-
PDF
- Split View
-
Views
-
Cite
Cite
The Steering Committee of the ISSUE 3 Study, International study on syncope of uncertain aetiology 3 (ISSUE 3): pacemaker therapy for patients with asystolic neurally-mediated syncope: rationale and study design, EP Europace, Volume 9, Issue 1, January 2007, Pages 25–30, https://doi.org/10.1093/europace/eul135
Close - Share Icon Share
Abstract
Aim To assess the effectiveness of pacing therapy for preventing syncope recurrence in patients with a high probability of cardio-inhibitory neurally-mediated syncope (NMS).
Methods Study design: Multi-centre, prospective, double-blind, randomized placebo-controlled study. Inclusion criteria: Eligible patients are at least 40 years of age and have suffered, in the prior 2 years, ≥ 3 syncope episodes of suspected NMS (with the exception of carotid sinus syndrome), which is considered by the attending physician to have a severe clinical presentation requiring treatment initiation. Patients with positive and negative tilt testing are included. Exclusion criteria: Patients with one or more of the following are excluded: carotid sinus syndrome; suspected or definite heart disease and high likelihood of cardiac syncope; symptomatic orthostatic hypotension diagnosed by standing blood pressure measurement; loss of consciousness different from syncope (e.g. epilepsy, psychiatric, metabolic, drop-attack, TIA, intoxication, cataplexy); subclavian steal syndrome. Study protocol: Eligible patients receive an Implantable Loop Recorder and are followed till the first documented syncopal recurrence or a significant asystolic event. Those patients who have an asystolic pause (sinus arrest or AV block) > 6 s or a syncopal asystolic pause ≥ 3 s receive a dual-chamber pacemaker implantation and are randomized to active therapy (Pm ON) or to placebo therapy (Pm OFF). End-points: Primary end-point is the first syncope recurrence after pacemaker implant. Sample size and duration: A maximum of 710 patients are to be enrolled during an anticipated period of 2 years to allow randomization of 60 patients in the Pm ON arm and 60 in the Pm OFF arm (total 120).
Introduction
The prospective International Study on Syncope of Uncertain Etiology 2 (ISSUE 2)1 assessed the effectiveness of a new strategy for managing patients with suspected neurally-mediated syncope (NMS)—apart from those with carotid sinus syndrome—according to criteria established in the European Society of Cardiology (ESC) guidelines (i.e. history, physical examination, electrocardiogram, and blood pressure measurements in both the supine and upright position).2,3 The strategy requires early application of an implantable loop recorder (ILR), irrespective of tilt testing results, and delay of therapy until after ILR documentation of recurrent syncope and establishment of a mechanism for the spontaneous syncope.
In ISSUE 2, only a minority of the NMS patients ultimately received ILR-based strategy. These were those who had both recurrences and a severe clinical presentation requiring treatment (high-risk or high-frequency settings as defined by ESC guidelines).2,3 A high mean age, a history of recurrent syncope beginning in middle or older age, and frequent injuries probably due to presentation without warning characterized the ISSUE-2 population and justify the need for a specific active treatment. In this population, about a half of the patients showed prolonged asystole at the time of syncope recurrence, which supports the indication for cardiac pacing therapy. In another study Brignole et al.,4 reported that older patients with unexplained syncope are more likely to have an indication for an ILR than those younger, and in these older patients ILR has a higher diagnostic value, with an arrhythmia more likely to be detected and successfully treated. These findings partially differentiated the ISSUE patients from the general population of patients affected by NMS and from the population of previous randomized controlled therapy trials.5–8
In asystolic NMS documented by ILR, ISSUE-2, an observational trial, showed that pacemakers were effective in reducing the 1-year first syncope recurrence rate from 33% rate before implant (ILR phase 1) to 5% rate after implant (phase 2). Moreover, the control non-asystolic group still continued to have a 41% recurrence rate after the first recurrence of syncope, supporting the fact that reduction with pacemakers was due to the beneficial effect of the pacemaker itself and not to other factors. However, a formal controlled trial is needed to confirm these findings.
