-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas W. Baron, Thomas S. Faber, Andreas Grom, Tillmann Schwab, Michael Brunner, Annette Geibel, Hanjörg Just, Christoph Bode, Manfred Zehender, Real-time assessment of acute myocardial ischaemia by an intra-thoracic 6-lead ECG: evaluation of a new diagnostic option in the implantable defibrillator, EP Europace, Volume 8, Issue 11, November 2006, Pages 994–1001, https://doi.org/10.1093/europace/eul104
Close - Share Icon Share
Abstract
Aim In the presence of coronary artery disease, implantable cardioverter-defibrillators (ICD) are used effectively for treating life-threatening tachyarrhythmias. Continuous monitoring of myocardial ischaemia would provide a new diagnostic option in future ICD generations.
Methods and results In 22 selected patients undergoing coronary angioplasty, percutaneous transluminal coronary angioplasty (PTCA), three electrodes, similar to those used in the ICD, were inserted aiming to create six intra-thoracic ECG (IT-ECG) leads according to Einthoven and Goldberger. In total, 27 PTCA were conducted. The diagnostic efficacy for ischaemia assessment was compared with the surface ECG.
The IT-ECG proved to be more sensitive than conventional ECG in early and overall ischaemia assessment. At 30 s of coronary artery occlusion, ischaemic ST-segment alterations (≥0.25 mV) were present in the IT-ECG 2.3 times more often (23 vs. 10/27 PTCA attempts, P<0.01) and at 90 s 1.4 times more often compared with conventional ECG leads (18 vs. 26/27, P<0.05). Intra-thoracic Einthoven 2 (SVC+RVA vs. ICD-housing) and Goldberger 3 (SVC+ICD-housing vs. RVA) had the highest sensitivity (88/85%). Using ≥4 IT-ECG, ischaemia monitoring was independent of severity and site of origin. IT-ECG signals showed double ST-T signal amplitude (4.19±0.6 vs. 2.15±0.3 mV, ratio: 1.95, P<0.01) at a QRS/ST amplitude ratio similar in the two ECG techniques.
Conclusion This study provides strong evidence that the ICD-based IT 6-lead ECG would provide a new and efficient means of assessing a patient's daily ischaemic burden.
Introduction
The implantable cardioverter-defibrillator (ICD) is well established in the treatment of life-threatening ventricular tachyarrhythmias.1–3 Once implanted, the device continuously monitors the patient for the occurrence of brady- and tachyarrhythmic complications, and in case of meeting predefined event criteria, it treats the arrhythmia by a variety of pacing, cardioversion, or defibrillation modes. As a result, sudden cardiac death remains a rare event in patients with an implanted cardioverter-defibrillator.3,4
With the possibility of effectively preventing sudden cardiac death, it is the progression of the underlying heart disease that becomes the prime determinant of the patient's prognosis. In the majority of ICD-treated patients suffering from coronary artery disease, such progression is characterized by new-onset ischaemia, acute myocardial infarction, and/or the development of ischaemic cardiomyopathy.5,6 Ischaemia monitored by the implanted device would therefore provide an interesting new diagnostic option in these patients. New-onset ischaemia, any change in the frequency, and severity of ischaemic episodes, as well as the patient's daily ischaemic burden would become routinely assessable whenever necessary. In addition, ischaemia monitoring in ICDs would further improve the overall and individual understanding of the mechanisms underlying the often-fatal interaction of ischaemia and spontaneous ventricular tachyarrhythmias.
However, ICD-based ischaemia monitoring must be simple, reliable, and feasible with a minimum of additional expense, electrode and device requirements, and energy consumption. These criteria are best met by the established method of ST-segment analysis. Intra-thoracic, IT-ECG leads derived from presently available electrodes in ICDs [located in the right ventricle, the superior vena cava (SVC), and at the generator site] may provide the structural basis for monitoring ischaemia in ICD recipients.7–10
This prospective study in 22 patients undergoing percutaneous transluminal coronary angioplasty (PTCA) at 27 coronary artery sites aimed to evaluate the feasibility and diagnostic accuracy of six temporarily established IT-ECG leads to monitor myocardial ischaemia continuously in comparison with information available via conventional surface ECG. None of the patients had a permanently implanted defibrillator at the time of this study.
