-
PDF
- Split View
-
Views
-
Cite
Cite
Carsten W. Israel, The role of pacing mode in the development of atrial fibrillation, EP Europace, Volume 8, Issue 2, February 2006, Pages 89–95, https://doi.org/10.1093/europace/euj038
Close - Share Icon Share
Abstract
Asynchronous ventricular pacing has been shown to increase the risk of development of atrial fibrillation (AF) because of various mechanisms: retrograde atrioventricular (AV) conduction with increase in atrial pressure causing acute atrial stretch and reverse flow in the pulmonary veins, mitral regurgitation, reduced coronary blood flow, adverse neuroendocrine reactions, etc. Dual-chamber pacing preserves atrioventricular synchrony. However, in randomized multicentre trials comparing VVI(R) with DDD(R) pacing, AF is only slightly less frequent in the dual-chamber mode. This is most likely due to unnecessary ventricular pacing, which is frequent in dual-chamber pacing. At nominal values, dual-chamber devices usually do not permit intrinsic AV conduction but promote delivery of the ventricular stimulus at an inappropriate time in an inappropriate place. Programming of long AV delays facilitates spontaneous AV conduction but usually cannot completely avoid unnecessary ventricular pacing and causes other problems in the dual-chamber mode. Atrial septal lead placement can improve left-sided AV synchrony and promote spontaneous AV conduction. Programming of the AAI(R) mode is superior to the dual-chamber mode but cannot be used if AV conduction is impaired intermittently or permanently. Therefore, dedicated algorithms enhancing spontaneous AV conduction in the dual-chamber mode are desirable for a large proportion of pacemaker patients.
Introduction
Atrial fibrillation (AF) is a common complication in patients with permanent pacemaker therapy. Although its incidence is influenced by the presence of structural heart disease, hypertension, congestive heart failure, or AF before pacemaker implantation, the pacing mode may also have a significant influence on the development of AF. This review provides insights into the haemodynamic properties of VVI and DDD pacing modes with regard to their potential to promote the development of AF.
Haemodynamic consequences of VVI pacing
Adverse haemodynamic and electrophysiological effects of VVI pacing during sinus rhythm have been well appreciated. Twenty years ago, the pacemaker syndrome was described,1 which is caused by haemodynamic deterioration due to loss of AV synchrony (or even due to VA synchrony), mitral regurgitation, inter-/intraventricular asynchrony, arrhythmia induction, and neuroendocrine reflexes. Adverse haemodynamic consequences of VVI pacing during sinus rhythm are most prominent if retrograde conduction is present. Particularly, at a VA interval of 100 ms, there may be a strong ‘negative atrial kick’ caused by atrial contraction against closed AV valves. This may reduce cardiac output and lead to pacemaker syndrome due to reflexes causing a fall in peripheral vascular resistance, and it should also be noted that ventricular pacing with retrograde 1:1 conduction causes a strong increase in atrial pressure and regurgitation into the pulmonary veins (Fig. 1). These so-called z-waves or ‘cannon waves’ may lead to a considerable distension of pulmonary veins, which may represent a potent trigger for AF. Even if no retrograde conduction is present, interference between sinus rate and VVI pacing rate leads to a periodicity in which P-waves shift until they appear after a ventricular pace, mimicking retrograde conduction (Fig. 2). As focal electrical activity in the pulmonary veins and acute atrial stretch have been recognized as two of the most important AF triggers in patients with and without structural heart disease,2 it may be concluded that asynchronous ventricular pacing in the VVI mode with or without retrograde conduction is a potent trigger for AF induction.
