantly associated with the failure of the pacing system at univariate analysis and age at implantation.
Pacing parameters of endocardial leads were compared with those of steroid-eluting epicardial leads8,10 with the two-sample Wilcoxon rank-sum (Mann–Whitney) test. A P-value <0.05 was considered significant.
Threshold data were reported only as pulse amplitude (in volts) with the earlier-mentioned pulse width5 rather than as energy threshold.1,3,10
Results
Patient demographics and clinical characteristics
Between 1984 and 1991, only five PM with epicardial leads have been implanted in infants. These patients were excluded from this study to minimize the effect of the oldest pacing technologies.
Between 1992 and 2004, 56 patients (19 girls) underwent permanent PM implantation at an age of 4.4 ± 3.8 months (median 4 months, range 1 day–12 months) with a weight of 4.1 ± 1.6 (median 3.7, 1.5–7.5) kg.
According to national and international guidelines,11–13 permanent PMs were implanted for complete or advanced atrio-ventricular block (AVB), either congenital or postoperative, and for sinus node dysfunction (SND) (Table 1).
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Patients | 37 (14 girls) | 19 (5 girls) |
| Weight at implantation | 3.8 ± 1.7 kg (3.4, 1.5–7.5 kg) | 4.9 ± 1.2 kg (4.8, 3–7 kg) |
| Age at implantation | 3.4 ± 3.7 months (2 months, 1 day–12 months) | 6.2 ± 3.2 months (7 months, 1 day–12 months) |
| Age at implantation <1 month | 16 | 1 |
| Congenital AVB | 16 | 11 |
| Postoperative AVB | 18 | 7 |
| SND | 3 | 1 |
| CHD | 28 | 9 |
| No structural HD | 9 | 10 |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Patients | 37 (14 girls) | 19 (5 girls) |
| Weight at implantation | 3.8 ± 1.7 kg (3.4, 1.5–7.5 kg) | 4.9 ± 1.2 kg (4.8, 3–7 kg) |
| Age at implantation | 3.4 ± 3.7 months (2 months, 1 day–12 months) | 6.2 ± 3.2 months (7 months, 1 day–12 months) |
| Age at implantation <1 month | 16 | 1 |
| Congenital AVB | 16 | 11 |
| Postoperative AVB | 18 | 7 |
| SND | 3 | 1 |
| CHD | 28 | 9 |
| No structural HD | 9 | 10 |
AVB, atrioventricular block; CHD, congenital heart defect; HD, heart disease; SND, sinus node dysfunction. Data are reported as mean ± SD (median, range).
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Patients | 37 (14 girls) | 19 (5 girls) |
| Weight at implantation | 3.8 ± 1.7 kg (3.4, 1.5–7.5 kg) | 4.9 ± 1.2 kg (4.8, 3–7 kg) |
| Age at implantation | 3.4 ± 3.7 months (2 months, 1 day–12 months) | 6.2 ± 3.2 months (7 months, 1 day–12 months) |
| Age at implantation <1 month | 16 | 1 |
| Congenital AVB | 16 | 11 |
| Postoperative AVB | 18 | 7 |
| SND | 3 | 1 |
| CHD | 28 | 9 |
| No structural HD | 9 | 10 |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Patients | 37 (14 girls) | 19 (5 girls) |
| Weight at implantation | 3.8 ± 1.7 kg (3.4, 1.5–7.5 kg) | 4.9 ± 1.2 kg (4.8, 3–7 kg) |
| Age at implantation | 3.4 ± 3.7 months (2 months, 1 day–12 months) | 6.2 ± 3.2 months (7 months, 1 day–12 months) |
| Age at implantation <1 month | 16 | 1 |
| Congenital AVB | 16 | 11 |
| Postoperative AVB | 18 | 7 |
| SND | 3 | 1 |
| CHD | 28 | 9 |
| No structural HD | 9 | 10 |
AVB, atrioventricular block; CHD, congenital heart defect; HD, heart disease; SND, sinus node dysfunction. Data are reported as mean ± SD (median, range).