Significance of ISSUE 3
ISSUE 3 is a multi-centre, prospective, randomized, controlled, double-blind study aimed to assess the effectiveness of pacemaker therapy for prevention of asystolic NMS.
Study objectives
The main objective of ISSUE 3 is to assess the effectiveness of pacing therapy for preventing syncope recurrence in patients with a high probability of NMS different from carotid sinus syndrome.
ISSUE 3 has two secondary objectives:
To evaluate the value of asystolic tilt testing responses in predicting spontaneous asystolic events.
To perform an observational evaluation of the feasibility and efficacy of physical manoeuvres in middle age and old patients with likely hypotensive NMS (PC study 2)
Study design
The patients undergoing randomization are identified by ILR diagnostic observations among patients who met the ESC criteria for a diagnosis of suspected NMS. The strategy requires early application of an ILR, irrespective of tilt testing results (phase 1), and delay of therapy until after ILR documentation of occurrence of an asystolic neurally-mediated episode (phase 2) (Figure 1).
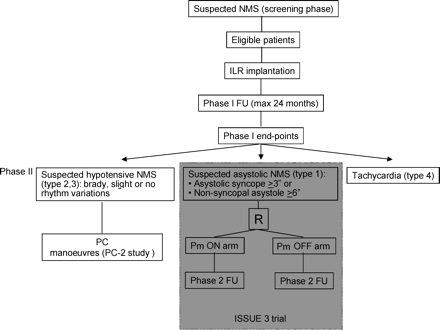
The patients who have a likely hypotensive NMS after the diagnostic ILR evaluation in phase 1 has excluded asystolic syncope, are instructed to perform physical counterpressure (PC) manoeuvre therapy (PC-2 study).
Study size and duration
The study will enrol a maximum of 710 patients in 60 centres in Europe, Canada, and USA with at least 100–200 patient attendances for syncope per year. This means an average of 12 enrolled patients per centre. No centre is allowed to recruit more than 10% of the total number of the study population.
Patient enrolment time is anticipated to last 2 years. Since it is anticipated that the study continues for a period of ∼24 months after the enrolment of the last patient, total study duration is ∼4 years.
Inclusion criteria
Patients must fulfil ALL of the following inclusion criteria:
Suspected or certain NMS, based on the Guidelines recently published by the Task Force on Syncope of the ESC2,3 (appendix);
≥ 3 syncope episodes in the last 2 years (minimum interval between the first and last episode ≥ 1 month);
Clinical presentation of syncope of sufficient severity requiring treatment initiation in the physician's and the patient's judgement. The final assessment whether the severity of the clinical presentation warrants treatment is left to the discretion of the physician and the patient, but the following definitions of high-risk or high-frequency settings are provided from guidelines:2,3
syncope is very frequent, e.g. alters the quality of life;
syncope is recurrent and unpredictable (absence of premonitory symptoms) and exposes patients at ‘high risk’ of trauma;
syncope occurs during the prosecution of a ‘high risk’ activity (e.g. driving, machine operator etc.);
Age > 40;
Negative carotid sinus massage;
Patients accept to have an ILR implantation.
