-
PDF
- Split View
-
Views
-
Cite
Cite
Jeppe Hagstrup Christensen, Sam Riahi, Erik Berg Schmidt, Henning Mølgaard, Anders Kirstein Pedersen, Finn Heath, Jens Cosedis Nielsen, Egon Toft, n-3 Fatty acids and ventricular arrhythmias in patients with ischaemic heart disease and implantable cardioverter defibrillators, EP Europace, Volume 7, Issue 4, 2005, Pages 338–344, https://doi.org/10.1016/j.eupc.2005.02.118
Close - Share Icon Share
Abstract
To investigate the relationship between serum content of n-3 polyunsaturated fatty acids (PUFA) and the incidence of ventricular arrhythmias in patients with an implantable cardioverter defibrillator (ICD).
We included 98 patients with ischaemic heart disease and an ICD. The numbers of ventricular fibrillation (VF) and ventricular tachycardia (VT) events were assessed during a 12-month period and related to the concentration of n-3 PUFA in serum phospholipids.
Patients with more than one arrhythmic event had significantly lower n-3 PUFA levels compared with patients without arrhythmias (mean 7.1% vs 9.2%, P < 0.01). Dividing the patients into quintiles according to their n-3 PUFA level those with the lowest content of n-3 PUFA had more ventricular arrhythmias than patients with the highest concentration of n-3 PUFA (mean 1.3 event vs 0.2 event, P < 0.05).
Patients with a low content of n-3 PUFA in serum had a higher incidence of ventricular arrhythmias compared with patients with high serum levels of n-3 PUFA. The data suggest that the protection offered by n-3 PUFA against sudden cardiac death observed in previous studies is mediated by a direct antiarrhythmic effect of n-3 PUFA.
Introduction
Sudden cardiac death (SCD) is the most common cause of death in Western countries and recent data suggest that the incidence of SCD is not declining [1] . The introduction of the implantable cardioverter defibrillator (ICD) is unlikely to reduce the incidence of SCD substantially, because in the majority of cases of SCD it is often the first manifestation of ischaemic heart disease (IHD). Although the individual risk is low the absolute figure is high, e.g. approximately 300 000 SCD per year in the US population [1] .
A search for preventive measures is needed, and during the past years increased attention has been paid to a reduced incidence of SCD among people eating fish or taking supplements of n-3 polyunsaturated fatty acids (PUFA) [2 – 6] . The effect of marine n-3 PUFA on SCD seems to be due to an antiarrhythmic effect of n-3 PUFA, an effect so far mainly demonstrated in in-vitro experiments and in animal studies [7] . However, a direct antiarrhythmic effect of n-3 PUFA has only been sparsely investigated in humans. The aim of the present study, therefore, was to investigate a possible relationship between the number of ventricular arrhythmic events in patients with IHD and an ICD and the concentration of n-3 PUFA in serum phospholipids.
Methods
Study population
Patients with an ICD treated in the out-patient clinics at the Department of Cardiology, Aalborg Hospital, Århus University Hospital, Aalborg, and the Department of Cardiology, Skejby Hospital, Århus University Hospital, Århus, were asked to participate. All the patients suffered from IHD, and the indications for ICD implantation were according to international guidelines [8] . Patients with known diabetes mellitus, atrial flutter or fibrillation were not asked to participate. Of 139 patients invited, 98 patients agreed to participate. Statin medication was noted.
The procedures followed in the study were approved by the regional ethical committee and signed informed consent was obtained from all the patients.
Laboratory analysis
Blood samples were drawn in the morning in the supine position after at least 10 h of fasting. Total lipids were extracted from serum [9] and the phospholipids were separated from other lipid classes [10] and transesterified. The content of marine n-3 PUFA was then measured by gas chromatography using a Chrompack CP-9002 gas chromatograph (Chrompack International, Middelburg, The Netherlands), and expressed as the percent of total fatty acids. The approach used permits quantification of fatty acids methyl esters with up to 24 carbon atoms. The three marine n-3 PUFA eicosapentaenoic acid (EPA), docosapentaenoic acid, and docosahexaenoic acid (DHA) were measured and the sum of these three fatty acids was regarded as total marine n-3 PUFA. The interassay CVs (variation coefficients) were 0.9%, 3.3%, and 1.3% for EPA, DPA, and DHA, respectively.
