-
PDF
- Split View
-
Views
-
Cite
Cite
Marjatta Strandberg, M.J. Pekka Raatikainen, Matti Niemelä, Matti Luotolahti, Jaakko Hartiala, K.E. Juhani Airaksinen, Clinical practicality and predictive value of transoesophageal echocardiography in early cardioversion of atrial fibrillation, EP Europace, Volume 8, Issue 6, June 2006, Pages 408–412, https://doi.org/10.1093/europace/eul034
Close - Share Icon Share
Abstract
Aims The objective of this study is to evaluate the feasibility of transoesophageal echocardiography (TOE)-guided cardioversion (CV) of atrial fibrillation (AF) in daily clinical practice.
Methods and results Transthoracic echocardiography and TOE were performed in 346 consecutive patients with AF lasting longer than 48 h or of unknown duration. If no intracavitary thrombus was found, CV was performed within 24 h of the TOE examination. Anticoagulation with subcutaneous low-molecular-weight heparin and warfarin was always started before CV. Warfarin was continued for at least 1 month after CV. The predictive value of several echocardiographic parameters including peak left atrial appendage emptying velocity (PLAAEV), left ventricular ejection fraction, left atrial diameter, and spontaneous echo contrast for the initial and long-term success of CV were evaluated. Transoesophageal echocardiography revealed no thrombus or other contraindications to CV in 274/346 (79%) patients. Early CV restored normal sinus rhythm or pacemaker rhythm in 90% (246/274) of the patients. One patient (0.3%) had a stroke within 30 days after CV. Peak left atrial appendage emptying velocity was significantly lower in patients with contraindications to early CV (P<0.001). However, neither PLAAEV nor any other echocardiographic parameter predicted the initial success of CV and the maintenance of sinus rhythm during long-term follow-up.
Conclusion Early TOE-guided CV with short-term anticoagulation is a safe and clinically effective alternative in treatment of AF lasting longer than 48 h or of unknown duration. The initial and long-term success of CV cannot be reliably predicted by echocardiographic parameters.
Introduction
Cardioversion (CV) of atrial fibrillation (AF) is frequently performed to relieve symptoms and improve cardiac performance. Because of the increased risk of stroke due to embolization of pre-existing intracavitary thrombus, patients with AF lasting more than 48 h or of unknown duration should be treated with therapeutic anticoagulation for at least 3 weeks before CV.1 Although the risk of stroke and other embolic events is low,2–4 the conventional approach exposes the patient to prolonged anticoagulation and postpones CV and symptom relief for up to several weeks.5 Given these limitations, several investigators have proposed that transoesophageal echocardiography (TOE) with short-term anticoagulation could be used as an alternative management strategy in patients with symptomatic AF.6–9 The premise of this approach is that TOE is a highly sensitive and specific method of detecting thrombus in the left atrium and left atrial appendage.10–12
The results of recent studies, including the ACUTE trial, have shown that the thromboembolic rate in early TOE-guided CV is comparable with that in conventional therapy.2–4,13–16 It is obvious that TOE-guided CV shortens the overall duration of AF and anticoagulation and may lower the risk of bleeding.2,7–9 However, its impact on restoration and maintenance of normal sinus rhythm is controversial.2,8,15,16 Likewise, the data on the predictive value of echocardiographic parameters (e.g. peak left atrial appendage emptying velocity, PLAAEV) on the short- and long-term success of CV are discordant.17–23
In this prospective study, we evaluated the safety and usefulness of TOE-guided CV of AF lasting longer than 48 h or of unknown duration in daily clinical practice. Moreover, the role of various echocardiographic parameters including PLAAEV in predicting the short- and long-term success of CV was analyzed.
Methods
Patients
This prospective study was carried out as part of normal clinical practice in the Oulu and Turku University Hospitals in Finland. A total of 354 consecutive patients with electrocardiographically documented AF lasting longer than 48 h or of unknown duration and willing to choose TOE-guided CV strategy were studied. Patients with acute myocardial infarction, acute myocarditis, acute pulmonary embolism, hyperthyroidism, congenital heart disease, mitral stenosis, pericarditis, ≥3 weeks effective anticoagulation, or contraindications to TOE or CV were excluded from the study. All patients gave oral informed consent for the data collection.