Exclusion criteria
Carotid sinus hypersensitivity;
Suspected or certain heart disease and high likelihood of cardiac syncope;
Syncope during exercise;
Overt heart failure;
Ejection fraction ≤ 40%;
Old or recent myocardial infarction;
Hypertrophic cardiomyopathy;
Dilated cardiomyopathy;
Significant valvular disease;
Sinus bradycardia < 50 bpm or sino-atrial block;
Mobitz I second degree atrioventricular block;
Mobitz II second or third-degree atrioventricular block;
Bundle branch block;
Rapid paroxysmal supraventricular tachycardia or ventricular tachycardia;
Pre-excited QRS complexes;
Prolonged QT interval;
Right bundle branch block pattern with ST-elevation in leads V1–V3 (Brugada syndrome);
Negative T-waves in right precordial leads, epsilon waves; and ventricular late potentials suggestive of arrhythmogenic right ventricular dysplasia;
Symptomatic orthostatic hypotension diagnosed by standing blood pressure measurement;
Loss of consciousness different from syncope (e.g. epilepsy, psychiatric, metabolic, drop-attack, TIA, intoxication, cataplexy);
Subclavian steal syndrome;
Psychologically or physically (due to any other illness) or cognitively unfit for participation in the study according to the opinion of the investigator;
Patient compliance doubtful;
Patient geographically or otherwise inaccessible for follow-up;
Patient unwilling or unable to give informed consent;
Life expectancy < 1 year.
Screening phase and enrolment
All syncope patients are consecutively included in the pre-study screening phase, which will determine each patient's eligibility for enrolment in the ISSUE 3 study. A screening flow chart is shown in Table 1.
| Age > 40 | If yes, continue |
| ≥ 3 syncope during last 2 years | If yes, continue |
| Severe presentation requiring treatment, if any | If yes, continue |
| Non-syncopal disorders mimicking syncope: | If no, continue |
| Epilepsy likely; | |
| Psychiatric disorder likely, for example, somatization, hysteria, conversion reaction | |
| Metabolic disorder likely, for example, hypoglycaemia, hypoxia, hyperventilation | |
| Drop attacks likely | |
| Intoxication likely | |
| Transient ischaemic attack likely | |
| Cataplexy likely | |
| Symptomatic orthostatic hypotensiona | If no, continue |
| Suspected or certain heart disease and high likelihood of cardiac syncopeb | If no, continue |
| Syncope during exercise; | |
| Overt heart failure; | |
| Ejection fraction ≤ 40%; | |
| Old or recent myocardial infarction; | |
| Hypertrophic cardiomyopathy; | |
| Dilated cardiomyopathy; | |
| Significant valvular disease; | |
| Sinus bradycardia < 50 b.p.m. or sino-atrial block; | |
| Mobitz I second degree atrioventricular block; | |
| Mobitz II second or third-degree atrioventricular block; | |
| Bundle branch block; | |
| Rapid paroxysmal supraventricular tachycardia or ventricular tachycardia; | |
| Pre-excited QRS complexes; | |
| Prolonged QT interval; | |
| Right bundle branch block pattern with ST-elevation in leads V1–V3 (Brugada syndrome); | |
| Negative T-waves in right precordial leads, epsilon waves, and ventricular late potentials suggestive of arrhythmogenic right ventricular dysplasia | |
| Steal syndrome | If no, continue |
| Carotid sinus hypersensitivityc | If no, continue |
| Tilt test performed)d | Irrespective of result, continue |
| Patient eligible |
| Age > 40 | If yes, continue |
| ≥ 3 syncope during last 2 years | If yes, continue |
| Severe presentation requiring treatment, if any | If yes, continue |
| Non-syncopal disorders mimicking syncope: | If no, continue |
| Epilepsy likely; | |
| Psychiatric disorder likely, for example, somatization, hysteria, conversion reaction | |
| Metabolic disorder likely, for example, hypoglycaemia, hypoxia, hyperventilation | |
| Drop attacks likely | |
| Intoxication likely | |
| Transient ischaemic attack likely | |
| Cataplexy likely | |
| Symptomatic orthostatic hypotensiona | If no, continue |
| Suspected or certain heart disease and high likelihood of cardiac syncopeb | If no, continue |
| Syncope during exercise; | |