Ventricular arrhythmia data
ICD stored episodes of ventricular fibrillation (VF) and ventricular tachycardia (VF) were registered for a 12 month period. The blood sample was taken at the end of the 12 months observation period. The ventricular arrhythmic events were verified by an experienced cardiologist. Only ventricular arrhythmias with documented episodes of ICD intervention by antitachycardia pacing or shocks were used for analysis. Episodes of inappropriate ICD therapy due to supraventricular arrhythmias, device malfunction, oversensing, lead problems and sinus tachycardia were excluded from the data analysis.
The ICDs were programmed individually. VT zones, with RR-intervals usually between 380 ms and 330 ms, were added in patients presenting with monomorphic VTs, RR-intervals above 330 ms and benign symptoms. VF zones (RR-interval < 330 ms) were programmed in all cases and was the only zone in the remaining patients. ECG strips showing monomorphic tachycardia were classified as VT and polymorphic tachycardia as VF. This classification was made by a specialist in cardiology and electrophysiology.
Statistical analysis
Comparisons of differences between two groups were tested by non-paired t -test for continuous variables (expressed as mean values and SD). The Chi square test or Fishers Exact test was used for discrete variables and frequencies. Also, the Mantel–Haenszel Chi square test was used to assess a possible trend (dose–response) between n-3 PUFA level and ventricular arrhythmic events. A P value below 0.05 (two-tailed) was considered significant.
Results
Patient data
Patient characteristics are given in Table 1 . Eight women and 90 men were included. The mean age was 64.5 years, and 92 patients had suffered from a myocardial infarction. Thirty-six had undergone coronary artery by-pass grafting and the mean left ventricular ejection fraction was 38%.
Patient characteristics (all patients in the last column) after dividing the patients according to quintiles of total marine n-3 polyunsaturated fatty acids in serum phospholipids
| Total marine n-3 PUFA quintiles . | ||||||
|---|---|---|---|---|---|---|
| . | First . | Second . | Third . | Fourth . | Fifth . | All . |
| Total n-3 PUFA (%) | 5.5 (1.0) | 7.7 (0.4) | 8.7 (0.5) | 10.4 (0.6) | 13.0 (1.9) | 9.1 (2.8) |
| N | 20 | 19 | 19 | 20 | 20 | 98 |
| Females ( n ) | 2 | 4 | 0 | 0 | 2 | 8 |
| Age (years) | 61.7 (8.9) | 66.9 (7.1) | 66.5 (8.4) | 64.3 (11.5) | 63.0 (7.5) | 64.5 (8.8) |
| n-3 Fatty acids | ||||||
| EPA (%) | 1.2 (0.4) | 1.8 (0.3) | 2.1 (0.4) | 2.6 (0.5) | 4.1 (1.4) | 2.4 (1.2) |
| DHA (%) | 3.5 (0.9) | 5.0 (0.4) | 5.7 (0.6) | 6.7 (0.5) | 7.8 (1.1) | 5.7 (1.6) |
| Heart related | ||||||