Methods
This prospective study was performed at the Department of Cardiology at the University of Freiburg after review by the University's Ethics Committee. Informed consent was obtained from all patients prior to investigation.
Patients
A total of 22 patients with high-grade coronary artery stenosis and an indication for PTCA were evaluated for the diagnostic accuracy of six IT-ECG leads to assess transient myocardial ischaemia resulting from acute coronary artery occlusion.
Patients were included in the study (i) if at least one high-grade coronary artery stenosis was present, as well as corresponding evidence of exercise-inducible myocardial ischaemia, (ii) the patient seemed suitable to undergo a standard PTCA protocol, (iii) no factors were present limiting the assessment of the ST-segment in the 12-lead surface ECG (patients with atrial fibrillation, pacemaker rhythm, bundle-branch block were excluded), and (iv) the patient agreed to take part in the study. Clinical data of all patients are summarized in Table 1.
Baseline characteristics of the 22 patients considered in evaluating the diagnostic efficacy of the 6-lead IT-ECG for assessing myocardial ischaemia induced by PTCA. LVEF, left ventricular ejection fraction.
| Clinical characteristics | |
| Age (years) | 64.5±9.6 |
| Range (years) | 35–77 |
| No. of men/women | 18/4 |
| Blood pressure | |
| systolic (mmHg) | 133±17 |
| diastolic (mmHg) | 81± 10 |
| Heart rate (/min) | 75± 9 |
| LVEF | 42± 6 |
| Coronary artery disease | |
| Previous infarction | 13 (59%) |
| Target artery site | |
| LAD | 9 |
| Left circumflex | 14 |
| Right coronary | 4 |
| Clinical characteristics | |
| Age (years) | 64.5±9.6 |
| Range (years) | 35–77 |
| No. of men/women | 18/4 |
| Blood pressure | |
| systolic (mmHg) | 133±17 |
| diastolic (mmHg) | 81± 10 |
| Heart rate (/min) | 75± 9 |
| LVEF | 42± 6 |
| Coronary artery disease | |
| Previous infarction | 13 (59%) |
| Target artery site | |
| LAD | 9 |
| Left circumflex | 14 |
| Right coronary | 4 |
Baseline characteristics of the 22 patients considered in evaluating the diagnostic efficacy of the 6-lead IT-ECG for assessing myocardial ischaemia induced by PTCA. LVEF, left ventricular ejection fraction.
| Clinical characteristics | |
| Age (years) | 64.5±9.6 |
| Range (years) | 35–77 |
| No. of men/women | 18/4 |
| Blood pressure | |
| systolic (mmHg) | 133±17 |
| diastolic (mmHg) | 81± 10 |
| Heart rate (/min) | 75± 9 |
| LVEF | 42± 6 |
| Coronary artery disease | |
| Previous infarction | 13 (59%) |
| Target artery site | |
| LAD | 9 |
| Left circumflex | 14 |
| Right coronary | 4 |
| Clinical characteristics | |
| Age (years) | 64.5±9.6 |
| Range (years) | 35–77 |
| No. of men/women | 18/4 |
| Blood pressure | |
| systolic (mmHg) | 133±17 |
| diastolic (mmHg) | 81± 10 |
| Heart rate (/min) | 75± 9 |
| LVEF | 42± 6 |
| Coronary artery disease | |
| Previous infarction | 13 (59%) |
| Target artery site | |
| LAD | 9 |
| Left circumflex | 14 |
| Right coronary | 4 |
Method
Before undergoing PTCA, a hexapolar electrode catheter (Berkovits-Castellanos catheter, USCI, Covington, GA, USA) was inserted transvenously via the internal jugular vein and placed in the right ventricular apex (RVA, tip electrode), which also incorporated the border zone between the right atrium and the SVC (proximal electrode). In addition, a patch electrode (CPI-patch electrode, large, Model 066) was placed cutaneously at the typical location for an ICD generator implantation site (left pectoral, LMP). The resulting three electrodes were used to create the following six IT-ECG leads, according to Einthoven and Goldberger (Figure 1):
Einthoven 1 (E1): SVC vs. LMP Goldberger 1 (G1): RVA+LMP vs. SVC
Einthoven 2 (E2): SVC vs. RVA Goldberger 2 (G2): RVA+SVC vs. LMP
Einthoven 3 (E3): RVA vs. LMP Goldberger 3 (G3): SVC+LMP vs. RVA
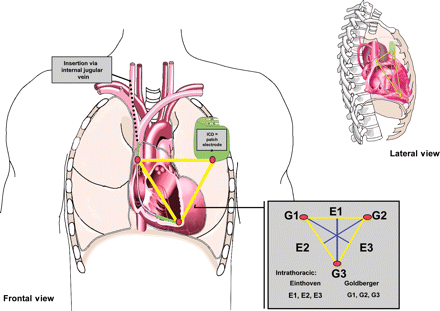
Assessment of the six IT-ECG leads (according to Einthoven, E1–E3 and Goldberger, G1–G3) by use of three electrodes placed in the RVA and SVA (inserted via the internal jugular vein) and the generator site (cutaneous patch). The frontal and lateral views are given.