| Overt heart failure; | |
| Ejection fraction ≤ 40%; | |
| Old or recent myocardial infarction; | |
| Hypertrophic cardiomyopathy; | |
| Dilated cardiomyopathy; | |
| Significant valvular disease; | |
| Sinus bradycardia < 50 b.p.m. or sino-atrial block; | |
| Mobitz I second degree atrioventricular block; | |
| Mobitz II second or third-degree atrioventricular block; | |
| Bundle branch block; | |
| Rapid paroxysmal supraventricular tachycardia or ventricular tachycardia; | |
| Pre-excited QRS complexes; | |
| Prolonged QT interval; | |
| Right bundle branch block pattern with ST-elevation in leads V1–V3 (Brugada syndrome); | |
| Negative T-waves in right precordial leads, epsilon waves, and ventricular late potentials suggestive of arrhythmogenic right ventricular dysplasia | |
| Steal syndrome | If no, continue |
| Carotid sinus hypersensitivityc | If no, continue |
| Tilt test performed)d | Irrespective of result, continue |
| Patient eligible |
aOrthostatic syncope is diagnosed when there is documentation of orthostatic hypotension associated with syncope or pre-syncope. Orthostatic blood pressure measurements are recommended after 5 min of lying supine, followed by measurements each minute, or more often, after standing for 3 min. Measurements may be continued for longer, if blood pressure is still falling at 3 min. If the patient does not tolerate standing for this period, the lowest systolic blood pressure during the upright posture should be recorded. A decrease in systolic blood pressure ≥ 20 mmHg or a decrease of systolic blood pressure to < 90 mmHg is defined as orthostatic hypotension.
bIn the case of suspected structural heart disease, an echocardiogram may be required to confirm or rule out the diagnosis of structural heart disease.
cCarotid sinus hypersensitivity must be ruled out by a carotid sinus massage, supine and upright. Carotid sinus hypersensitivity is defined as asystole ≥ 3 s and/or a fall in systolic blood pressure ≥ 50 mmHg.
dThe tilt test protocol comprises a 60°–70° passive tilt (20 min) followed by a 0.4 mg nitroglycerine challenge (15 min) when the passive phase fails to induce syncope.
| Age > 40 | If yes, continue |
| ≥ 3 syncope during last 2 years | If yes, continue |
| Severe presentation requiring treatment, if any | If yes, continue |
| Non-syncopal disorders mimicking syncope: | If no, continue |
| Epilepsy likely; | |
| Psychiatric disorder likely, for example, somatization, hysteria, conversion reaction | |
| Metabolic disorder likely, for example, hypoglycaemia, hypoxia, hyperventilation | |
| Drop attacks likely | |
| Intoxication likely | |
| Transient ischaemic attack likely | |
| Cataplexy likely | |
| Symptomatic orthostatic hypotensiona | If no, continue |
| Suspected or certain heart disease and high likelihood of cardiac syncopeb | If no, continue |
| Syncope during exercise; | |
| Overt heart failure; | |
| Ejection fraction ≤ 40%; | |
| Old or recent myocardial infarction; | |
| Hypertrophic cardiomyopathy; | |
| Dilated cardiomyopathy; | |
| Significant valvular disease; | |
| Sinus bradycardia < 50 b.p.m. or sino-atrial block; | |
| Mobitz I second degree atrioventricular block; | |
| Mobitz II second or third-degree atrioventricular block; | |
| Bundle branch block; | |
| Rapid paroxysmal supraventricular tachycardia or ventricular tachycardia; | |
| Pre-excited QRS complexes; | |
| Prolonged QT interval; | |
| Right bundle branch block pattern with ST-elevation in leads V1–V3 (Brugada syndrome); | |
| Negative T-waves in right precordial leads, epsilon waves, and ventricular late potentials suggestive of arrhythmogenic right ventricular dysplasia | |
| Steal syndrome | If no, continue |
| Carotid sinus hypersensitivityc | If no, continue |
| Tilt test performed)d | Irrespective of result, continue |
| Patient eligible |
| Age > 40 | If yes, continue |
| ≥ 3 syncope during last 2 years | If yes, continue |
| Severe presentation requiring treatment, if any | If yes, continue |
| Non-syncopal disorders mimicking syncope: | If no, continue |
| Epilepsy likely; | |