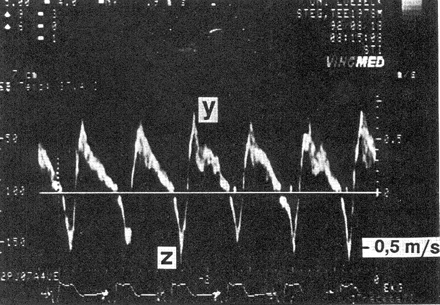
Ventricular pacing with 1:1 retrograde conduction. In this transoesophageal pulsed-Doppler echocardiographic recording, positive values depict antegrade flow in a pulmonary vein, whereas negative values depict retrograde flow. After each ventricular stimulus (see ECG at the bottom of the tracing), a retrograde P-wave appears, resulting in regurgitation into the pulmonary vein (z-wave). With permission from Stierle et al.37
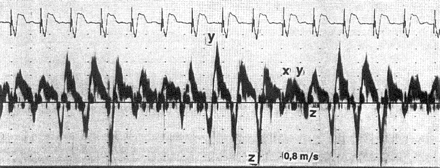
Ventricular pacing without retrograde conduction. Transoesophageal pulsed-Doppler echocardiographic recording as in Fig. 1. With VVI pacing rate (75 bpm) different from the sinus rate, P-waves appear shortly after the ventricular stimulus in three out of five cycles, resulting in regurgitation into the pulmonary vein (z-wave as in Fig. 1). With permission from Stierle et al.37
While permanent or intermittent atrial contraction against closed AV valves causes atrial/pulmonary vein distension as one trigger of AF, another AF trigger is represented by mitral regurgitation caused by right ventricular pacing,3–6 which can sometimes be reversed by dual-chamber pacing.3,5 Owing to mitral regurgitation, ventricular pacing leads to an elevation in pulmonary capillary wedge pressure and increases the risk of AF. Elevated atrial natriuretic peptide levels observed in patients with VVI pacing7 may further document atrial pressure increase with the potential to induce AF.
Likewise, it has been shown in animal and human studies that ventricular pacing reduces coronary blood flow,8–10 increases tissue norepinephrine levels,10 and deteriorates the relationship between left ventricular output and myocardial oxygen consumption.11 By these mechanisms, VVI pacing may produce ischaemia which may promote the development of AF. Finally, asynchronous ventricular pacing induces electrical and mechanical atrial remodelling, which may facilitate the induction of AF and thrombus formation in the left-atrial appendage.12,13
AF in VVI and DDD pacing: randomized and non-randomized data
In accordance with this picture of how VVI pacing may cause AF, retrospective and non-randomized studies show a yearly AF incidence of 7% with VVI pacing in contrast to 2% in patients with AAI or DDD pacing14–27 (Table 1). Also, several large-scale randomized trials confirm that AF develops more frequently in VVI(R) than in DDD(R) pacing mode28–35 (Table 2). However, in these studies, the absolute difference in AF development converges to 1.2% and is thus much smaller than expected, given the adverse effects of VVI pacing as outlined earlier in contrast to dual-chamber pacing which attempts to reproduce physiological atrioventricular timing.
Association between pacing mode and the rate of AF in non-randomized observational studies (meta-analysis by Barold and Israel59)
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Sutton and Kenny14 | 1986 | 1121 | 2118 | 1.4 | 6.8 |
| Markewitz et al.15 | 1986 | 360 | 232 | 3.1 | 10.0 |
| Ebagosti et al.16 | 1988 | 198 | 210 | 2.0 | 5.2 |
| Langenfeld et al.17 | 1988 | 115 | 1150 | 0.9 | 5.4 |
| Rosenqvist et al.18 | 1988 | 329 | 307 | 1.8 | 12.1 |
| Sasaki et al.19 | 1988 | 39 | 73 | 0 | 12.3 |
| Bianconi et al.20 | 1989 | 558 | 743 | 4.8 | 7.9 |