The IT-ECG leads were assessed using a modified Siemens (Sweden) Mingograf 7 with a range of 0.01–1000 Hz. E1–E3 and G1–G3 were simultaneously documented at a paper speed of 50 mm/s. In addition, six conventional surface ECG leads I–III, and V4–6 were simultaneously documented for intra-individual comparison of the ST-segment alterations resulting from coronary artery occlusion. We limited the simultaneously documented ECG leads to 12 for continuous monitoring to assess the six most important and sensitive conventional ECG leads on the same ECG machine, at the same time and with identical filter characteristics.
Documentation of the IT and the surface ECG leads was started 5 min prior to PTCA and lasted until 10 min after deflation of the PTCA balloon. In case of repetitive PTCAs, the first three attempts at any PTCA site were documented. All statistical analyses provided in this paper are based only on the first PTCA attempt at each coronary artery site.
Ischaemic ST-segment changes in the IT and surface ECG (measurements were made 60 ms after the J-point) were measured as absolute values (difference from the isoelectric line) as well as percentage change in the absolute QRS amplitude. The isoelectric line was defined for three regular consecutive beats and based on the PR segment. Calculations of all amplitudes and time intervals were based on these three regular complexes and the median value chosen for each parameter. The following parameters were used for primary analysis: (i) presence of ST-segement depression or elevation ≥0.25 mV (J-point+60 ms), (ii) time of onset of ST-segment depression or elevation ≥0.25 mV, (iii) number of leads showing significant ST-deviation (≥0.1 mV)
All analyses were made for the entire patient group; subanalysis considered the PTCA site (left anterior descending, LAD; left circumflex artery, LCX; right coronary artery, RCA).
Percutaneous transluminal coronary angioplasty
PTCA was performed in all patients using a standardized protocol at each target vessel site. All ST-segment measurements were taken at 30 s and at the end of PTCA (90 s of inflation), each attempt was followed by a control interval of at least 5 min.
Statistical methods
All data were given as median intervals ± 25/75 quartile, unless otherwise indicated as mean intervals ± standard deviation. Whenever appropriate, comparison of data was made by using the Wilcoxon test, the χ2 test, and the McNemar test.
Results
Assessment of ischaemic ST-segment changes by use of surface ECG leads I–III and V4-6
Based on conventional ECG leads I–III and precordial leads V4–6, 30 s of coronary artery occlusion resulted in ischaemic ST-segment changes (depression/elevation ≥0.25 mV in any of the six ECG leads) during 10/27 PTCA attempts (37%, Figure 2). After 90 s of coronary artery occlusion, ischaemic ST-segment changes were identified in at least one of the six surface ECG leads during 18/27 PTCA attempts (68%, Figure 2). Assessment of ischaemia showed a close relationship between the occlusion site and the ECG lead used for analysis (Figure 3, data not shown).
