-
PDF
- Split View
-
Views
-
Cite
Cite
Luigi Padeletti, Fabio Fantini, Antonio Michelucci, Paolo Pieragnoli, Andrea Colella, Nicola Musilli, Giuseppe Ricciardi, Trina A. Buhr, Sergio Valsecchi, Rate stabilization by right ventricular apex or His bundle pacing in patients with atrial fibrillation, EP Europace, Volume 7, Issue 5, 2005, Pages 454–459, https://doi.org/10.1016/j.eupc.2005.05.007
Close - Share Icon Share
Abstract
In patients with atrial fibrillation right ventricular pacing can block antegrade conduction at pacing intervals longer than the shortest spontaneous R–R interval, causing the stabilization of ventricular rhythm. In this study the effects of pacing at two sites were compared in order to evaluate the role of conduction times in determining the stabilization of ventricular rhythm.
In eight patients with permanent atrial fibrillation, the ventricular rate was recorded before and during pacing at the right ventricular apex and the His bundle with different cycle lengths.
In all patients, we obtained a reduction in spontaneous QRS complexes with respect to those anticipated at pacing rates slightly above the spontaneous mean rate, and the ventricular rhythm stabilized at pacing intervals longer than the spontaneous shortest R–R intervals. Between pacing sites we did not observe any difference in the reduction in spontaneous beats and the cycle stabilizing the rhythm. Moreover, simulation of the interaction between antegrade and retrograde impulses in a computer model confirmed that results obtained by pacing at the His bundle cannot be readily explained as a consequence of conduction delays.
This study suggests that the lag introduced by the His-Purkinje conduction cannot explain, as proposed, the stabilization of ventricular rhythm observed in patients with atrial fibrillation and right ventricular pacing.
Introduction
The irregular ventricular rhythm during atrial fibrillation (AF) has been explained in terms of repetitive concealment of electrical impulses of atrial origin within the atrioventricular (AV) node, as a result of decremental conduction [1] . This classical theory was challenged by Meijler et al. [2–,4] . Their hypothesis states that the ventricular response is dictated by automaticity of AV junctional tissue, electrotonically modulated by disorganized AF wavefronts.
In patients with stable AF, two phenomena have long been recognized: single ventricular beats are often followed by a lengthened ventricular cycle, an effect similar to the post-extrasystolic “compensatory pause” [5,,6] , and ventricular pacing prevents spontaneous ventricular complexes from occurring at cycle lengths shorter than the pacing cycle length [7,,8] . When spontaneous ventricular activity is nearly suppressed, this effect has been called “stabilization” of ventricular rhythm.
These findings have been considered by Meijler et al. to support their hypothesis. They postulated that properly timed ventricular complexes conduct retrogradely, penetrate into the AV node and reset the nodal pacemaker. Reset of the discharge sequence of the AV node will only occur if delivery of the ventricular impulse fortuitously falls within an interval between antegrade impulses that would otherwise have been long. Other retrograde impulses will simply collide with and eliminate the antegrade ones.
Conversely, in the context of the classical theory of decremental conduction, Watanabe and Watanabe ascribed the elimination of shorter R–R intervals to the His-Purkinje conduction delays [9] ; during ventricular on-demand pacing, the length of escape intervals (i.e. the intervals between paced complexes and following spontaneous impulses) is greater than or equal to the sum of the refractory period of the AV node (minimum observed spontaneous R–R interval) and the forward and retrograde His-Purkinje conduction times. As a consequence, the pacing cycle suppressing antegrade conduction will exceed the shortest R–R interval by the sum of forward and retrograde conduction times.
The objective of our work was to test both hypotheses by comparing the effects of right ventricle (RV) pacing at two sites, the RV apex (RVA) and the His bundle, evaluating in this way the role of conduction times in determining the stabilization effect.
Methods
Eight patients indicated for an intracardiac cardioversion were enrolled in the study. The study conforms with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board. Witnessed informed consent was obtained from each patient.
All patients ( Table 1 ) had lone permanent AF, as documented by clinical history, physical and electrocardiographic examination and thyroid function evaluation. They had intact AV conduction and no implanted pacing device. None of them had accessory pathways capable of retrograde conduction. No patient was on amiodarone therapy, and any antiarrhythmic drug or drugs affecting AV conduction were suspended at least 1 week before the study.