Echocardiography
The echocardiographic examinations were performed as part of normal clinical practice with the Acuson Sequeia (Acuson Corp., Mountain View, CA, USA) or the Hewlett Packard Sonos 5500 (Andover, MA, USA) echocardiographic system. Transthoracic echocardiography was performed before TOE in all patients using a 2.5–3.5 MHz scanning frequency linear array transducer. Atrial and ventricular dimensions and left ventricular ejection fraction were measured from the parasternal long-axis view.24 The TOE study was performed in the fasting state under local anaesthesia using a 5.0 MHz biplane or a 5.0 MHz multiplane transducer. The entire heart, especially the left atrial appendage, was screened for thrombus (Figure 1) and spontaneous echo contrast, ‘smoke-like’ echoes (Figure 2). Peak left atrial appendage emptying velocity was measured by pulsed-wave Doppler at the orifice of the left atrial appendage, as an average of three to five consecutive cycles.
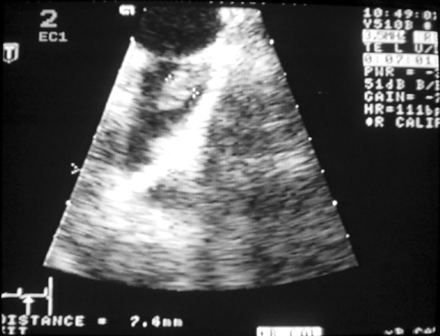
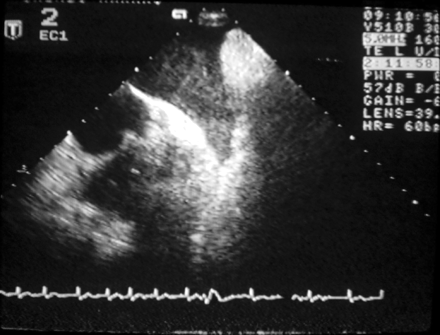
CV and follow-up
If no intracavitary thrombus was found (or suspected), electrical or pharmacological CV was done within 24 h from the TOE examination. Anticoagulation with subcutaneous low-molecular-weight heparin (LMWH) and warfarin was always started before CV. Warfarin was continued for at least 1 month after CV and subcutaneous LMWH (enoxaparin 1 mg/kg×2, maximum dosage 100 mg×2, or dalteparin 200 U/kg×1, maximum dosage 18 000 U×1) treatment during hospitalization. Spontaneous echo contrast and low PLAAEV were not considered as a contraindication to early CV. ECG was monitored continuously during the procedure and for several hours after the CV. Cardioversion was considered successful if the patient was in sinus or pacemaker rhythm on the next day. All thromboembolic events within 1 month from the procedure were regarded as being related to the CV. In patients with intracavitary thrombus, CV was postponed until anticoagulation with warfarin had reached therapeutic value (INR 2.0–3.0) for at least 3 weeks.
All patients were followed for 1 year after CV. Follow-up and monitoring of warfarin therapy were carried out in the outpatient clinic or in local health centres. The patients were instructed to contact their physician for ECG in the case of recurrence of arrhythmia symptoms. No attempt to document asymptomatic arrhythmias was made.
Statistical analysis
The statistical analyses were performed using SAS statistical software (version 9.1 for Windows, Cary, NC, USA). The results are given as mean±standard deviation or percentages where appropriate. Two-by-two tables were constructed to analyse associations between the groups. Significance was tested using Fisher's exact test. The level of significance for testing all null hypotheses was a two-tailed P-value <0.05. Odds ratios and 95% confidence intervals were calculated for the risk factors. The two sample t-test was used to compare the mean values.
Results
Clinical and echocardiographic findings
Transoesophageal echocardiography examination was technically successful in 346 patients (98%). In eight patients, the quality of TOE examination was inadequate and they were excluded from the study. The age of the study patients was 66±12 years (range 21–90 years) and 65% (225/346) of them were men. The exact duration of AF was unknown in 68% (235/346) of patients. In the others, AF had lasted 10±18 days (range 2–150 days) before hospitalization.