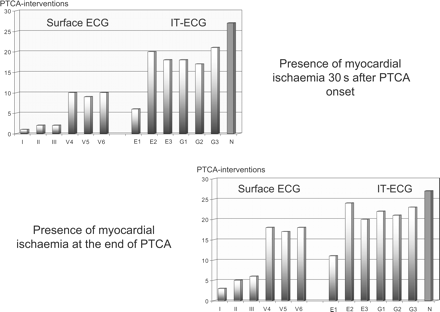
Presence of ST-segment changes ≥0.25 mV after 30 s (upper left site) and at the end of PTCA-induced coronary artery occlusion (90 s, lower right site) during 27 interventional attempts performed at different coronary artery sites. Data are shown for the conventional surface ECG leads I–III, V5–V6 vs. the reconstructed IT-ECG leads E1, E2, E3 and G1, G2, G3 (P<0.01).
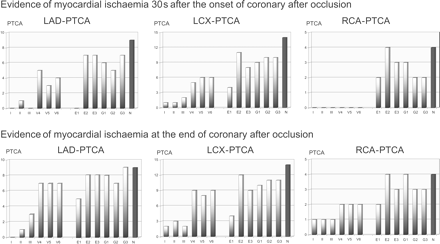
Presence of ST-segment changes ≥0.25 mV after 30 s (upper part) and at the end of PTCA-induced coronary artery occlusion (lower part) stratified according to the PTCA site (LAD, LCX, RCA). The IT-ECG was more sensitive in detecting myocardial ischaemia than conventional ECG for all PTCA sites. No difference was observed between the different sites of intervention for the IT-ECG leads.
Onset of ischaemic ST-segment changes (depression/elevation ≥0.25 mV) in any of the six surface ECG leads occurred as early as 29 ± 18 s after the onset of coronary artery occlusion and persisted after reperfusion for 47 ± 24 s. Maximum ST-segment deviation during PTCA was calculated at 0.05 ± 0.05 mV for surface ECG leads I–III and 0.22 ± 0.27 mV for ECG leads V4–6.
Assessment of ischaemic ST-segment changes by the IT-ECG leads
Thirty seconds after the onset of coronary occlusion, the predefined criteria for ischaemic ST-segment alterations (depression/elevation ≥0.25 mV) were met during 20/27 PTCA attempts using the intra-thoracic E1–E3 (74%, twice the number observed by the surface ECG leads, P<0.01) and during 21/27 PTCA attempts using the intra-thoracic G1–G3 (77%, twice the number observed by the surface ECG, P<0.01). Figure 2 demonstrates the increased sensitivity of the IT-ECG leads at 30 s of coronary artery occlusion compared with the conventional ECG leads. Of the six intra-thoracic leads, E2 and G3 were found to be most sensitive in assessing PTCA-induced ischaemia after 30 s of coronary occlusion (Figure 2).
At 90 s after the onset of coronary artery occlusion, the IT-ECG leads E1–E3 indicated myocardial ischaemia during 24/27 attempts (89%, a 31% increase compared with the six surface ECG leads, P<0.05), the intra-thoracic leads G1–G3 indicated ischaemia during 23/27 attempts (85%, a 25% increase compared with the six surface ECG leads, P<0.05). Again the intra-thoracic leads E2 and G3 were found to be the most sensitive in ischaemia detection (Figures 2 and 3).
Comparison of the diagnostic accuracy of the surface and IT-ECG leads for assessing myocardial ischaemia
Using predefined criteria for ischaemia assessment (ST-segment changes ≥0.25 mV in any of the six ECG leads) at 30 s after coronary artery occlusion, the IT-ECG leads were 2.3 times more sensitive than the conventional ECG leads in demonstrating myocardial ischaemia (23 vs. 10/27 PTCA attempts, P<0.01). At 90 s, conventional surface ECG leads I–III failed to demonstrate myocardial ischaemia during 9/27 PTCA attempts (33%), compared with only one attempt when the six IT-ECG leads were used (4%, P<0.01).