Other congenital heart defects (CHDs) were present in 37 patients. In these patients, the main anomaly was ventricular septal defect (n = 10), atrio-ventricular (AV) septal defect (n = 9), tetralogy of Fallot (n = 6), transposition of the great arteries (TGA) {S, D, D} (n = 5), congenitally corrected TGA {S, L, L} (n = 3), totally anomalous pulmonary venous return (n = 2), pulmonary stenosis and tricuspid atresia (n = 1 each). For statistical analysis, CHDs were divided as simple (no indication for surgery, ventricular or AV septal defect or congenitally corrected TGA {S, L, L} without other associated anomalies, pulmonary stenosis, n = 17) and complex (all others, n = 20). Seven patients also had trisomy 21. Nineteen patients had no structural heart disease.
Pacemaker implantation
The first pacing system was implanted using epicardial leads in 37 patients and endocardial leads in 19 patients. The epicardial leads were implanted by sternotomy (31 patients, 84%), lateral thoracotomy (4 patients, 11%), or by the subxiphoid approach (2 patients, 5%). Absolute indication for epicardial pacing in these patients was a right-to-left shunt or the need of surgery for other CHDs. Except in patients with congenital AVB, when epicardial pacing was performed at the time of first pericardial sac opening, in the other patients, the epicardial pacing was performed after a recent or distant surgery within the first year of life.
The majority of transvenous implants were performed in the years 1992–1999 (n = 17). Since 2000, 19 epicardial systems and only 2 transvenous system have been implanted, reflecting an institutional choice for an epicardial pacing system and DDD pacing mode in these small patients. In fact, in these latter two patients who received transvenous pacing systems, epicardial pacing was contraindicated because of recurrent infection of the abdominal pocket after antibiotic therapy and mediastinitis after surgical correction of tetralogy of Fallot. The reasons for this institutional preference for epicardial pacing since 2000 were the good results of epicardial pacing with steroid-eluting leads described in the paediatric population in that period,1–3,10 in addiction to some concerns about the outcome of transvenous leads in the follow-up.
Sixty implantation procedures were performed in the 56 patients in the first year of life (see Early complications section). A total of 30 dual-chamber PMs (DDD) and 30 single-chamber PMs (27 VVI/R, 3 AAI/R) were implanted in the 56 patients. All patients with transvenous pacing received VVI/R PM (Table 2). Seventeen patients were less than 1 month at the time of the operation.
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| VVI/R | 6 | 19 |
| DDD | 29 | — |
| AAI/R | 2 | — |
| Leads | Cordis Encor (UP); n = 5 A | Medtronic 4023 (steroid, t., UP, 3.6 Fr.); n = 9 |
| Medtronic 4951 (UP); n = 2 A, n = 9 V | Medtronic 4024 (steroid, t., BP, 5.8 Fr); n = 1 | |
| Medtronic 4965 (steroid, UP); n = 27 A, n = 28 V | Medtronic 4073 (steroid, t., UP, 3.6 Fr.); n = 1 | |
| Vitatron F6 ISP 19 (t., UP, 3.4 Fr.); n = 8 | ||
| Osypka KY 5 (UP, screw in, 5 Fr.); n = 1 |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| VVI/R | 6 | 19 |
| DDD | 29 | — |
| AAI/R | 2 | — |
| Leads | Cordis Encor (UP); n = 5 A | Medtronic 4023 (steroid, t., UP, 3.6 Fr.); n = 9 |
| Medtronic 4951 (UP); n = 2 A, n = 9 V | Medtronic 4024 (steroid, t., BP, 5.8 Fr); n = 1 | |
| Medtronic 4965 (steroid, UP); n = 27 A, n = 28 V | Medtronic 4073 (steroid, t., UP, 3.6 Fr.); n = 1 | |
| Vitatron F6 ISP 19 (t., UP, 3.4 Fr.); n = 8 | ||
| Osypka KY 5 (UP, screw in, 5 Fr.); n = 1 |
A, atrial; BP, bipolar; Fr., French; steroid, steroid-eluting; t., tined; UP, unipolar; V, ventricular. All transvenous leads are ventricular.