| Psychiatric disorder likely, for example, somatization, hysteria, conversion reaction | |
| Metabolic disorder likely, for example, hypoglycaemia, hypoxia, hyperventilation | |
| Drop attacks likely | |
| Intoxication likely | |
| Transient ischaemic attack likely | |
| Cataplexy likely | |
| Symptomatic orthostatic hypotensiona | If no, continue |
| Suspected or certain heart disease and high likelihood of cardiac syncopeb | If no, continue |
| Syncope during exercise; | |
| Overt heart failure; | |
| Ejection fraction ≤ 40%; | |
| Old or recent myocardial infarction; | |
| Hypertrophic cardiomyopathy; | |
| Dilated cardiomyopathy; | |
| Significant valvular disease; | |
| Sinus bradycardia < 50 b.p.m. or sino-atrial block; | |
| Mobitz I second degree atrioventricular block; | |
| Mobitz II second or third-degree atrioventricular block; | |
| Bundle branch block; | |
| Rapid paroxysmal supraventricular tachycardia or ventricular tachycardia; | |
| Pre-excited QRS complexes; | |
| Prolonged QT interval; | |
| Right bundle branch block pattern with ST-elevation in leads V1–V3 (Brugada syndrome); | |
| Negative T-waves in right precordial leads, epsilon waves, and ventricular late potentials suggestive of arrhythmogenic right ventricular dysplasia | |
| Steal syndrome | If no, continue |
| Carotid sinus hypersensitivityc | If no, continue |
| Tilt test performed)d | Irrespective of result, continue |
| Patient eligible |
aOrthostatic syncope is diagnosed when there is documentation of orthostatic hypotension associated with syncope or pre-syncope. Orthostatic blood pressure measurements are recommended after 5 min of lying supine, followed by measurements each minute, or more often, after standing for 3 min. Measurements may be continued for longer, if blood pressure is still falling at 3 min. If the patient does not tolerate standing for this period, the lowest systolic blood pressure during the upright posture should be recorded. A decrease in systolic blood pressure ≥ 20 mmHg or a decrease of systolic blood pressure to < 90 mmHg is defined as orthostatic hypotension.
bIn the case of suspected structural heart disease, an echocardiogram may be required to confirm or rule out the diagnosis of structural heart disease.
cCarotid sinus hypersensitivity must be ruled out by a carotid sinus massage, supine and upright. Carotid sinus hypersensitivity is defined as asystole ≥ 3 s and/or a fall in systolic blood pressure ≥ 50 mmHg.
dThe tilt test protocol comprises a 60°–70° passive tilt (20 min) followed by a 0.4 mg nitroglycerine challenge (15 min) when the passive phase fails to induce syncope.
Eligible patients, after giving informed consent, receive ILR implant and are followed till the first documented syncopal recurrence or a significant asystolic event or the end of the study, whichever comes first. The results of the carotid sinus massage and tilt test performed during the screening phase are collected. Tilt testing positive responses are classified according to the New VASIS classification.9
Phase-1 follow-up
After ILR implantation, all patients are followed-up quarterly during the first 24 months. If ILR battery exhaustion occurs before the completion of Phase I, ILR replacement is encouraged, but the decision is left to the investigator. The patient will keep a logbook for registration of symptoms as palpitations, dizziness, pre-syncope, and syncope. Through the whole study, the patient is instructed to activate the ILR and to contact a doctor in case of any syncope, in order to interrogate the device as soon as possible.
Phase-1 follow-up scheme will be continued until the first occurrence of one of the following events:
First syncope reported by the patient with ILR documentation, irrespective of ECG findings; or
Non-syncopal (asymptomatic or pre-syncopal) episodes with ILR documentation of an asystolic pause (sinus arrest or AV block) ≥ 6 s (type 1 of ISSUE classification); or
Completion of the 24-month follow-up period or ILR battery exhaustion (and decision not to replace the device).