| Ejection fraction (%) | 40 (15) | 34 (11) | 40 (14) | 38 (12) | 37 (13) | 38 (13) |
| Years with an ICD | 2.5 (1.7) | 2.3 (1.5) | 2.3 (1.0) | 2.3 (1.6) | 2.4 (1.7) | 2.4 (1.5) |
| Previous MI ( n ) | 18 | 19 | 18 | 18 | 19 | 92 |
| CABG ( n ) | 7 | 8 | 5 | 9 | 7 | 36 |
| Medication | ||||||
| β-blockers ( n ) | 15 | 18 | 13 | 15 | 16 | 77 |
| ACE-inhibitors ( n ) | 15 | 12 | 13 | 15 | 16 | 71 |
| Spironolactone ( n ) | 4 | 5 | 2 | 4 | 4 | 19 |
| Amiodarone ( n ) | 5 | 2 | 8 | 7 | 5 | 27 |
| Statins ( n ) | 10 | 13 | 10 | 14 | 13 | 60 |
| Arrhythmias | ||||||
| Mean number of VT | 0.55 (1.3) | 0.95 (2.9) | 0.22 (0.9) | 0.11 (0.3) | 0.16 (0.5) | 0.35 (1.1) |
| Mean number of VF | 0.75 (1.5) | 0.40 (1.6) | 0.22 (0.7) | 0.22 (0.7) | 0.05 (0.2) * | 0.41 (1.5) |
| Patients with arrhythmias ( n ) | 7 | 6 ** | 2 | 4 | 3 | 22 a |
| Total marine n-3 PUFA quintiles . | ||||||
|---|---|---|---|---|---|---|
| . | First . | Second . | Third . | Fourth . | Fifth . | All . |
| Total n-3 PUFA (%) | 5.5 (1.0) | 7.7 (0.4) | 8.7 (0.5) | 10.4 (0.6) | 13.0 (1.9) | 9.1 (2.8) |
| N | 20 | 19 | 19 | 20 | 20 | 98 |
| Females ( n ) | 2 | 4 | 0 | 0 | 2 | 8 |
| Age (years) | 61.7 (8.9) | 66.9 (7.1) | 66.5 (8.4) | 64.3 (11.5) | 63.0 (7.5) | 64.5 (8.8) |
| n-3 Fatty acids | ||||||
| EPA (%) | 1.2 (0.4) | 1.8 (0.3) | 2.1 (0.4) | 2.6 (0.5) | 4.1 (1.4) | 2.4 (1.2) |
| DHA (%) | 3.5 (0.9) | 5.0 (0.4) | 5.7 (0.6) | 6.7 (0.5) | 7.8 (1.1) | 5.7 (1.6) |
| Heart related | ||||||
| Ejection fraction (%) | 40 (15) | 34 (11) | 40 (14) | 38 (12) | 37 (13) | 38 (13) |
| Years with an ICD | 2.5 (1.7) | 2.3 (1.5) | 2.3 (1.0) | 2.3 (1.6) | 2.4 (1.7) | 2.4 (1.5) |
| Previous MI ( n ) | 18 | 19 | 18 | 18 | 19 | 92 |
| CABG ( n ) | 7 | 8 | 5 | 9 | 7 | 36 |
| Medication | ||||||
| β-blockers ( n ) | 15 | 18 | 13 | 15 | 16 | 77 |
| ACE-inhibitors ( n ) | 15 | 12 | 13 | 15 | 16 | 71 |
| Spironolactone ( n ) | 4 | 5 | 2 | 4 | 4 | 19 |
| Amiodarone ( n ) | 5 | 2 | 8 | 7 | 5 | 27 |
| Statins ( n ) | 10 | 13 | 10 | 14 | 13 | 60 |
| Arrhythmias | ||||||
| Mean number of VT | 0.55 (1.3) | 0.95 (2.9) | 0.22 (0.9) | 0.11 (0.3) | 0.16 (0.5) | 0.35 (1.1) |
| Mean number of VF | 0.75 (1.5) | 0.40 (1.6) | 0.22 (0.7) | 0.22 (0.7) | 0.05 (0.2) * | 0.41 (1.5) |
| Patients with arrhythmias ( n ) | 7 | 6 ** | 2 | 4 | 3 | 22 a |
*P < 0.05 (first quintile vs fifth).
**P < 0.05 (first quintile + second quintile vs the other quintiles, Fishers exact test).
Abbreviations: PUFA: Polyunsaturated fatty acids; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; VT: ventricular tachycardia; VF: ventricular fibrillation.
The arrhythmias given are those treated by the ICD. Values are means (SD) or exact numbers.
a Some patients had more than one episode of ventricular arrhythmias.