Patient data, distribution of R–R intervals before pacing and results of pacing protocol and computer simulation
| Patient . | Age (years) . | Sex . | HV (ms) . | RRmin (ms) . | RRmean (ms) . | RRmax (ms) . | SD (ms) . | CV . | PCL >95 . | PCL >95 −RRmin (ms) . | Optimal cond. delay (ms) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | RVA (ms) . | HIS (ms) . | . | . |
| 1 | 56 | M | 35 | 489 | 923 | 2200 | 275 | 0.30 | 750 | 750 | 261 | 216 |
| 2 | 70 | F | 45 | 418 | 879 | 1606 | 185 | 0.21 | 750 | 750 | 332 | 193 |
| 3 | 76 | M | 40 | 567 | 822 | 1423 | 126 | 0.15 | 750 | 750 | 183 | 139 |
| 4 | 56 | M | 50 | 489 | 734 | 1293 | 131 | 0.18 | 600 | 600 | 111 | 50 |
| 5 | 65 | M | 35 | 412 | 711 | 1131 | 158 | 0.22 | 600 | 600 | 188 | 225 |
| 6 | 73 | F | 40 | 322 | 564 | 1222 | 158 | 0.28 | 500 | 500 | 178 | 300 |
| 7 | 67 | M | 43 | 363 | 563 | 1137 | 129 | 0.23 | 500 | 500 | 137 | 215 |
| 8 | 58 | F | 42 | 358 | 646 | 1387 | 206 | 0.32 | 500 | 500 | 142 | 215 |
| Patient . | Age (years) . | Sex . | HV (ms) . | RRmin (ms) . | RRmean (ms) . | RRmax (ms) . | SD (ms) . | CV . | PCL >95 . | PCL >95 −RRmin (ms) . | Optimal cond. delay (ms) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | RVA (ms) . | HIS (ms) . | . | . |
| 1 | 56 | M | 35 | 489 | 923 | 2200 | 275 | 0.30 | 750 | 750 | 261 | 216 |
| 2 | 70 | F | 45 | 418 | 879 | 1606 | 185 | 0.21 | 750 | 750 | 332 | 193 |
| 3 | 76 | M | 40 | 567 | 822 | 1423 | 126 | 0.15 | 750 | 750 | 183 | 139 |
| 4 | 56 | M | 50 | 489 | 734 | 1293 | 131 | 0.18 | 600 | 600 | 111 | 50 |
| 5 | 65 | M | 35 | 412 | 711 | 1131 | 158 | 0.22 | 600 | 600 | 188 | 225 |
| 6 | 73 | F | 40 | 322 | 564 | 1222 | 158 | 0.28 | 500 | 500 | 178 | 300 |
| 7 | 67 | M | 43 | 363 | 563 | 1137 | 129 | 0.23 | 500 | 500 | 137 | 215 |
| 8 | 58 | F | 42 | 358 | 646 | 1387 | 206 | 0.32 | 500 | 500 | 142 | 215 |
F, female; M, male; HV, HV interval; RRmin, minimum R–R interval; RRmean, mean R–R interval; RRmax, maximum R–R interval; SD, standard deviation; CV, coefficient of variation; PCL >95 , the longest pacing cycle that eliminates more than 95% of antegrade impulses; RVA, RV apex pacing; HIS, His bundle pacing; optimal cond. delay, the minimum value of the sum of retrograde and antegrade conduction delay that, when set in simulation, returns the values of PCL >95 obtained during the in vivo trial with His bundle pacing.