At the end of Phase 1, ILR is left in situ in patients undergoing PC-2 study for documentation of PC effect. ILR can be explanted in patients entering the randomized phase of the ISSUE 3 trial after pacemaker implantation; however, investigators are encouraged to leave the ILR inserted if the patient permits in order to collect further follow-up clinical data.
The end-points of Phase I are:
2-year incidence of syncope recurrence, expressed as proportion of patients experiencing a recurrent syncopal episode;
2-year incidence of asystolic (≥3 s) syncope or ≥ 6 s non-syncopal episodes (estimated 2-year pacemaker implantation incidence according to ISSUE 3 criteria);
Value of asystolic tilt testing responses in predicting spontaneous asystolic events.
Phase 2
The second follow-up phase will document the patient outcome after administration of ILR-guided therapy according to the schema shown in the Table 2, which is based on the ILR findings (see ISSUE classification10).
| ILR findings in Phase 1 . | ISSUE classification . | Therapy . | Phase 2 . |
|---|---|---|---|
| ECG documentation of non-syncopal (asymptomatic or pre-syncopal) asystolic events of ≥ 6 s | Type 1 | Pacemaker | RCT |
| Syncope, asystolic | Type 1 | Pacemaker | RCT |
| Syncope, bradycardia | Type 2 | CPM | PC 2 study |
| Syncope, slight or no rhythm variations | Type 3 | CPM | PC 2 study |
| Syncope, sinus tachycardia | Type 4A | CPM | PC 2 study |
| Syncope, tachycardia | Type 4B,C,D | Individual | Noa |
| ILR findings in Phase 1 . | ISSUE classification . | Therapy . | Phase 2 . |
|---|---|---|---|
| ECG documentation of non-syncopal (asymptomatic or pre-syncopal) asystolic events of ≥ 6 s | Type 1 | Pacemaker | RCT |
| Syncope, asystolic | Type 1 | Pacemaker | RCT |
| Syncope, bradycardia | Type 2 | CPM | PC 2 study |
| Syncope, slight or no rhythm variations | Type 3 | CPM | PC 2 study |
| Syncope, sinus tachycardia | Type 4A | CPM | PC 2 study |
| Syncope, tachycardia | Type 4B,C,D | Individual | Noa |
RCT, randomized controlled trial; CPM, counterpressure manoeuvres.
aUsual clinical management outside the trial.
| ILR findings in Phase 1 . | ISSUE classification . | Therapy . | Phase 2 . |
|---|---|---|---|
| ECG documentation of non-syncopal (asymptomatic or pre-syncopal) asystolic events of ≥ 6 s | Type 1 | Pacemaker | RCT |
| Syncope, asystolic | Type 1 | Pacemaker | RCT |
| Syncope, bradycardia | Type 2 | CPM | PC 2 study |
| Syncope, slight or no rhythm variations | Type 3 | CPM | PC 2 study |
| Syncope, sinus tachycardia | Type 4A | CPM | PC 2 study |
| Syncope, tachycardia | Type 4B,C,D | Individual | Noa |
| ILR findings in Phase 1 . | ISSUE classification . | Therapy . | Phase 2 . |
|---|---|---|---|
| ECG documentation of non-syncopal (asymptomatic or pre-syncopal) asystolic events of ≥ 6 s | Type 1 | Pacemaker | RCT |
| Syncope, asystolic | Type 1 | Pacemaker | RCT |
| Syncope, bradycardia | Type 2 | CPM | PC 2 study |
| Syncope, slight or no rhythm variations | Type 3 | CPM | PC 2 study |
| Syncope, sinus tachycardia | Type 4A | CPM | PC 2 study |
| Syncope, tachycardia | Type 4B,C,D | Individual | Noa |
RCT, randomized controlled trial; CPM, counterpressure manoeuvres.
aUsual clinical management outside the trial.