| Feuer et al.21 | 1989 | 367 | 440 | 2.5 | 4.5 |
| Santini et al.22 | 1990 | 1091 | 491 | 1.4 | 11.8 |
| Stangl et al.23 | 1990 | 477 | 504 | 1.5 | 4.2 |
| Zanini et al.24 | 1990 | 199 | 190 | 1.0 | 5.3 |
| Nürnberg et al.25 | 1991 | 126 | 318 | 4.8 | 11.0 |
| Hesselsson et al.26 | 1992 | 1453 | 1045 | 1.4 | 5.3 |
| Sgarbossa et al.27 | 1993 | 2173 | 616 | a | a |
| Total/mean | 9152 | 9233 | 1.9 | 7.0 | |
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Sutton and Kenny14 | 1986 | 1121 | 2118 | 1.4 | 6.8 |
| Markewitz et al.15 | 1986 | 360 | 232 | 3.1 | 10.0 |
| Ebagosti et al.16 | 1988 | 198 | 210 | 2.0 | 5.2 |
| Langenfeld et al.17 | 1988 | 115 | 1150 | 0.9 | 5.4 |
| Rosenqvist et al.18 | 1988 | 329 | 307 | 1.8 | 12.1 |
| Sasaki et al.19 | 1988 | 39 | 73 | 0 | 12.3 |
| Bianconi et al.20 | 1989 | 558 | 743 | 4.8 | 7.9 |
| Feuer et al.21 | 1989 | 367 | 440 | 2.5 | 4.5 |
| Santini et al.22 | 1990 | 1091 | 491 | 1.4 | 11.8 |
| Stangl et al.23 | 1990 | 477 | 504 | 1.5 | 4.2 |
| Zanini et al.24 | 1990 | 199 | 190 | 1.0 | 5.3 |
| Nürnberg et al.25 | 1991 | 126 | 318 | 4.8 | 11.0 |
| Hesselsson et al.26 | 1992 | 1453 | 1045 | 1.4 | 5.3 |
| Sgarbossa et al.27 | 1993 | 2173 | 616 | a | a |
| Total/mean | 9152 | 9233 | 1.9 | 7.0 | |
aMultivariate analysis identified ventricular pacing as an independent predictor of chronic AF.
Association between pacing mode and the rate of AF in non-randomized observational studies (meta-analysis by Barold and Israel59)
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Sutton and Kenny14 | 1986 | 1121 | 2118 | 1.4 | 6.8 |
| Markewitz et al.15 | 1986 | 360 | 232 | 3.1 | 10.0 |
| Ebagosti et al.16 | 1988 | 198 | 210 | 2.0 | 5.2 |
| Langenfeld et al.17 | 1988 | 115 | 1150 | 0.9 | 5.4 |
| Rosenqvist et al.18 | 1988 | 329 | 307 | 1.8 | 12.1 |
| Sasaki et al.19 | 1988 | 39 | 73 | 0 | 12.3 |
| Bianconi et al.20 | 1989 | 558 | 743 | 4.8 | 7.9 |
| Feuer et al.21 | 1989 | 367 | 440 | 2.5 | 4.5 |
| Santini et al.22 | 1990 | 1091 | 491 | 1.4 | 11.8 |
| Stangl et al.23 | 1990 | 477 | 504 | 1.5 | 4.2 |
| Zanini et al.24 | 1990 | 199 | 190 | 1.0 | 5.3 |
| Nürnberg et al.25 | 1991 | 126 | 318 | 4.8 | 11.0 |
| Hesselsson et al.26 | 1992 | 1453 | 1045 | 1.4 | 5.3 |
| Sgarbossa et al.27 | 1993 | 2173 | 616 | a | a |
| Total/mean | 9152 | 9233 | 1.9 | 7.0 | |
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Sutton and Kenny14 | 1986 | 1121 | 2118 | 1.4 | 6.8 |
| Markewitz et al.15 | 1986 | 360 | 232 | 3.1 | 10.0 |
| Ebagosti et al.16 | 1988 | 198 | 210 | 2.0 | 5.2 |
| Langenfeld et al.17 | 1988 | 115 | 1150 | 0.9 | 5.4 |
| Rosenqvist et al.18 | 1988 | 329 | 307 | 1.8 | 12.1 |
| Sasaki et al.19 | 1988 | 39 | 73 | 0 | 12.3 |
| Bianconi et al.20 | 1989 | 558 | 743 | 4.8 | 7.9 |
| Feuer et al.21 | 1989 | 367 | 440 | 2.5 | 4.5 |
| Santini et al.22 | 1990 | 1091 | 491 | 1.4 | 11.8 |
| Stangl et al.23 | 1990 | 477 | 504 | 1.5 | 4.2 |
| Zanini et al.24 | 1990 | 199 | 190 | 1.0 | 5.3 |
| Nürnberg et al.25 | 1991 | 126 | 318 | 4.8 | 11.0 |
| Hesselsson et al.26 | 1992 | 1453 | 1045 | 1.4 | 5.3 |
| Sgarbossa et al.27 | 1993 | 2173 | 616 | a | a |
| Total/mean | 9152 | 9233 | 1.9 | 7.0 | |
aMultivariate analysis identified ventricular pacing as an independent predictor of chronic AF.