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| VVI/R | 6 | 19 |
| DDD | 29 | — |
| AAI/R | 2 | — |
| Leads | Cordis Encor (UP); n = 5 A | Medtronic 4023 (steroid, t., UP, 3.6 Fr.); n = 9 |
| Medtronic 4951 (UP); n = 2 A, n = 9 V | Medtronic 4024 (steroid, t., BP, 5.8 Fr); n = 1 | |
| Medtronic 4965 (steroid, UP); n = 27 A, n = 28 V | Medtronic 4073 (steroid, t., UP, 3.6 Fr.); n = 1 | |
| Vitatron F6 ISP 19 (t., UP, 3.4 Fr.); n = 8 | ||
| Osypka KY 5 (UP, screw in, 5 Fr.); n = 1 |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| VVI/R | 6 | 19 |
| DDD | 29 | — |
| AAI/R | 2 | — |
| Leads | Cordis Encor (UP); n = 5 A | Medtronic 4023 (steroid, t., UP, 3.6 Fr.); n = 9 |
| Medtronic 4951 (UP); n = 2 A, n = 9 V | Medtronic 4024 (steroid, t., BP, 5.8 Fr); n = 1 | |
| Medtronic 4965 (steroid, UP); n = 27 A, n = 28 V | Medtronic 4073 (steroid, t., UP, 3.6 Fr.); n = 1 | |
| Vitatron F6 ISP 19 (t., UP, 3.4 Fr.); n = 8 | ||
| Osypka KY 5 (UP, screw in, 5 Fr.); n = 1 |
A, atrial; BP, bipolar; Fr., French; steroid, steroid-eluting; t., tined; UP, unipolar; V, ventricular. All transvenous leads are ventricular.
Leads implanted are reported in Table 2. Thirty-four atrial leads, all epicardial, and 57 ventricular leads, 20 endocardial, were implanted. The majority (55/71, 77%) of epicardial leads were steroid-eluting. All except one of the transvenous leads were tined (passive fixation) and unipolar, and 11 of 20 (55%) were steroid-eluting. Tined and unipolar leads were chosen at the beginning of our experience for their smaller size (3.4–3.6 Fr.). The endocardial pacing leads were inserted through the left subclavian vein in 18 patients and through the right subclavian vein in the remaining 2 patients (in presence of persistent left superior vena cava).
Follow-up
The duration of the follow-up was 4.5 ± 3.5 years (median 4 years, range 3 months–13 years). Follow-up of endocardial systems was longer than that of epicardial systems. Four patients, all with epicardial pacing, died of causes not related to pacing and seven patients (four with epicardial pacing and three with endocardial pacing) were lost to follow-up. There were 16 PM generator replacements for end of battery life. During PM replacement, transvenous leads were not advanced. Complications are reported in Table 3.
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Follow-up | 3.5 ± 3.0 years (3, 0.3–13 years) | 6.0 ± 3.8 years (6, 1–11 years) |
| Cumulative complications requiring pacing system re-implantation | 13 | 2 |
| Infection | 4 early (3 successfully treated with antibiotic therapy), 1 late | 1 late, treated with antibiotic therapy |
| Erosion | — | 1 early |
| Haemothorax | — | 1, at implantation, requiring chest drainage |
| Lead malfunction | 16 leads (2 early) in 15 patients | 1 lead (late) in 1 patient |
| Lead dislodgement | — | 1 (early, requiring lead repositioning on the first postoperative day) |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Follow-up | 3.5 ± 3.0 years (3, 0.3–13 years) | 6.0 ± 3.8 years (6, 1–11 years) |
| Cumulative complications requiring pacing system re-implantation | 13 | 2 |
| Infection | 4 early (3 successfully treated with antibiotic therapy), 1 late | 1 late, treated with antibiotic therapy |
| Erosion | — | 1 early |
| Haemothorax | — | 1, at implantation, requiring chest drainage |
| Lead malfunction | 16 leads (2 early) in 15 patients | 1 lead (late) in 1 patient |
| Lead dislodgement | — | 1 (early, requiring lead repositioning on the first postoperative day) |
Data are reported as mean ± SD (median, range).