Randomized double-blind trial
Pacemaker therapy for patients with asystolic (type 1) NMS: ISSUE- 3
Eligible patients (Table 2) receive a dual-chamber pacemaker implantation with rate drop response (RDR) features and are randomized to active therapy (Pm ON) or to placebo therapy (Pm OFF). Randomization 1:1, Pm DDD with RDR vs. Pm ODO with diagnostic functions, mode of pacing blind to the patient and to the follow-up physician. Randomization is made centrally and is assigned automatically to each patient, via the web, online. The randomization list is blocked per centre. Randomization, pacemaker implantation and programming of the pacemaker ON or OFF are performed in close sequence during the same day in order to avoid the risk of occurrence of end-point events in between. The pacemaker is programmed by the implanting electrophysiologist and mode of pacing is kept blind to the patient and to the follow-up physician and to all other study personnel. Blinded study personnel and physician are asked not to perform routine electrocardiograms.
In patients randomized to pacemaker ON, the pacemaker is programmed with the RDR feature ON. The RDR feature institutes rapid DDD pacing if the device detects a rapid decrease in heart rate. On the basis of a post hoc analysis of spontaneous asystolic episodes documented by ILR in the ISSUE 2 study, the initial programmed setting must be as follows:
Detection options: both (drop detect + low-rate detect);
Drop size: 20 bpm;
Drop rate: 50 bpm;
Detection window: 1 min;
Intervention rate: 90 bpm;
Intervention duration: 1 min;
Lower rate: 40 bpm;
Confirmation beats: 2.
RDR function is utilized in the DDD mode with an AV delay sufficient to minimize unnecessary ventricular pacing, as the aim is to combine minimal ventricular pacing with an effective AV delay during intervention.
At any time during follow-up, if this programmed mode causes discomfort for the patient, the pacemaker may be reprogrammed to:
Detection options: low-rate detect only;
Intervention rate: 90 bpm;
Intervention duration: 1 min;
Lower rate: 40 bpm;
Confirmation beats: 2.
In patients randomized to pacemaker OFF, pacemaker is programmed in ODO mode with default diagnostic functions. After the first syncopal recurrence in Pm OFF arm, the pacemaker is switched ON, using the programmed parameters as described first in Phase II (above) and follow-up continues for secondary end-points.
After pacemaker implantation, all patients are followed-up quarterly during the first 24 months or until the study ends by a physician who is blind to the pacemaker mode. The patient keeps a logbook for registration of symptoms as palpitations, dizziness, pre-syncope, and syncope. Through the whole study, the patient is instructed to contact a doctor as soon as possible in case of any syncope in order to insert the event in the database, to interrogate the pacemaker (and ILR if still implanted) and to switch the pacemaker ON in the patients in the pm OFF arm.
Primary end-point is the comparison of the time to first syncope recurrence in the two study arms according to the intention-to-treat assignment. Secondary end-points are: ILR findings at the time of syncopal recurrence in the control group (reproducibility of responses), and predictive value of tilt testing.
PC-2 observational study
Feasibility and efficacy of physical manoeuvres in hypotensive NMS in middle age and old patients
The patients who have a likely hypotensive NMS after the diagnostic evaluation are instructed to perform PC manoeuvre therapy and lifestyle changes and are followed-up in an observational trial (Figure 1 and Table 2).
The randomized controlled PC trial11 recently showed that education and PC manoeuvres performed at the time of appearance of symptoms of impending syncope are effective in reducing syncopal recurrences in young patients affected by vasovagal syncope.
The objective of the PC-2 study is to evaluate feasibility and efficacy of PC in a different subset of patients such as those of the ISSUE 3 population. An additional objective is to find an ECG documentation (by means of the already inserted ILR) of the cardiac rhythm before and during the execution of PC.
Treatment consists of isometric leg and arm counterpressure manoeuvres. Patients are advised to use leg-crossing as a preventive measure and to use lower body muscle tensing including ‘buttock-clenching’ with leg- and abdominal muscle tensing, or use hand-gripping with a rubber ball or arm tensing in case of the occurrence of symptoms of impending syncope. The patients are instructed to maintain the manoeuvre they choose as long as possible and eventually to move on to a second or third manoeuvre if useful. Patients are allowed to choose the manoeuvre and the sequence of their administration, but they must make note of them in the logbook.