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/ DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Andersen et al.28 | 1997 | 110 | 115 | 4.1 | 6.6 |
| Wharton et al.29 | 1998 | 100 | 98 | 48 | 43 |
| Lamas et al.30 | 1998 | 203 | 204 | 6.9 | 7.5 |
| Connolly et al.31 | 2000 | 1094 | 1474 | 5.3 | 6.6 |
| Lamas et al.32 | 2002 | 1014 | 996 | 7.8 | 9.9 |
| Kerr et al.33 | 2004 | (1014)a | (996)a | 4.5 | 5.7 |
| Toff et al.34 | 2003 | 1012 | 1009 | b | b |
| Wilkoff et al.35 | 2002 | 250 | 256 | 0 | 2.1 |
| Total/mean | 3783 | 4152 | 6.6 | 7.8 | |
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/ DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Andersen et al.28 | 1997 | 110 | 115 | 4.1 | 6.6 |
| Wharton et al.29 | 1998 | 100 | 98 | 48 | 43 |
| Lamas et al.30 | 1998 | 203 | 204 | 6.9 | 7.5 |
| Connolly et al.31 | 2000 | 1094 | 1474 | 5.3 | 6.6 |
| Lamas et al.32 | 2002 | 1014 | 996 | 7.8 | 9.9 |
| Kerr et al.33 | 2004 | (1014)a | (996)a | 4.5 | 5.7 |
| Toff et al.34 | 2003 | 1012 | 1009 | b | b |
| Wilkoff et al.35 | 2002 | 250 | 256 | 0 | 2.1 |
| Total/mean | 3783 | 4152 | 6.6 | 7.8 | |
a2002 patients of the CTOPP trial were eligible for the extended follow-up study.
bNo significant difference between AAI(R)/DDD(R) and VVI(R).
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/ DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Andersen et al.28 | 1997 | 110 | 115 | 4.1 | 6.6 |
| Wharton et al.29 | 1998 | 100 | 98 | 48 | 43 |
| Lamas et al.30 | 1998 | 203 | 204 | 6.9 | 7.5 |
| Connolly et al.31 | 2000 | 1094 | 1474 | 5.3 | 6.6 |
| Lamas et al.32 | 2002 | 1014 | 996 | 7.8 | 9.9 |
| Kerr et al.33 | 2004 | (1014)a | (996)a | 4.5 | 5.7 |
| Toff et al.34 | 2003 | 1012 | 1009 | b | b |
| Wilkoff et al.35 | 2002 | 250 | 256 | 0 | 2.1 |
| Total/mean | 3783 | 4152 | 6.6 | 7.8 | |
| Author . | Year . | Patients with AAI/DDD (n) . | Patients with VVI(R) (n) . | AF in AAI/ DDD pacing (% per year) . | AF in VVI pacing (% per year) . |
|---|---|---|---|---|---|
| Andersen et al.28 | 1997 | 110 | 115 | 4.1 | 6.6 |
| Wharton et al.29 | 1998 | 100 | 98 | 48 | 43 |
| Lamas et al.30 | 1998 | 203 | 204 | 6.9 | 7.5 |
| Connolly et al.31 | 2000 | 1094 | 1474 | 5.3 | 6.6 |
| Lamas et al.32 | 2002 | 1014 | 996 | 7.8 | 9.9 |
| Kerr et al.33 | 2004 | (1014)a | (996)a | 4.5 | 5.7 |
| Toff et al.34 | 2003 | 1012 | 1009 | b | b |
| Wilkoff et al.35 | 2002 | 250 | 256 | 0 | 2.1 |
| Total/mean | 3783 | 4152 | 6.6 | 7.8 | |
a2002 patients of the CTOPP trial were eligible for the extended follow-up study.