Transoesophageal echocardiography revealed no contraindications to early CV in 79% (274/346) of the patients. An obvious or suspected left atrial appendage or left atrial thrombus was observed in 20% (70/346) of the cases. In two other patients, early CV was not performed, because of severe mitral valve prolapse and regurgitation. Previous stroke was significantly more common among the patients with a contraindication to early CV (P=0.011). Contraindications to early CV were equally common in patients with AF and atrial flutter (21.3 vs. 19.0%). There were no differences between the groups in the other clinical characteristics (e.g. age and duration of AF) (Table 1). In the TOE examination, spontaneous echo contrast was significantly more common in the patients with intracavitary thrombus (P<0.001) (Table 2). The mean PLAAEV in the entire study population was 0.42±0.19 m/s. Peak left atrial appendage emptying velocity was significantly lower in the patients with a contraindication to early CV (P<0.001) (Table 2). Low PLAAEV (<0.40 m/s) was associated with the presence of a left atrial or left atrial appendage thrombus (OR 2.3, 95% CI 1.2–4.5, P=0.013) and the presence of left atrial spontaneous echo contrast (OR 3.9, 95% CI 2.2–6.7, P<0.001).
Comparison of the clinical parameters in the patients undergoing TOE-guided CV and the patients with contraindications to early cardioversion
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Age (years) | 65 ± 12 | 66 ± 12 | 0.789 |
| Males | 43 (60%) | 182 (66%) | 0.331 |
| Females | 29 (40%) | 92 (34%) | 0.331 |
| Coronary artery disease | 30 (42%) | 111 (41%) | 0.893 |
| Hypertension | 30 (42%) | 108 (39%) | 0.787 |
| Diabetes | 10 (14%) | 34 (12%) | 0.695 |
| Congestive heart failure | 18 (25%) | 54 (20%) | 0.331 |
| Previous stroke | 9 (13%) | 11 (4.0%) | 0.011 |
| Mitral valve regurgitation (grade ≥2) | 15 (21%) | 44 (16%) | 0.379 |
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Age (years) | 65 ± 12 | 66 ± 12 | 0.789 |
| Males | 43 (60%) | 182 (66%) | 0.331 |
| Females | 29 (40%) | 92 (34%) | 0.331 |
| Coronary artery disease | 30 (42%) | 111 (41%) | 0.893 |
| Hypertension | 30 (42%) | 108 (39%) | 0.787 |
| Diabetes | 10 (14%) | 34 (12%) | 0.695 |
| Congestive heart failure | 18 (25%) | 54 (20%) | 0.331 |
| Previous stroke | 9 (13%) | 11 (4.0%) | 0.011 |
| Mitral valve regurgitation (grade ≥2) | 15 (21%) | 44 (16%) | 0.379 |
Comparison of the clinical parameters in the patients undergoing TOE-guided CV and the patients with contraindications to early cardioversion
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Age (years) | 65 ± 12 | 66 ± 12 | 0.789 |
| Males | 43 (60%) | 182 (66%) | 0.331 |
| Females | 29 (40%) | 92 (34%) | 0.331 |
| Coronary artery disease | 30 (42%) | 111 (41%) | 0.893 |
| Hypertension | 30 (42%) | 108 (39%) | 0.787 |
| Diabetes | 10 (14%) | 34 (12%) | 0.695 |
| Congestive heart failure | 18 (25%) | 54 (20%) | 0.331 |
| Previous stroke | 9 (13%) | 11 (4.0%) | 0.011 |
| Mitral valve regurgitation (grade ≥2) | 15 (21%) | 44 (16%) | 0.379 |
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Age (years) | 65 ± 12 | 66 ± 12 | 0.789 |
| Males | 43 (60%) | 182 (66%) | 0.331 |
| Females | 29 (40%) | 92 (34%) | 0.331 |