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Follow-up | 3.5 ± 3.0 years (3, 0.3–13 years) | 6.0 ± 3.8 years (6, 1–11 years) |
| Cumulative complications requiring pacing system re-implantation | 13 | 2 |
| Infection | 4 early (3 successfully treated with antibiotic therapy), 1 late | 1 late, treated with antibiotic therapy |
| Erosion | — | 1 early |
| Haemothorax | — | 1, at implantation, requiring chest drainage |
| Lead malfunction | 16 leads (2 early) in 15 patients | 1 lead (late) in 1 patient |
| Lead dislodgement | — | 1 (early, requiring lead repositioning on the first postoperative day) |
| . | Epicardial pacing . | Transvenous pacing . |
|---|---|---|
| Follow-up | 3.5 ± 3.0 years (3, 0.3–13 years) | 6.0 ± 3.8 years (6, 1–11 years) |
| Cumulative complications requiring pacing system re-implantation | 13 | 2 |
| Infection | 4 early (3 successfully treated with antibiotic therapy), 1 late | 1 late, treated with antibiotic therapy |
| Erosion | — | 1 early |
| Haemothorax | — | 1, at implantation, requiring chest drainage |
| Lead malfunction | 16 leads (2 early) in 15 patients | 1 lead (late) in 1 patient |
| Lead dislodgement | — | 1 (early, requiring lead repositioning on the first postoperative day) |
Data are reported as mean ± SD (median, range).
Pacing system failures
Fifteen system failures occurred among the 56 patients (26%) implanted in the first year of life at 4.5 ± 3.2 years (1 day–11 years) after implantation.
PM generator failures did not occur. Generator pocket problems (infection/erosion) accounted for 3 (of 15) failures (2 with epicardial pacing).
In the 56 patients, the log-rank test for equality of survivor functions referred to the pacing system implanted in the first year of life (and not to the patient), showed no significant differences for sex, arrhythmias, pacing mode, or type of leads. The outcome was significantly worse for epicardial vs. endocardial systems (P < 0.0001, Figure 1) even after stratification for the presence of other CHDs, with the worst outcome for complex CHD (P = 0.02). Using a Cox regression model, the only significant variable for predicting outcome is the presence of epicardial leads, with a 21-fold increase in the risk of failure of the pacing system implanted in the first year of age (hazard ratio 21.0, 95% confidence interval 2.3–191.9, P = 0.007).
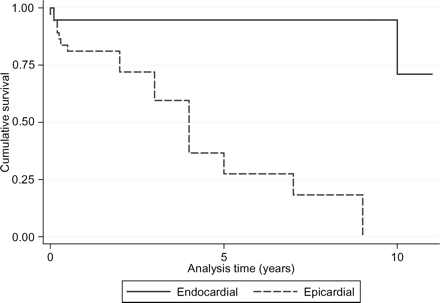
Kaplan–Meier survival estimates of the pacing systems implanted in all neonates and infants, divided by pacing approach (endocardial and epicardial) (P < 0.0001).
Endocardial vs. epicardial pacing in patients without surgery for CHD
In this subgroup of 25 patients (13 with endocardial and 12 with epicardial pacing), the log-rank test for equality of survivor functions referred to the pacing system implanted in the first year of life, showed a significant better outcome for endocardial pacing in comparison with epicardial pacing (P = 0.03, Figure 2).
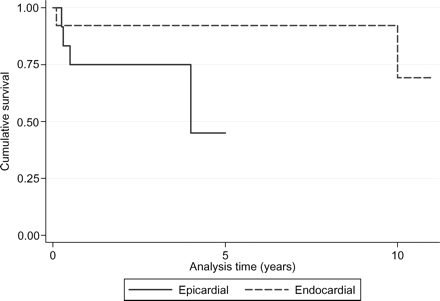
Kaplan–Meier survival estimates of pacing systems implanted in neonates and infants without associated heart surgery for other CHDs, divided by pacing approach (P = 0.03).
Lead failure
Sixteen leads failed among the 91 implanted (18%). Six failures occurred with atrial leads, all epicardial (18%), and 10 failures (1 endocardial) occurred with ventricular leads (24% of epicardial, 5% of endocardial leads). The risk of failure was greater for complex CHD (P = 0.01) and for epicardial systems (P = 0.0002). There were no significant differences in the outcome of steroid-eluting and non-steroid-eluting epicardial leads and in the stratification according to the type of surgical procedure.
Early complications
Two patients with an epicardial pacing system (one with an atrial and one with a ventricular lead) underwent implantation of new epicardial systems because of early lead malfunction. One patient with a DDD PM underwent implantation of a transvenous pacing system because of recurrent abdominal pocket infection after a period of temporary pacing and antibiotic therapy. One patient with an endocardial pacing system underwent implantation of an epicardial DDD system because of pocket erosion. This patient was the only patient who underwent implantation of an endocardial pacing system under 1 month of age (Table 3).