Patient data, distribution of R–R intervals before pacing and results of pacing protocol and computer simulation
| Patient . | Age (years) . | Sex . | HV (ms) . | RRmin (ms) . | RRmean (ms) . | RRmax (ms) . | SD (ms) . | CV . | PCL >95 . | PCL >95 −RRmin (ms) . | Optimal cond. delay (ms) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | RVA (ms) . | HIS (ms) . | . | . |
| 1 | 56 | M | 35 | 489 | 923 | 2200 | 275 | 0.30 | 750 | 750 | 261 | 216 |
| 2 | 70 | F | 45 | 418 | 879 | 1606 | 185 | 0.21 | 750 | 750 | 332 | 193 |
| 3 | 76 | M | 40 | 567 | 822 | 1423 | 126 | 0.15 | 750 | 750 | 183 | 139 |
| 4 | 56 | M | 50 | 489 | 734 | 1293 | 131 | 0.18 | 600 | 600 | 111 | 50 |
| 5 | 65 | M | 35 | 412 | 711 | 1131 | 158 | 0.22 | 600 | 600 | 188 | 225 |
| 6 | 73 | F | 40 | 322 | 564 | 1222 | 158 | 0.28 | 500 | 500 | 178 | 300 |
| 7 | 67 | M | 43 | 363 | 563 | 1137 | 129 | 0.23 | 500 | 500 | 137 | 215 |
| 8 | 58 | F | 42 | 358 | 646 | 1387 | 206 | 0.32 | 500 | 500 | 142 | 215 |
| Patient . | Age (years) . | Sex . | HV (ms) . | RRmin (ms) . | RRmean (ms) . | RRmax (ms) . | SD (ms) . | CV . | PCL >95 . | PCL >95 −RRmin (ms) . | Optimal cond. delay (ms) . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | . | . | RVA (ms) . | HIS (ms) . | . | . |
| 1 | 56 | M | 35 | 489 | 923 | 2200 | 275 | 0.30 | 750 | 750 | 261 | 216 |
| 2 | 70 | F | 45 | 418 | 879 | 1606 | 185 | 0.21 | 750 | 750 | 332 | 193 |
| 3 | 76 | M | 40 | 567 | 822 | 1423 | 126 | 0.15 | 750 | 750 | 183 | 139 |
| 4 | 56 | M | 50 | 489 | 734 | 1293 | 131 | 0.18 | 600 | 600 | 111 | 50 |
| 5 | 65 | M | 35 | 412 | 711 | 1131 | 158 | 0.22 | 600 | 600 | 188 | 225 |
| 6 | 73 | F | 40 | 322 | 564 | 1222 | 158 | 0.28 | 500 | 500 | 178 | 300 |
| 7 | 67 | M | 43 | 363 | 563 | 1137 | 129 | 0.23 | 500 | 500 | 137 | 215 |
| 8 | 58 | F | 42 | 358 | 646 | 1387 | 206 | 0.32 | 500 | 500 | 142 | 215 |
F, female; M, male; HV, HV interval; RRmin, minimum R–R interval; RRmean, mean R–R interval; RRmax, maximum R–R interval; SD, standard deviation; CV, coefficient of variation; PCL >95 , the longest pacing cycle that eliminates more than 95% of antegrade impulses; RVA, RV apex pacing; HIS, His bundle pacing; optimal cond. delay, the minimum value of the sum of retrograde and antegrade conduction delay that, when set in simulation, returns the values of PCL >95 obtained during the in vivo trial with His bundle pacing.
Using a femoral approach, two 6 Fr, 1 cm spaced, quadripolar electrode catheters (USCI Division, C.R. Bard Inc., Billerica, MA, USA) were placed in the right ventricle: at the RVA and at the His bundle, respectively. RV apical lead placement was confirmed through fluoroscopy. His bundle lead placement was confirmed by recording of a His-Purkinje mediated cardiac activation and repolarization ( Fig. 1 ).
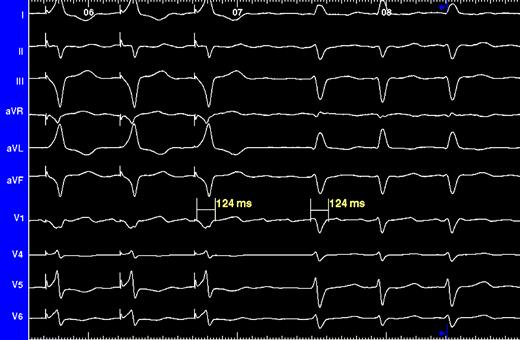
Surface ECG. Three leftmost QRS complexes resulted from His bundle pacing, whereas the remaining ones are intrinsically conducted. ECG concordance provides evidence of successful His bundle pacing.