| Coronary artery disease | 30 (42%) | 111 (41%) | 0.893 |
| Hypertension | 30 (42%) | 108 (39%) | 0.787 |
| Diabetes | 10 (14%) | 34 (12%) | 0.695 |
| Congestive heart failure | 18 (25%) | 54 (20%) | 0.331 |
| Previous stroke | 9 (13%) | 11 (4.0%) | 0.011 |
| Mitral valve regurgitation (grade ≥2) | 15 (21%) | 44 (16%) | 0.379 |
Documented arrhythmia and echocardiographic parameters in patients undergoing TOE-guided CV and the patients with contraindications to early CV
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Atrial fibrillation | 62 (86%) | 227 (83%) | 0.595 |
| Atrial flutter | 10 (14%) | 47 (17%) | 0.595 |
| PLAAEV (m/s ± SD) | 0.35 ± 0.16 | 0.44 ± 0.20 | <0.001 |
| LAD (mm ± SD) | 46 ± 7 | 44 ± 7 | 0.194 |
| LVEF (% ± SD) | 51 ± 14 | 51 ± 14 | 0.822 |
| SEC | 40 (56%) | 70 (26%) | <0.001 |
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Atrial fibrillation | 62 (86%) | 227 (83%) | 0.595 |
| Atrial flutter | 10 (14%) | 47 (17%) | 0.595 |
| PLAAEV (m/s ± SD) | 0.35 ± 0.16 | 0.44 ± 0.20 | <0.001 |
| LAD (mm ± SD) | 46 ± 7 | 44 ± 7 | 0.194 |
| LVEF (% ± SD) | 51 ± 14 | 51 ± 14 | 0.822 |
| SEC | 40 (56%) | 70 (26%) | <0.001 |
LAD, left atrial diastolic diameter; LVEF, left ventricular ejection fraction; SEC, spontaneous echo contrast.
Documented arrhythmia and echocardiographic parameters in patients undergoing TOE-guided CV and the patients with contraindications to early CV
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Atrial fibrillation | 62 (86%) | 227 (83%) | 0.595 |
| Atrial flutter | 10 (14%) | 47 (17%) | 0.595 |
| PLAAEV (m/s ± SD) | 0.35 ± 0.16 | 0.44 ± 0.20 | <0.001 |
| LAD (mm ± SD) | 46 ± 7 | 44 ± 7 | 0.194 |
| LVEF (% ± SD) | 51 ± 14 | 51 ± 14 | 0.822 |
| SEC | 40 (56%) | 70 (26%) | <0.001 |
| . | No CV (n=72) . | CV (n=274) . | P-value . |
|---|---|---|---|
| Atrial fibrillation | 62 (86%) | 227 (83%) | 0.595 |
| Atrial flutter | 10 (14%) | 47 (17%) | 0.595 |
| PLAAEV (m/s ± SD) | 0.35 ± 0.16 | 0.44 ± 0.20 | <0.001 |
| LAD (mm ± SD) | 46 ± 7 | 44 ± 7 | 0.194 |
| LVEF (% ± SD) | 51 ± 14 | 51 ± 14 | 0.822 |
| SEC | 40 (56%) | 70 (26%) | <0.001 |
LAD, left atrial diastolic diameter; LVEF, left ventricular ejection fraction; SEC, spontaneous echo contrast.
Short- and long-term success of CV
Transoesophageal echocardiography-guided CV was successful in 90% (246/274) of the cases. Pharmacological CV was used in 24 patients (9%) and all others underwent electrical CV. None of the clinical and echocardiographic variables predicted the initial success of CV. At 1 month, 77% (190/246) of patients with successful CV had remained in sinus rhythm. Patients on antihypertensive medication had significantly less recurrences of AF at 1 month (P=0.029) than the normotensive patients (Table 3). In the other clinical (Table 3) and echocardiographic variables (Table 4), there were no significant differences between the patients who remained in sinus rhythm and the patients with recurrence of AF. Antiarrhythmic medication did not differ significantly between the groups. No major haemorrhagic complications appeared during the 1-month follow-up period. At 1 year, only 46% (114/246) of the patients were in sinus rhythm. No clinical or echocardiographic variable predicted the recurrence of AF.