Late complications
In 10 of 37 patients (27%) with an epicardial pacing systems, the first pacing system implanted failed: 9 patients underwent new system implantations for lead malfunction (8 ventricular leads and 1 atrial and ventricular lead) and 1 patient for pocket infection. In three of these re-implantations, the pacing system was switched to transvenous system (Table 3).
Four patients with a DDD pacing system were downgraded to VVIR/VDD mode for atrial lead malfunction.
One patient with an endocardial ventricular lead developed an abnormal threshold increase 10 years after the initial implant. There was an absence of spontaneous junctional rhythm with PM inhibition, and high pacing output caused pectoral muscle stimulation. This patient underwent new VDD lead implantation in the same subclavian vein as the initial implantation, and the old lead was abandoned.14 Venous patency was previously evaluated with Doppler echo according to our protocol for PM upgrading.8,9,14 After 12 months of follow-up, the new PM and lead are functioning normally; there are neither echo signs of tricuspid valve dysfunction nor echo signs of subclavian vein occlusion.
Thirteen patients with transvenous leads, including the one with a bipolar lead, underwent Doppler echo evaluation of the subclavian vein, and no evidence for venous occlusion was found.
In 2 patients with ventricular endocardial leads, at 5 and 10 years after implantation, the chest X-ray shows loss of lead slack due to somatic growth, but the lead function remains good.
Pacing parameters
The maximum duration of follow-up of epicardial steroid-eluting leads is only 4 years. Data for endocardial leads extend to 10 years. Median endocardial ventricular pacing thresholds were stable and excellent (generally between 0.5 and 1.5 V) for the whole period, and significantly lower than those for epicardial steroid-eluting leads at implantation, 12, 24, 36, 48 months. Median epicardial atrial and ventricular pacing thresholds showed a U-shaped curve, with a reduction of voltage in the first year after implantation, followed by an increase (∼1 V for atrial and between 1.5 and 2 V for ventricular thresholds). Median P-wave sensing was excellent (generally between 2 and 4 mV). Also, R-wave sensing was satisfactory (between 7 and 15 V) and not significantly different for endocardial and epicardial leads, with some fluctuations due to the small numbers of patients and the low escape rates in some patients that precluded the analysis of spontaneous rhythm at all follow-up visits. Pacing impedances dropped after implantation and then remained stable with endocardial leads having higher impedance than atrial and ventricular epicardial leads.
Discussion
Permanent cardiac pacing continues to have a high complication rate even in adults,15 but especially in infants and children.1,2,4–8,16 In our study of infants and neonates, almost 1 in 4 underwent placement of a new pacing system within 4–5 years because of complications. The likelihood of system replacement was higher in patients with an epicardial pacing system: most complications occurred in patients with epicardial pacing systems as other authors reported in older patients.16
A worse outcome of the epicardial pacing system was found also in the subgroup of patients with congenital AVB without other heart surgery. No difference was found according to the surgical approach, but this might be due to the small numbers of these subgroups. Epicardial leads also failed more frequently, especially in the presence of complex CHD. A better outcome for pacing systems implanted using a subxiphoid approach was reported in older age patients.1 The reasons for these findings might include trauma or traction imposed on epicardial leads by thoraco-abdominal movement and the numerous cardiac operations that patients with CHD often undergo with consequent presence of inflamed or scarred epicardium. However, the results of patients without associated heart surgery and the lack of statistically significant differences of the results of steroid-eluting and non-steroid-eluting epicardial leads might imply that the effects of trauma and traction on the epicardial leads might be more important for the system outcome in these very young patients. However, caution must be applied in the interpretation of these data, as the numbers of the observations are small.
The more favourable outcome with endocardial pacing was also observed in our total paediatric population8 as well as by other investigators,4,5,16 although comparable system performances between steroid-eluting and thin transvenous pacing leads were observed.10,17,18
The efficacy of endocardial pacing in very young patients has already been described during short-term5 and medium-term7 follow-up.