Figure 4A and B show the original surface and intra-thoracic ECG registrations at baseline and immediately before the onset of reperfusion for patients with coronary artery occlusion studied in the LCX (Figure 4A), the LAD, and the RCA (Figure 4B). Ischaemic ST-segment changes are most impressive in the IT-ECG. As single IT-ECG leads, Einthoven lead E2 and Goldberger lead G3 were found to be the most sensitive in indicating PTCA-induced ischaemia after 30 and 90 s of balloon-induced myocardial ischaemia (Figures 3 and 4). No difference was observed between the different sites of intervention for the IT-ECG leads themselves
![Original ECG registration comparing the ST-segment changes in the conventional surface ECG leads I–III, V4–V6 and IT-ECG leads E1–E3 and G1–G3 during baseline and at the end of PTCA performed in the LCX [1 mV signal amplitude is marked at the right end of each tracing sequence (A)]. Figure 4B summarizes the ST-segment changes resulting from PTCA performed in the LAD and in the RCA. Ischaemia-induced ST-segment changes are most impressive in the IT-ECG leads (marked area).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/europace/8/11/10.1093/europace/eul104/2/m_eul10404.gif?Expires=1748073654&Signature=y8nj3RIueBAxdnuajKZqhegMqmXuGvSyj3DJXD6KieWpzQzV9GcJ8HQA6ClWsbYrMW1N5tRPmYGSEjtNf53rn~Afj0XTgSBn59AzFXpXQyQw4sVtNvnB2aLds5wWmpuIkr8ovYRtqZJUD6CDbIydjOfk8mQ4aQaEShKGerHEyKxvH6M9xTwFPQq4NLCJsXwmFEJ4gcTD99iV5m0K6j5iMTdnuQvbqr4vBEL-psa3GO1bAn0OQl3amWlB2jHrnQM7iFSE~aS1dHKvdSfl2qo93Jnuw22CoY5n3rQgIqyG7MEQ7LLaQ1R3-2Px42dBoj7xIz0bKCpUYpUWmOZHsxoS0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Original ECG registration comparing the ST-segment changes in the conventional surface ECG leads I–III, V4–V6 and IT-ECG leads E1–E3 and G1–G3 during baseline and at the end of PTCA performed in the LCX [1 mV signal amplitude is marked at the right end of each tracing sequence (A)]. Figure 4B summarizes the ST-segment changes resulting from PTCA performed in the LAD and in the RCA. Ischaemia-induced ST-segment changes are most impressive in the IT-ECG leads (marked area).
Diagnostic accuracy of multiple ECG-leads and a standard 0.1 mV criterion for ischaemia-induced ST-segment deviation
Several ECG-leads are necessary for early and reliable detection of ST-segment deviation independent of the site of origin. In 11/27 (40.7%) cases, none of the surface ECG-leads showed significant ST-segment deviation of ≥0.1 mV after 30 s. Conversely, ischaemic ST-segment deviation ≥0.1 mV was present in at least one IT-ECG-lead after 30 s of all PTCAs (27/27, 100.0%, P<0.01, Figure 5).
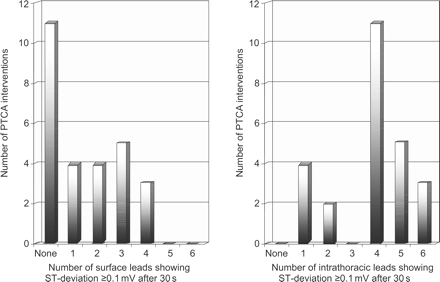
Comparison of the number of surface and IT-ECG leads showing significant ST-segment deviation (≥0.1 mV) after 30 s of coronary occlusion.
Aiming to identify the most sensitive combination of intra-thoracic leads, we tested all permutations. The combination of E2 and G3 [each of which detected 21/27 (77.8%) ischaemic episodes] yielded the highest sensitivity by detecting 26/27 (96.3%) episodes.
Marked differences were found in QRS and ST-segment morphology and vector when the six surface and six IT-ECG leads were compared (Figure 4A and B). Additional analysis of the QRS amplitudes (mean absolute value: 4.193 ± 0.6 mV vs. 2.15 ± 0.3 mV in the surface ECG leads, ratio: 1.95) and the ST-segment deviation/QRS amplitude ratio (Figure 6) demonstrate that the IT-ECG can use higher signal amplitudes for subsequent analysis, whereas the QRS/ST-segment amplitude ratio does not provide evidence of differences compared with the surface ECG.