Clinical parameters in patients with and without recurrence of atrial fibrillation at 1 month
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| Age (years ± SD) | 67 ± 11 | 63 ± 13 | 0.065 |
| Males | 125 (66%) | 38 (68%) | 0.873 |
| Females | 65 (34%) | 18 (32%) | 0.873 |
| Coronary artery disease | 79 (42%) | 18 (32%) | 0.217 |
| Hypertension | 83 (44%) | 15 (27%) | 0.029 |
| Diabetes | 22 (12%) | 7 (13%) | 0.817 |
| Congestive heart failure | 35 (18%) | 13 (23%) | 0.445 |
| Previous stroke | 8 (4.2%) | 2 (3.6%) | 1.000 |
| Antiarrhythmic drugsa | 131 (69%) | 45 (80%) | 0.129 |
| Mitral valve regurgitation (grade ≥2) | 32 (17%) | 6 (11%) | 0.301 |
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| Age (years ± SD) | 67 ± 11 | 63 ± 13 | 0.065 |
| Males | 125 (66%) | 38 (68%) | 0.873 |
| Females | 65 (34%) | 18 (32%) | 0.873 |
| Coronary artery disease | 79 (42%) | 18 (32%) | 0.217 |
| Hypertension | 83 (44%) | 15 (27%) | 0.029 |
| Diabetes | 22 (12%) | 7 (13%) | 0.817 |
| Congestive heart failure | 35 (18%) | 13 (23%) | 0.445 |
| Previous stroke | 8 (4.2%) | 2 (3.6%) | 1.000 |
| Antiarrhythmic drugsa | 131 (69%) | 45 (80%) | 0.129 |
| Mitral valve regurgitation (grade ≥2) | 32 (17%) | 6 (11%) | 0.301 |
SR, sinus rhythm; FA, atrial fibrillation.
aAntiarrhythmic drugs included digoxin (n=41), sotalol (n=40), beta-blockers (n=125), flecainide (n=4), amiodarone (n=2), propafenone (n=2), and calcium channel antagonists (n=4).
Clinical parameters in patients with and without recurrence of atrial fibrillation at 1 month
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| Age (years ± SD) | 67 ± 11 | 63 ± 13 | 0.065 |
| Males | 125 (66%) | 38 (68%) | 0.873 |
| Females | 65 (34%) | 18 (32%) | 0.873 |
| Coronary artery disease | 79 (42%) | 18 (32%) | 0.217 |
| Hypertension | 83 (44%) | 15 (27%) | 0.029 |
| Diabetes | 22 (12%) | 7 (13%) | 0.817 |
| Congestive heart failure | 35 (18%) | 13 (23%) | 0.445 |
| Previous stroke | 8 (4.2%) | 2 (3.6%) | 1.000 |
| Antiarrhythmic drugsa | 131 (69%) | 45 (80%) | 0.129 |
| Mitral valve regurgitation (grade ≥2) | 32 (17%) | 6 (11%) | 0.301 |
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| Age (years ± SD) | 67 ± 11 | 63 ± 13 | 0.065 |
| Males | 125 (66%) | 38 (68%) | 0.873 |
| Females | 65 (34%) | 18 (32%) | 0.873 |
| Coronary artery disease | 79 (42%) | 18 (32%) | 0.217 |
| Hypertension | 83 (44%) | 15 (27%) | 0.029 |
| Diabetes | 22 (12%) | 7 (13%) | 0.817 |
| Congestive heart failure | 35 (18%) | 13 (23%) | 0.445 |
| Previous stroke | 8 (4.2%) | 2 (3.6%) | 1.000 |
| Antiarrhythmic drugsa | 131 (69%) | 45 (80%) | 0.129 |
| Mitral valve regurgitation (grade ≥2) | 32 (17%) | 6 (11%) | 0.301 |
SR, sinus rhythm; FA, atrial fibrillation.
aAntiarrhythmic drugs included digoxin (n=41), sotalol (n=40), beta-blockers (n=125), flecainide (n=4), amiodarone (n=2), propafenone (n=2), and calcium channel antagonists (n=4).