bNo significant difference between AAI(R)/DDD(R) and VVI(R).
For instance, in patients with sick sinus syndrome, the MOST trial showed little difference between VVIR and DDDR pacing for various clinical endpoints.32 The development of AF differed (absolutely) by only 2.1%. However, patients required antibradycardia pacing for only 58% of the time in VVIR mode, whereas ventricular pacing was present for 90% of the time in patients randomized to DDDR pacing.36 This is even more remarkable as only patients with sinus node disease were included; therefore, ventricular pacing was unnecessary in the vast majority of patients and was due to programming of a short AV delay. In a subanalysis, Sweeney et al.36 observed an association between the percentage of ventricular pacing and the development of AF. Patients paced in VVIR mode for <10% of the time developed AF in only 21%, whereas VVIR pacing for 50–90% of the time was associated with AF 29% of patients. Interestingly, a similar and even stronger association between ventricular pacing and development of AF was found for the DDDR mode where ventricular pacing for <10% of the time was associated with AF in 16% of the patients in contrast to AF in 32% of patients with ventricular pacing for 50–90% of the time.36 In this subanalysis, it looked as if ventricular pacing was even more deleterious in the DDDR mode than in the VVIR mode: The risk of AF increased by 1% for each per cent increase in ventricular pacing in the DDDR mode when compared with only 0.7% for each per cent increase in the VVIR mode. This observation poses the question why right ventricular pacing in atrioventricular synchronous or sequential pacing may be at least as harmful as asynchronous ventricular pacing.
Consequences of DDD pacing with nominal AV delay
To study the haemodynamic consequences of ventricular pacing in the dual-chamber mode, a study using Doppler ultrasound in the pulmonary veins is quite revealing.37 If a short AV interval was programmed, 2 of 14 patients had retrograde pulmonary vein flow (z-waves in Fig. 3) similar to VVI pacing. In both patients, symptoms of pacemaker syndrome appeared despite dual-chamber pacing which were reversible by prolonging the AV delay. The appearance of pacemaker syndrome in the dual-chamber mode can be explained by recordings of left-atrial activity, e.g. by transoesophageal ECGs38. These show an interatrial conduction delay (IACD) in a significant number of patients, particularly if they are elderly39 or if left-atrial dilatation is present.40 In 100 patients, the mean IACD was 70 ms in sinus rhythm and 120 ms if the right atrium was paced.40 However, IACD can postpone left-atrial activation by 130 ms or more.38,39,41 Thus, with a programmed AV delay of 120 ms (nominal value in many dual-chamber pacemakers), left-sided AV intervals can be very short if intraventricular conduction is normal (Fig. 4) and may even be negative (Fig. 5),38 i.e. left-atrial activation occurs after ventricular pacing. Therefore, seemingly ‘physiological’ dual-chamber pacing can lead to a considerably unfavourable haemodynamic situation. The association of IACD and atrial tachyarrhythmias has been well established42,43 as has been the development of atrial electrical remodelling in response to even small increases in left-atrial pressure.44 Even though adverse haemodynamic effects of ‘untimely’ ventricular pacing in the dual-chamber mode have, therefore, long been recognized and several formulae for optimizing the AV delay with the use of echocardiography have been proposed,45 this has not been considered in large multicentre trials comparing VVI(R) with DDD(R) pacing. Additionally, pacemaker manufacturers have obviously not been aware of the potential implications of inappropriately programmed AV intervals because even today most dual-chamber pacemakers are still shipped with nominal AV delays of 120 ms or similar. Some pacemaker and ICD devices even force programming of rate adaptive AV delays of 30 ms as an interlock if the mode switching algorithm is activated.