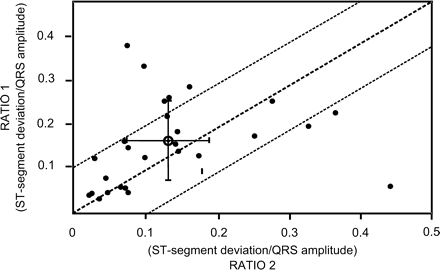
Comparison of the ST-segment deviation amplitude/QRS amplitude ratio using the IT-ECG leads (RATIO 1) and the conventional surface ECG leads (RATIO 2). All 27 PTCA attempts are included, the mean value of RATIO 1/RATIO 2 is marked with its standard deviation showing a moderate linear relationship despite an overall double QRS and ST-segment amplitude in the IT-ECG leads.
Discussion
The majority of patients treated today by an ICD suffer from coronary artery disease.1–3 Once sudden arrhythmic death is no longer of clinical relevance, progression of coronary atherosclerosis and subsequent fatal or non-fatal ischaemic events will become the major prognostic determinants in these patients. Thus, myocardial reinfarction or need for revascularization occurs in up to 32–50% within the first 5 years after ICD implantation, a time interval that is survived by 64–75% of all ICD-recipients today.5,6
On the basis of these considerations, continuous ICD-monitoring of myocardial ischaemia would markedly increase the diagnostic impact of this device.7,8 Currently, the most simple and reliable technique for monitoring ischaemia is based on the ST-segment analysis. Algorithms directed to the assessment of ischaemic ST-segment changes are well established.8,9 The present study proposes an IT 6-lead ECG according to Einthoven and Goldberger for ST-segment monitoring. This IT-ECG is based on the technological platform of today's ICD and uses three electrodes—one in the RVA, one in the SVC, and one at the generator site—for assessing the six IT-ECG leads. To prove the feasibility and diagnostic accuracy of the IT-ECG leads for ischaemia monitoring, we selected the model of PTCA as a standardized method for inducing and monitoring myocardial ischaemia originating at different sites in the heart.
The present study provides convincing evidence that the IT-ECG leads are much more sensitive than the limb and chest leads for monitoring the onset and time-course of PTCA-induced ischaemia. Thus, 30 s after coronary artery occlusion, myocardial ischaemia is twice as frequent in the intra-thoracic as in conventional surface ECG leads. Even after 90 s of coronary artery occlusion, there is a 30% higher incidence of ischaemic ST-segment alterations present in the IT-ECG than in the conventional ECG used as a standard. We also demonstrated that by using all six IT-ECG leads, ischaemia detection was independent of the site of origin. One strong argument for this observation is the fact that the three electrodes used for the IT-ECG leads are well-placed to surround the heart anatomically and electrically (SVC electrode: superior and dorsal to the heart, generator site electrode: superior and anterior, RV electrode: diaphragmatic and anterior); another argument is the close contact of all electrodes to the heart which allows assessment of electrical signals of excellent quality and unaffected by signal alterations due to the skin–electrode interface.
However, one must consider that the IT-ECG leads provide information that is in principle very similar to the conventional ECG leads. Overall, the IT-ECG amplitudes' greater sensitivity seems to derive mainly from the position inside the chest and its closer proximity to the heart. Thus, the amplitude of ST-segment alterations consistently remained twice as great as those of the surface ECG, without affecting the QRS/ST-segment amplitude ratio. Thus, first, the IT-ECG was more sensitive in early detection of ST-segment alterations ≥0.1 mV, but this difference became smaller when ischaemia persisted longer and, second, the ST-segment alteration was always more pronounced in the IT-ECG leads.
Subanalysis focused on the question of which IT-ECG leads provide the greatest diagnostic impact demonstrated that the intra-thoracic Einthoven ECG lead E2 and the Goldberger ECG lead G3 were most sensitive in assessing PTCA-induced ischaemia. The combination of these two leads yields 96.3% sensitivity. However the potential and anticipatable loss of specificity remains unclear.