Echocardiographic parameters in patients with and without recurrence of atrial fibrillation in 1 month
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| PLAAEV (m/s ± SD) (n=180) | 0.43 ± 0.20 | 0.47 ± 0.21 | 0.196 |
| LAD (mm ± SD) (n=181) | 45 ± 8 | 43 ± 5 | 0.167 |
| LVEF (% ± SD) (n=175) | 52 ± 14 | 51 ± 13 | 0.621 |
| SEC (n=246) | 53 (28%) | 9 (16%) | 0.082 |
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| PLAAEV (m/s ± SD) (n=180) | 0.43 ± 0.20 | 0.47 ± 0.21 | 0.196 |
| LAD (mm ± SD) (n=181) | 45 ± 8 | 43 ± 5 | 0.167 |
| LVEF (% ± SD) (n=175) | 52 ± 14 | 51 ± 13 | 0.621 |
| SEC (n=246) | 53 (28%) | 9 (16%) | 0.082 |
AF, atrial fibrillation; SR, sinus rhythm; LAD, left atrial diastolic diameter; LVEF, left ventricular ejection fraction; SEC, spontaneous echo contrast.
Echocardiographic parameters in patients with and without recurrence of atrial fibrillation in 1 month
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| PLAAEV (m/s ± SD) (n=180) | 0.43 ± 0.20 | 0.47 ± 0.21 | 0.196 |
| LAD (mm ± SD) (n=181) | 45 ± 8 | 43 ± 5 | 0.167 |
| LVEF (% ± SD) (n=175) | 52 ± 14 | 51 ± 13 | 0.621 |
| SEC (n=246) | 53 (28%) | 9 (16%) | 0.082 |
| . | SR (n=190) . | AF (n=56) . | P-value . |
|---|---|---|---|
| PLAAEV (m/s ± SD) (n=180) | 0.43 ± 0.20 | 0.47 ± 0.21 | 0.196 |
| LAD (mm ± SD) (n=181) | 45 ± 8 | 43 ± 5 | 0.167 |
| LVEF (% ± SD) (n=175) | 52 ± 14 | 51 ± 13 | 0.621 |
| SEC (n=246) | 53 (28%) | 9 (16%) | 0.082 |
AF, atrial fibrillation; SR, sinus rhythm; LAD, left atrial diastolic diameter; LVEF, left ventricular ejection fraction; SEC, spontaneous echo contrast.
Thromboembolic complications
Warfarin and LMWH were started in the majority of the patients on the same day or 1 day before the CV and the duration of the oral anticoagulation and LMWH before CV was on average <24 h. One patient (0.3%) had a stroke within 30 days after CV. She was an 86-year-old woman who had coronary artery disease, paroxysmal AF, and mild mitral valve stenosis. She had warfarin therapy, but the INR was below 2 when she came to hospital. Because of severe palpitations and unstable haemodynamics, she underwent TOE examination and early CV. Although TOE did not reveal any obvious or suspected thrombi in left atrium or left atrial appendage, she had a stroke the night following the CV.
Discussion
The results of our study indicate that TOE-guided early CV can be safely performed when implemented in daily clinical practice, in the majority of patients with AF lasting longer than 48 h or of unknown duration. There were no relationships between the echocardiographic findings (e.g. PLAAEV) and the initial and long-term success of CV in our material. Hence, TOE-guided CV in concert with short-term anticoagulation is a safe and feasible alternative to the conventional strategy in the management of AF, but the predictive value of the echocardiographic measurements is limited.
TOE-guided CV
Left atrial appendage thrombi are presumably responsible for the majority of stroke and other embolic events after CV. Transoesophageal echocardiography can detect thrombi in both the left atrium and left atrial appendage with a sensitivity and specificity of almost 100%.10–12 Therefore, it has been proposed that TOE could be used to guide early CV in patients with AF lasting >48 h or of unknown duration.6–9 This approach consists of screening for existing thrombus and other predictors of thrombo-embolic events (e.g. spontaneous echo contrast and PLAAEV) by TOE and short-term anticoagulation therapy to reduce the post-CV thrombo embolic complications. In agreement with the results of previous studies,8,14–16,25 Transoesophageal echocardiography revealed no contraindication to early CV in the majority of our patients. The only difference in the clinical characteristics of the patients was that previous stroke was significantly more common among the subjects with a contraindication to early CV.