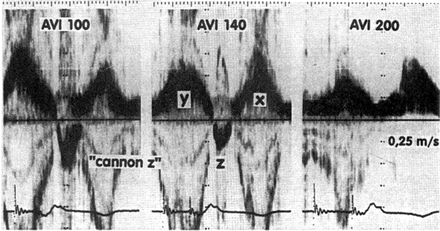
Dual-chamber pacing causing retrograde flow in the pulmonary vein. Transoesophageal pulsed-Doppler echocardiographic recording as in Figs. 1 and 2. With an AV delay of 100 ms (AVI 100, left panel), a large z-wave representing retrograde flow in the pulmonary vein appears. This z-wave is diminished but not abolished by reprogramming the AV delay to 140 ms (AVI 140, middle panel). Only after prolonging the paced AV delay to 200 ms (AVI 200, right panel), there is no longer retrograde flow in the pulmonary veins. With permission from Stierle et al.37
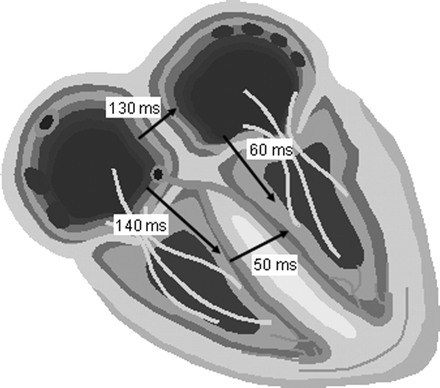
Example of the left-sided AV interval in IACD. In this case, an IACD of 130 ms, an interventricular conduction delay of 50 ms (mean value in Chirife et al.41), and a programmed AV delay of 140 ms would lead to a left-sided AV interval of only 60 ms.
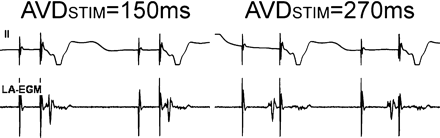
Left-atrial activation in relation to ventricular pacing in the DDDR/DDIR mode. After atrial pacing, left-atrial activity as measured with a transoesophageal left-atrial electrogram (LA-EGM) is delayed. Thus, in this case, ventricular stimulation occurs before left-atrial activity if the AV delay is programmed to 150 ms. Only after prolongation of the AV delay to 270 ms, left-atrial activation occurs just before the ventricular pacing spike. With permission from Ismer et al.38
Therefore, instead of delivering atrioventricular activation sequences that mimic physiological behaviour, poorly programmed dual-chamber devices may apply a ventricular stimulus at a time when the left atrium is activated. This is haemodynamically equivalent to VVI pacing with 1:1 retrograde conduction. Due to maintenance of short nominal AV delays, patients with dual-chamber devices received much more unnecessary ventricular pacing than patients with VVI devices in the MOST and DAVID trials.35,36 As a result, many patients with dual-chamber devices may have experienced AV desynchronization, and AF may have developed much less frequently if spontaneous AV conduction had been permitted.