In implantable devices, therefore, a detection mode, e.g. when a certain heart rate criterion is met, would be most effective to combine maximum diagnostic accuracy with minimal energy consumption.
Our data are well in line with previous experimental and clinical studies using various intra-cardiac uni- and bipolar electrodes to assess transient myocardial ischaemia during coronary artery occlusion.11–13 The key advantage of the IT 6-lead-ECG as described by us7 and the working groups of Theres et al.9 and Ghuran et al.10 is the heart-surrounding position of the leads in the present ICD generation. Theres et al.9 used a similar study protocol in a smaller patient group and compared the IT-ECG only with the surface ECG leads I, II, and V2. Nevertheless, they reported similar diagnostic accuracy of the IT-ECG leads, as well as the doubling of resulting ECG amplitudes in comparison with the surface ECG. The technical background for monitoring ischaemic ST-segment alterations in implantable devices has also been recently established.8
The benefit associated with the permanent availability of a 6-lead IT-ECG in implantable defibrillation devices does not only result from the possibility of ischaemia monitoring. Once this diagnostic option is established, the 6-lead IT-ECG may also be used for continuous, periodical, or episodical monitoring of other parameters such as T-wave alternans, QT-duration, and QT-dispersion, and ECG information may also be used to differentiate better between supraventricular and ventricular tachycardia, or to provide the basis of AV-sequential pacing in single-chamber ICDs without an atrial lead by P-wave monitoring.
Limitations of the study
The present study is based on patients with symptomatic myocardial ischaemia undergoing PTCA intervention. This, a well-standardized situation for ischaemia induction and monitoring, was chosen as the first step in evaluating the feasibility of the 6-lead IT-ECG for monitoring myocardial ischaemia. The extent, severity, and time-course of demand-ischaemia during exertion might differ from PTCA-induced ischaemia, and this might influence the diagnostic accuracy of the IT-ECG. Although not observed in this study, noise, possibly introduced by muscle tremor and other confounders during daily activities, might also influence diagnostic accuracy. Regarding data interpretation, it should be considered that we did not aim to provide data from a certain vascular region of the heart.
In our study, the ICD-like, 6-lead IT-ECG was implanted only temporarily in patients with symptomatic coronary artery disease considered for PTCA. Due to this aspect, the hypothesis raised herein would require testing in a real population of those patients who would normally receive an ICD device (e.g. patients with poor left-ventricular function, broad QRS complex, digitalis therapy, atrial fibrillation, etc.).
Due to restrictions in simultaneous documentation with identical filtering characteristics on the same ECG machine, conventional ECG leads were restricted to six (+ six IT-ECG leads resulting in a total of 12 simultaneous ECG leads). Overall, restriction of the five conventional ECG leads known to be the most sensitive may influence the diagnostic efficacy of the conventional ECG. When used for analysis, there is no evidence from the data documented in this study for such a hypothesis. Ischaemic ST-segment alterations were based on predefined ≥0.25 and ≥0.10 mV criteria. Whereas the 0.1 mV threshold is well established for ischaemia detection by surface ECG, no threshold is known for the intra-thoracic lead with their higher amplitudes. Therefore, the specificity of the ST-changes demonstrated here for ischaemia is not known.
Conclusions
The ICD has become a very effective device for treating life-threatening ventricular tachyarrhythmias in patients suffering from coronary artery disease. Implementation of additional diagnostic and therapeutic tools aiming to control disease-related complications such as myocardial ischaemia and heart failure would indicate a shift in the future from the simple defibrillator introduced in the mid-1980s towards highly sophisticated devices to reduce morbidity effectively and overall mortality resulting from underlying coronary artery disease and its various complications.
The present study provides promising evidence that, with the use of several IT-ECG leads according to Einthoven and Goldberger, reliable assessment of myocardial ischaemia during daily life may become feasible, providing an interesting diagnostic option for future ICD generations. Patients with severe coronary artery disease might benefit from the availability of real-time ischaemia-monitoring.
Acknowledgements
The study was supported by the International LEG Prize 2001.
References
Data from this study were presented during the annual scientific sessions of the AHA 2000 and 2001