All patients are followed-up quarterly during the first 24 months or until the study ends. The patient keeps a logbook for registration of symptoms as palpitations, dizziness, pre-syncope, and syncope.
The end-points are: time to first syncope recurrence; total burden of syncope; correlation between syncope recurrence and execution of PC manoeuvre; and correlation of PC manoeuvres with ILR findings at the time of aborting syncope.
Statistics
On the basis of ISSUE 2 results, the control arm will have 1-year 35% recurrence rate (40% observed minus 5% potential placebo effect) and active arms 10% recurrence rate (5% observed plus 5% potential unblindness bias).
A further 15% increase in sample size is scheduled to account for: censored data (5%), cross-over after randomization and violation of assigned treatment (5%), and any other factor (5%).
Since with a sequential study design, an ad interim analysis with an alpha error of 1% is planned, the final sample size, calculated with a confidence interval of 96% with a power of 80%, gives a result of 60 patients in the Pm ON arm and 60 in the Pm OFF arm (total 120).
Thus a total of 27 patients (6 in Pm ON arm and 21 in Pm OFF arm) are required to have recurrence of syncope during the follow-up. The ad interim analysis will be performed when 75% of total primary end-points (total 20 patients) will be obtained. If the primary objective is reached, the study immediately stops. The stop date of Phase 2 study is the date of insertion in the database of the last syncopal recurrence.
ISSUE 2 showed that 18 asystolic target episodes (14 syncopal and 4 non-syncopal) were recorded every 100 patients receiving an ILR during a mean of 12 months of observation after ILR implantation. This means that 667 patients need an ILR implantation to reach the number of 120 asystolic target events. Since it is anticipated a 6% rate of patients lost to follow-up after ILR implantation (Phase I), the total maximum number of patients to be enrolled and implanted is to be increased to 710.
In the EGSYS 2 study,12 NMS accounted for 66% of patients referred to the emergency department for syncope. Among these, 9% (6% of total patients referred for syncope) had had a median of six syncopal episodes during life and were ≥ 40 years old, features that matched those of the ISSUE population. In another study,13 5% of patients referred to two syncope units finally received an ILR implantation. Therefore, it is anticipated that ISSUE 3 eligible patients are 9% of all patients affected by NMS and 6% of all patients affected by syncope seeking medical assistance. This means that about 6000 patients affected by suspected NMS (or 9000 patients affected by syncope) need to be screened in order to find the required number of patients eligible for inclusion.
The primary analysis of ISSUE 3 is planned as a comparison of the cumulative risk of syncope between the two treatment arms using a log-rank test. An one-sided test is used for primary analysis because there is no plausible potential for an increase in syncope to occur with pacing.
All randomized patients are analysed according to the intention-to-treat principle. Thus, all outcomes are attributed to the randomly assigned treatment group regardless of compliance with assigned treatment.
During ISSUE 3 Phase-2 follow-up, the cumulative number of patients with syncopal recurrence, but not the relative distribution of these episodes between the two randomized arms (Pm ON or Pm OFF), is made available to the End-point Committee. Statistical analysis is performed by an independent statistician not involved in the study. Neither the End-point Committee nor the Steering Committee are informed of the results before study closure.
Appendix
Steering committee: Michele Brignole (Chairman), Dietrich Andresen, David Benditt, Jean Jacques Blanc, Roberto Garcia-Civera, Andrew Khran, Carlo Menozzi, Angel Moya, Richard Sutton, Panos Vardas, Wouter Wieling.
End-point committee: Michele Brignole, Richard Sutton, Carlo Menozzi, Angel Moya.
Statistical analysis: Erik Cobo.
Overall study management responsibilities: Nicoletta Grovale.
Sponsor: Medtronic Bakken Research Center.