Dual-chamber pacing with long AV delay
In the light of the disappointing results of dual-chamber pacing and for the reasons just outlined, attempts have been made to avoid unnecessary ventricular pacing in patients without high-degree AV block by programming long AV delays (e.g. 300 ms) and DDI(R) instead of DDD(R) mode. In a study of 177 patients with sick sinus syndrome and normal AV conduction, patients were randomized to AAI(R) and DDD(R) with short AV delay (≤150 ms) or long AV delay (300 ms).46 Interestingly, patients randomized to DDD(R) with long AV delay still received 17% ventricular pacing (most likely fusion and pseudo-fusion), and this percentage was sufficient to cause significantly more AF than AAI(R) pacing and only moderately less than DDD(R) pacing with a short AV delay, which led to ventricular pacing for 90% of the time. Intrinsic AV conduction may vary considerably and can be unexpectedly long in atrial paced rhythm and during night.47 Also, an inappropriate atrial rate increase (e.g. due to sensor over-reactivity or atrial preventive pacing algorithms) increases the intrinsic AV delay and promotes ventricular pacing (Fig. 6). Of note, most multicentre trials comparing single- and dual-chamber pacing used rate responsive pacing for all patients even without any clinical signs of chronotropic sinus node incompetence (e.g. DAVID, PASE, MOST).30,32,35 Also, in trials on atrial preventive pacing algorithms, the ventricle was typically paced for 70–95% of the time despite the fact that most patients only had sinus node disease with normal AV conduction. In the PIPAF study, the subgroup of patients with a low percentage of ventricular pacing (mean 26%) due to programming of the AV delay to a mean of 261 ms received a benefit from atrial preventive pacing, whereas in patients with a high percentage of ventricular pacing (mean 99%) due to programming of a relatively short AV delay (mean 176 ms), the time in AF even increased slightly during atrial preventive pacing.48
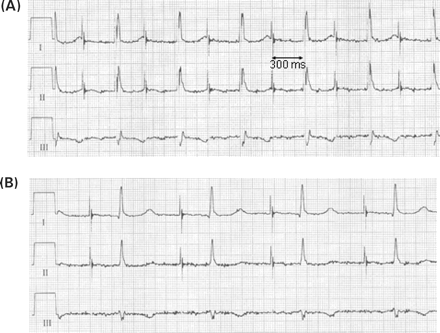
Example of a prolongation of the intrinsic AV conduction due to inappropriately fast atrial pacing. In this patient, an automatic sensor adjustment algorithm has increased the pacing rate to approximately 90 bpm at rest. This prolonged spontaneous AV conduction to approximately 300 ms causing ventricular fusion beats even with an AV delay programmed to 300 ms (A). After reprogramming the sensor, dual-chamber pacing at rest occurs at the lower rate limit of 70 bpm (B). Now, the spontaneous AV delay shortens again to 220 ms. Leads I–III, paper speed 50 mm/s.
Finally, programming of long AV delays in DDD(R) or DDI(R) modes may cause additional problems such as a limitation of the maximum tracking rate, non-re-entrant VA synchrony,49 and pacing in the vulnerable phase of the T-wave after a ventricular premature beat which coincides with the atrial stimulus (Fig. 7).
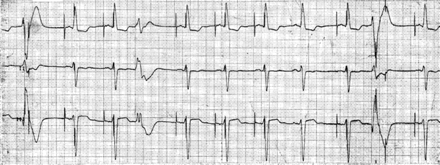
Ventricular pacing in the T-wave after a ventricular premature beat. If a ventricular premature beat coincides with the atrial stimulus, it may be sensed by the ventricular lead causing safety window pacing (first complex in the tracing) or it may be completely blanked out and the ventricular pace appears at the programmed AV delay (second complex from the right). The longer the AV delay is programmed, the more like the ventricular pace may capture in the ventricular vulnerable phase.
In conclusion, dual-chamber pacing with long AV delay does not seem to avoid unnecessary ventricular pacing sufficiently to be as effective in preventing AF as AAIR pacing in patients with normal AV conduction, and causes new problems.