In the largest series with endocardial pacing, including 39 patients from two centres, 10 (28%) required lead or generator interventions prior to battery replacement, whereas 17/36 (47%) had the initial lead in situ and in use after a median follow-up duration of 4.3 years.7 Our data, based on a single-centre experience, demonstrate a reduced rate of complications requiring pacing system replacement, with 15/17 (88%) of the initial endocardial leads in use at last follow-up visit (median 6 years, or 7 years not considering the 3 patients lost to follow-up). Only 2 patients with transvenous pacing systems underwent system replacement: the youngest patient implanted at 2 days of age because of pocket erosion, and another patient because of lead malfunction 10 years after the initial implantation. Even if we consider the two patients with implant-related complications (haemothorax, lead dislodgement, and repositioning) that did not require system replacement, endocardial pacing has good results in this age group. However, it must be kept in mind that the potentially more serious complications of transvenous leads, including lead malfunction7,16,19 and valvular damage, may develop later. Moreover, as already underlined, the number of patients is small and results should be interpreted with caution.
Other authors7 described lead advancement for lead stretch during follow-up. We have no experience with this procedure. We think that the slowly absorbable ligature used for placement of endocardial leads20 alone is not the ideal solution to the problem of growth in our population, as it really does not allow the lead to advance into the vein over several years because of the creation of adherences between the lead and the subcutaneous tissues and/or the venous system, a fact that also reduces the risk of late lead dislodgement. However, this procedure seems sufficient to allow good pacing performance in the time-period described in our study (at least in the first 10 years of life of these patients), but lead malfunction seems to occur after the lead stretched with further somatic growth.8,14 Thus, in our last patients, we added an atrial loop to increase longevity.7,21
Our results indicate that in these very young patients, VVI/R PMs with transvenous leads seem a good solution, as previously reported7 also in older patients,22,23 and the findings from this study will likely influence our institutional preference from this point on.
No venous occlusion was found in this group of patients. Regarding venous occlusion,6,7,9,24,25 Doppler echo is not as reliable for the evaluation of innominate/superior vena cava occlusion as with venography or intravascular ultrasound.26 Other authors27 have recently described mild stenosis of the subclavian vein in 2/12 (17%) newborns and infants with permanent transvenous ventricular leads detected with Doppler sonographic examination. In our experience,9,14 Doppler echo examination of the subclavian vein before upgrading procedures was effective in identifying subclavian patency/occlusion subsequently confirmed by wire/lead insertion and/or venography, without false-positive or -negative results. Thus, although our approach may be less sensitive, we think that in absence of clinical or echo signs of venous obstruction, an invasive procedure is not to be indicated simply for evaluating venous patency during follow-up examination of children.27 The majority of our patients received thin unipolar and tined endocardial leads (Table 2). The smaller lead size seems the most important difference according to Kammeraad et al.,7 who described an 11% of asymptomatic subclavian vein occlusion. However, tined leads are more difficult to extract, and this might be relevant in the context of the patient who had a lead abandoned in the venous system and a second lead inserted.14
Pacing parameters were acceptable for all endocardial leads and epicardial steroid-eluting leads, although generally better for the former at 3–4 years of follow-up, as already described after nearly 2–4 years of follow-up.5,17 The U-shaped curve found for the thresholds of unipolar, epicardial steroid-eluting leads has not been described previously,1,3,5,10 and the long-term evolution of this pattern has yet to be defined.
Study limitations
This study is a single-centre, retrospective analysis, and there is a clear institutional bias in the choice of the pacing system implanted in the years 2000–04. Some patients were lost to follow-up. In the analysis of results, caution must be applied as the numbers are small. The venous patency could be examined only in 13 of the 16 patients at the completion of follow-up and only with non-invasive technique. Some of the pacing parameters, especially sensing values, were not always recorded. Finally, the PM generator and pacing lead technology improved during the years of the observation.
Conclusions
In infants who need PM therapy, there is a high frequency of complications often leading to implantation of new pacing systems, especially in epicardially paced patients. This study demonstrated that endocardial pacing is feasible and shows good results in experienced centres, even in infants. In this group of patients with thin transvenous leads, no venous occlusion was found during follow-up. The efficacy and safety with the current available medium-term follow-up indicate that single-lead, VVIR endocardial pacing is probably the best choice outside the neonatal period and this finding will likely influence our institutional preference from this point on. However, because of the small number of patients and the lack of long-term follow-up, these findings should be treated with caution.
Acknowledgements
The authors thank Professor Stephen P. Sanders, MD, for his help in reviewing the manuscript.