The electrocardiogram was continuously recorded by means of a Bard LabSystem Duo recording system (C. R. Bard, Inc., Murray Hill, NJ, USA). Signal was bandpass filtered (30–400 Hz), amplified, and then analogue-to-digital (A/D) converted at 16 bits, 1000 Hz. Each recording was manually reviewed and R–R intervals measured.
At each pacing site (RVA and His bundle), a 4-min recording of the patient's intrinsic ventricular rate during AF (no-pacing period) was performed to provide baseline data, then four 4-min periods of pacing were initiated and recorded at different pacing rates (60, 80, 100, and 120 ppm) with no intermediate pauses. Pacing sites and rates were presented in random order and the pulse amplitude was set at twice the diastolic threshold.
In all patients, the serial autocorrelogram and the histogram of R–R intervals recorded during the no-pacing phase were computed to verify the random pattern of the ventricular rhythm [2] .
For each patient the mean, the maximum, the minimum R–R interval (respectively RRmean, RRmax and RRmin) and the coefficient of variation (CV) were estimated, the latter defined as standard deviation/RRmean.
The longest pacing cycle that eliminates more than 95% of spontaneous QRS complexes (PCL >95 ) [7] was estimated for both pacing sites.
Having stimulated our patients with an on-demand ventricular pacing, if no regularization was exerted, only spontaneous cycles longer than the pacing interval would have been eliminated. Taking into account the distribution of ventricular cycles during the no-pacing period, it is possible to predict the percentage of spontaneous beats that will be recorded at any pacing rate.
Therefore, we estimated the actual amount of spontaneous QRS for each pacing cycle step, when stimulation was delivered at both sites, and we compared this value with the expected number of antegrade impulses at the same pacing rate, considering the distribution of spontaneous intervals.
We reproduced the computer model proposed by Wittkampf et al. [10,,11] to simulate the behaviour of the AV node during AF and RV pacing. This model was designed in the hypothesis of an electrotonically modulated pacemaker, and taking into account the extinction of conduction due to the interception of antegrade impulses by relatively late ventricular complexes. In this model the histogram of spontaneous R–R intervals of each patient is used to simulate the random discharge sequence of the AV node. The transit time through the His-Purkinje system, which can be freely set in the simulation, is assumed constant, so the ventricular rhythm during AF reflects the rhythm of the AV nodal generator. The model also permits simulation of the delivery of ventricular stimuli. When a ventricular stimulus occurs relatively late in the random timing cycle, a collision of antegrade and retrograde impulses will take place causing the extinction of both impulses, without affecting the timing cycle of the AV node. However, when the ventricular stimulus falls early in the timing cycle, then the AV nodal generator will be reset by retrograde activation.
In each patient we estimated the “optimal” value of the antegrade-retrograde conduction delay, defined as the minimum interval that, when set in simulation, achieves the regularization of rhythm at the same pacing rate (PCL >95 ) obtained during the in vivo trial.
A P value of 0.05 was used as the level of statistical significance. Correlation coefficients were calculated using linear regression. All data are reported as mean±standard deviation. Statistical analyses were made with commercial software and the simulation was performed with a custom-made software developed in LabVIEW 6i (National Instruments, Houston, TX, USA).
Results
In all patients the distribution of the consecutive R–R intervals showed a random pattern, the RRmean ranged from 563±129 ms in patient no. 7 to 923±275 ms in patient no. 1, with a CV ranging from 0.15 (patient no. 3) to 0.32 (patient no. 8); the mean value of RRmean in our population was 730±137 ms ( Table 1 ).
Pacing effects
Over 95% of the QRS complexes were pacemaker originated in all patients at pacing intervals that were longer than the duration of the spontaneous shortest R–R intervals (i.e. PCL >95 >RRmin) ( Table 1 ).
A direct correlation ( r =0.96, P <0.01) between RRmean and the PCL >95 was observed. The mean difference between RRmean and PCL >95 was 112±41 ms and the mean difference PCL >95 −RRmin was 192±72 ms.
A correlation resulted between the PCL >95 −RRmin and RRmean ( r =0.70, P <0.05).
We did not observe any difference in PCL >95 between pacing sites.