Atrial septal pacing in DDD mode
Apart from the pacing mode, the atrial lead position may determine the risk of AF development. Lead implantation in the right atrial appendage or high lateral right atrium seems to promote electrical desynchronization of the atria,50–52 which becomes visible by a prolongation of the paced P-wave compared with the sinus P-wave (Fig. 8). At the same time, induction of AF by an extrastimulus is easier if the atrium is paced from these positions than from the low atrial septum near the coronary sinus ostium or from the high septum near the right atrial insertion of Bachmann's bundle.53 Pacing at these atrial septal positions is associated with a shorter P-wave than in sinus rhythm (Fig. 8) due to electrical connections of right and left atrium via Bachmann's bundle or connecting fibres in the coronary sinus.54 As a result, pacing near Bachmann's bundle was associated with less frequent development of AF during DDDR pacing than pacing from the right atrial appendage.55 Similarly, the effect of atrial overdrive pacing to prevent AF was more pronounced if the atrial electrode was implanted at the low atrial septum than in the right atrial appendage56. Also, AAIR pacing from the right atrial appendage or high lateral right atrium may be associated with a prolonged intrinsic AV conduction and may produce a ‘pseudo-pacemaker syndrome’ with left-atrial contraction coincident with left ventricular contraction (atrial pacing spike typically within the T-wave).57,58 Of note, especially pacing at the low atrial septum is associated with a short intrinsic AV interval. Thus, for identical AV delays, ventricular pacing is less likely for atrial electrodes positioned at the low atrial septum when compared with the high right atrium. Prevention of unnecessary ventricular pacing should also be included as a mechanism for how atrial septal pacing may prevent AF.
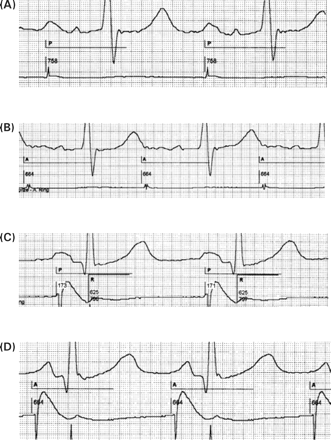
Intrinsic vs. paced P-wave duration and morphology: impact of atrial pacing site. In a patient with IACD (A, intrinsic P-wave duration 210 ms), P-wave duration is prolonged to 240 ms and P-wave morphology is further distorted if atrial pacing is performed from the lateral high right atrium (B). Any AV delay <240 ms will pace the ventricle before the end of atrial depolarization. In a patient with an intrinsic P-wave of 130 ms (C), atrial septal pacing near Bachmann's bundle synchronizes right- and left-atrial activation leading to a rather narrow peak of the P-wave and in this case to a small decrease of P-wave duration to 100 ms (D). Paper speed 100 mm/s, lead II and atrial electrograms. A: atrial pace; P: atrial-sensed event; R: ventricular sensed event; numbers: atrial cycle length, AV delay, VA interval and ventricular cycle length in ms.
Conclusion
Ventricular pacing in the VVI(R) mode and also in the DDD(R)/DDI(R) modes seems to be associated with the development of AF. In the VVI(R) mode, AF may be triggered by retrograde conduction or simple coincidence of sinus beat and ventricular stimulus and atrial stretch. In the dual-chamber mode, inappropriate timing of ventricular pacing causes inappropriate left-sided AV intervals, in the worst case synchronizing left-atrial contraction with left ventricular activation. Thus, inappropriately programmed dual-chamber devices may cause AV dissociation or VA synchrony similar to VVI pacing with 1:1 retrograde conduction. Unnecessary (right) ventricular pacing, even if it causes only fusion or pseudo-fusion, should therefore be avoided as much as possible, and intrinsic AV conduction should usually be preferred to ventricular pacing. Inappropriate, fast atrial pacing rates (over-reactive sensor, preventive pacing algorithm) can significantly prolong intrinsic AV conduction and should be avoided. Programming of a long AV interval or DDI(R) instead of DDD(R) mode only partially solves this problem. Atrial septal lead position may compensate for IACD and thus promote more physiological left-sided AV intervals; by a low septal atrial lead position also, the intrinsic AV conduction may be improved. In patients with intact AV conduction, the AAI(R) mode is superior to dual-chamber modes and should be the mode of choice whenever possible. However, if AV conduction is impaired intermittently or permanently, other measures have to be sought to minimize unnecessary ventricular pacing.