Anticoagulation and thromboembolic complications
In contrast to most previous studies,2,8,14,15 we used LMWH (enoxaparin or dalteparin) as a bridge to effective oral anticoagulation. To our knowledge, there is only one previous study using LMWH (dalteparin) instead of intravenous unfractionated heparin in this context.16 In our study, only one patient receiving dalteparin and warfarin had a stroke within 30 days after TOE-guided CV. Thus, the occurrence of thromboembolic complications (0.3%) was similar to that reported earlier for the conventional strategy2–4 and TOE-guided CV with intravenous unfractionated heparin (0.35%).7 We feel that this provides indirect evidence that LMWH can be used as an adjunct antithrombotic therapy instead of intravenous unfractionated heparin in patients undergoing TOE-guided CV. Its easier application may make it the preferred alternative also in this setting, similar to that in patients with acute myocardial infarction, unstable angina pectoris, pulmonary embolism, and deep venous thrombosis.26–28 In the present study, 10 patients with atrial flutter had thrombus on TOE, supporting the view that the risk of thromboembolic complications in CV of atrial flutter in non-anticoagulated patients is comparable with that in patients with AF.
Clinical findings and the success of CV
Early CV restored normal sinus rhythm or pacemaker rhythm in 90% of the patients. Thus, the immediate success rate of TOE-guided CV in our population was comparable with previous reports.2,13–15 Although it has previously been shown that the duration of the arrhythmia is a strong predictor of initial and long-term success of CV,2,8,15,16 here the initial success of CV was not related to any of the clinical or echocardiographic findings. In view of the important role of hypertension in the pathogenesis of AF, it was surprising that the patients with hypertension had significantly less clinical recurrences of AF than the normotensive patients at 1 month. Although this might be coincidental, it may also be related to the frequent use of beta-blockers, ACE-inhibitors (ACE-I), and angiotensin II receptor blockers (ARBs) in patients with hypertension. Beta-blockers prevent the recurrence of AF and reduce the symptoms of recurrences.29 Furthermore, both ACE-Is and ARBs have been reported to reduce the occurrence of AF in patients with congestive heart failure or hypertension.30,31
Echocardiographic findings and the success of CV
It has been suggested that measurement of left atrial appendage flow velocities by pulsed-wave Doppler can be used to predict the success of CV and maintenance of normal sinus rhythm during long-term follow-up. Recently, some investigators reported that low PLAAEV was associated with decreased success of CV.17,18 However, others did not find any relationship between PLAAEV and restoration of normal sinus rhythm.19,23 Likewise, the role of PLAAEV in the maintenance of normal sinus rhythm is controversial.19–23 In our study, PLAAEV was significantly lower in patients with contraindications to early CV, but neither low PLAAEV nor any other echocardiographic parameter predicted the initial success of the CV or the maintenance of sinus rhythm at 1 and 12 months of follow-up.
Limitations of the study
This study was not a randomized comparison of TOE-guided and conventional CV strategies. In addition, because our clinical follow-up did not allow documentation of short, asymptomatic episodes of AF, it is possible that some differences between the groups were underestimated. The conclusions of our study are dependent on the resources and skills to perform the TOE-guided approach, although the studies were performed by multiple operators in normal daily practice.
Conclusions
Our data confirm that early TOE-guided CV with short-term anticoagulation is a safe and clinically effective alternative in the treatment of symptomatic AF. It should be considered especially when the clinical situation warrants rapid restoration of sinus rhythm or prolonged anticoagulation is considered hazardous. The value of PLAAEV and other echocardiographic measurements in predicting the success of CV and maintenance of normal sinus rhythm seems to be limited.
Acknowledgement
We would like to thank medical students Elise Saarela and Virpi Koskela for data collection in Oulu University Hospital.
References
- anticoagulation
- artificial cardiac pacemaker
- atrial fibrillation
- warfarin
- left ventricular ejection fraction
- echocardiography
- transesophageal echocardiography
- electric countershock
- left atrium
- left auricular appendage
- cerebrovascular accident
- ischemic stroke
- low-molecular-weight heparin
- follow-up
- toes
- sinus rhythm
- thrombus
- echocardiography, transthoracic
- examination of toe
- diameter
- rhythm