The regularization phenomenon was present also at intermediate pacing rates. In our patients, with a pacing interval longer than the mean spontaneous R–R, no significant difference was found between the percentage of expected spontaneous beats and those actually recorded during pacing. Whereas, at pacing rates slightly above the spontaneous mean rate, we observed a reduction of spontaneous QRS complexes with respect to those expected (12.7±6.5% with RVA pacing and 16.3±5.1% with His bundle pacing, both P <0.01) ( Fig. 2 ). In all patients, no significant difference in spontaneous beat reduction was apparent between sites.
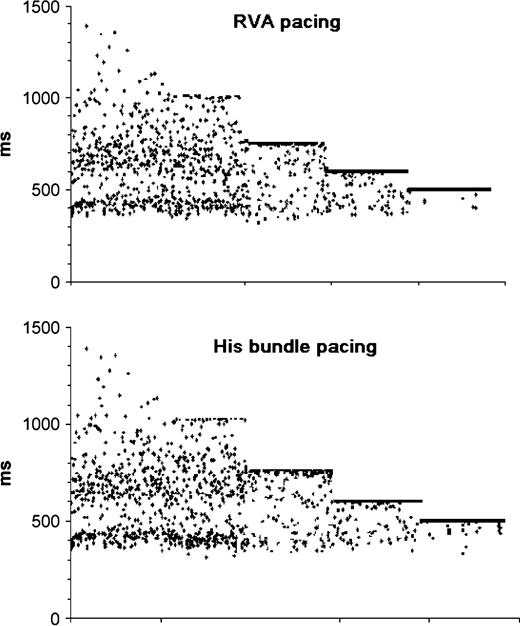
Successive R–R intervals during RVA and His bundle pacing at 60, 80, 100, and 120 bpm. The regularization of ventricular rhythm becomes evident at pacing rates slightly above the mean spontaneous rate. Further increase of pacing rate results in blocking all antegrade impulses.
Using the computer simulation of the Wittkampf model and considering the values of PCL >95 obtained imodelur patients with His bundle pacing, we calculated the minimum value of the sum of retrograde and antegrade conduction delay and we obtained a mean value of 194±73 ms, with values longer than 200 ms in five patients ( Table 1 ).
Discussion
Watanabe and Watanabe explained the elimination of shorter R–R intervals by RV pacing, ascribing the difference PCL >95 −RRmin to the sum of forward and retrograde “AV node-pacing site” conduction delays [9,,12] .
In agreement with Vereckei et al. [13] , they pointed out that the study by Wittkampf et al. [7] , which found differences PCL >95 −RRmin greater than 120 ms, used patients with slow ventricular rates. They speculated that, in patients with normal ventricular rates, the differences could have been shorter and thus compatible with the decremental conduction hypothesis.
In all our patients with stable AF, no pacemaker implanted and normal ventricular rate (730±137 ms), RV pacing achieved more than 95% of ventricular captures at a pacing interval that was shorter than mean spontaneous R–R but longer than shortest spontaneous interval, with a difference of over 120 ms between PCL >95 and RRmin (192±72 ms). Thus, our findings seem to support the hypothesis of electrotonic modulation.
Moreover, our results confirm previous experimental data showing that the difference between PCL >95 and RRmin directly correlates with the mean spontaneous heart cycle [13] . We think that, in accordance with the theory of Meijler et al., this finding can be easily explained by a facilitated penetration of retrograde beats into the AV node in patients with longer intervals between antegrade beats.
If the His-Purkinje conduction delays represented the only cause of ventricular rhythm stabilization, as stated by Watanabe and Watanabe, His bundle pacing should produce a reduced elimination effect with respect to the RVA pacing (i.e. PCL >95 (HIS)<PCL >95 (RVA) and PCL >95 (HIS)≈RRmin).
On the contrary, considering the model of Meijler et al., pacing from the His bundle should make it easier to reset the putative junctional pacemaker compared with RVA pacing, since His pacing would circumvent the conduction delays, reducing the proportion of retrograde beats colliding with the antegrade ones.
In this study, we tested directly these hypotheses by comparing the degree of suppression of spontaneous ventricular activity produced by His bundle and RVA pacing.
We found no measurable difference of regularization between the two pacing sites.
The regularization of ventricular rhythm, which is evident at PCL >95 when spontaneous impulses are no longer conducted, is already present at pacing rates slightly above the spontaneous mean rates. In fact, comparing the percentage of expected beats and those actually recorded during pacing, we verified a significant reduction of spontaneous beats caused by pacing, though not observing a significant difference between pacing sites.
These findings, showing similar effects with both pacing sites, do not provide evidence to support definitively one of the two hypotheses. However, they permit exclusion of the possibility that conduction delays play any role in determining the stabilization effect, by either prevention or facilitation.
In 1990, Wittkampf et al. proposed a model to describe the behaviour of AV node during AF in the hypothesis of electrotonic modulation [10,,11] . According to this model, the authors considered two different mechanisms: relatively early retrogradely conducted impulses penetrate into the AV node and reset its cycle, whereas later retrograde impulses simply intercept antegrade ones below the AV node causing cancellation of both. Thus, the conduction delays determine the degree of interaction between antegrade and retrograde impulses and, also in this model, have a key role in producing the stabilization effect.
In this model, short “AV node-pacing site” conduction delays, resulting from His bundle pacing, should reduce the frequency of collisions of antegrade and retrograde beats with respect to RVA pacing and increase the probability to reset the AV node, stabilizing the ventricular rhythm.
However, the escape intervals (that also in this model are equal to the sum of spontaneous beats and conduction delays) are shorter during His pacing than with RVA pacing, facilitating in this way the occurrence of spontaneous beats, and thus counterbalancing the former effect.
Using the distribution of spontaneous R–R intervals to simulate the discharge sequence of the putative pacemaker, we calculated the minimum value of delay that returns the values of PCL >95 obtained with His bundle pacing in our patients, in order to verify whether this conduction time was consistent with the site of pacing.
We obtained intervals that seem too long to represent the antegrade and retrograde conduction delay between a putative nodal pacemaker and the pacing site at the His bundle. Therefore, in our opinion, this result confirms that also this model, requiring long delays to justify the regularization effect, does not explain in vivo observations.
Limitations of the study
The pacing protocol adopted in our patients was based on a few, fixed steps of pacing intervals, from 1000 to 500 ms.
This limitation affected the evaluation of PCL >95 , whose estimates have to be considered as approximations. The relationship between PCL >95 and the mean heart rate, however, was quite strict in our patients, reproducing the results previously obtained with more accurate pacing protocols [7,,13] .
Although there was no difference in the ventricular rate stabilization effects between the two pacing sites, it remains unclear whether the mechanisms of ventricular rate stabilization during pacing at these two sites are similar. It is possible that the electronic modulation effects of the AV node is larger during His bundle pacing than during RVA pacing such that the conduction delay at the His-Purkinje system during RVA pacing might still contribute to the ventricular rate stabilization effect.
Previous studies, however, investigating the phenomenon of rate stabilization used similar study sample size, which does not allow us to exclude the possibility that failure to detect differences between RVA and His bundle pacing may be due to insufficient power of this study.
Conclusions
Meijler et al., studying the effect of RV pacing on the ventricular response during AF, concluded that the stabilization of rhythm cannot readily be explained by the classical concept of decremental AV nodal conduction.
In the context of the classical theory, Watanabe and Watanabe explained the elimination of shorter R–R intervals, ascribing the difference between the pacing cycle stabilizing the rhythm and the shortest spontaneous R–R interval to the sum of retrograde and forward His-Purkinje conduction times, but direct evidence was lacking.
These hypotheses were directly tested in the present study. The effects of pacing at two sites, the RVA and the His bundle, were compared in order to evaluate the role of conduction delays in determining the stabilization of ventricular rhythm in patients with AF and intact AV conduction.
The results of this study, obtained in patients with normal ventricular rate, confirm the existence of the rate stabilization phenomenon. Moreover, although not providing a clear explanation of the electrophysiological mechanism determining the stabilization of ventricular rhythm, we have provided evidence that this effect cannot be attributed to the transit time of antegrade and retrograde impulses.
References
Author notes
1 Trina A. Buhr and Sergio Valsecchi are employees of Medtronic, Inc.