Patient characteristics (all patients in the last column) after dividing the patients according to quintiles of total marine n-3 polyunsaturated fatty acids in serum phospholipids
| Total marine n-3 PUFA quintiles . | ||||||
|---|---|---|---|---|---|---|
| . | First . | Second . | Third . | Fourth . | Fifth . | All . |
| Total n-3 PUFA (%) | 5.5 (1.0) | 7.7 (0.4) | 8.7 (0.5) | 10.4 (0.6) | 13.0 (1.9) | 9.1 (2.8) |
| N | 20 | 19 | 19 | 20 | 20 | 98 |
| Females ( n ) | 2 | 4 | 0 | 0 | 2 | 8 |
| Age (years) | 61.7 (8.9) | 66.9 (7.1) | 66.5 (8.4) | 64.3 (11.5) | 63.0 (7.5) | 64.5 (8.8) |
| n-3 Fatty acids | ||||||
| EPA (%) | 1.2 (0.4) | 1.8 (0.3) | 2.1 (0.4) | 2.6 (0.5) | 4.1 (1.4) | 2.4 (1.2) |
| DHA (%) | 3.5 (0.9) | 5.0 (0.4) | 5.7 (0.6) | 6.7 (0.5) | 7.8 (1.1) | 5.7 (1.6) |
| Heart related | ||||||
| Ejection fraction (%) | 40 (15) | 34 (11) | 40 (14) | 38 (12) | 37 (13) | 38 (13) |
| Years with an ICD | 2.5 (1.7) | 2.3 (1.5) | 2.3 (1.0) | 2.3 (1.6) | 2.4 (1.7) | 2.4 (1.5) |
| Previous MI ( n ) | 18 | 19 | 18 | 18 | 19 | 92 |
| CABG ( n ) | 7 | 8 | 5 | 9 | 7 | 36 |
| Medication | ||||||
| β-blockers ( n ) | 15 | 18 | 13 | 15 | 16 | 77 |
| ACE-inhibitors ( n ) | 15 | 12 | 13 | 15 | 16 | 71 |
| Spironolactone ( n ) | 4 | 5 | 2 | 4 | 4 | 19 |
| Amiodarone ( n ) | 5 | 2 | 8 | 7 | 5 | 27 |
| Statins ( n ) | 10 | 13 | 10 | 14 | 13 | 60 |
| Arrhythmias | ||||||
| Mean number of VT | 0.55 (1.3) | 0.95 (2.9) | 0.22 (0.9) | 0.11 (0.3) | 0.16 (0.5) | 0.35 (1.1) |
| Mean number of VF | 0.75 (1.5) | 0.40 (1.6) | 0.22 (0.7) | 0.22 (0.7) | 0.05 (0.2) * | 0.41 (1.5) |
| Patients with arrhythmias ( n ) | 7 | 6 ** | 2 | 4 | 3 | 22 a |
| Total marine n-3 PUFA quintiles . | ||||||
|---|---|---|---|---|---|---|
| . | First . | Second . | Third . | Fourth . | Fifth . | All . |
| Total n-3 PUFA (%) | 5.5 (1.0) | 7.7 (0.4) | 8.7 (0.5) | 10.4 (0.6) | 13.0 (1.9) | 9.1 (2.8) |
| N | 20 | 19 | 19 | 20 | 20 | 98 |
| Females ( n ) | 2 | 4 | 0 | 0 | 2 | 8 |
| Age (years) | 61.7 (8.9) | 66.9 (7.1) | 66.5 (8.4) | 64.3 (11.5) | 63.0 (7.5) | 64.5 (8.8) |
| n-3 Fatty acids | ||||||
| EPA (%) | 1.2 (0.4) | 1.8 (0.3) | 2.1 (0.4) | 2.6 (0.5) | 4.1 (1.4) | 2.4 (1.2) |
| DHA (%) | 3.5 (0.9) | 5.0 (0.4) | 5.7 (0.6) | 6.7 (0.5) | 7.8 (1.1) | 5.7 (1.6) |
| Heart related | ||||||
| Ejection fraction (%) | 40 (15) | 34 (11) | 40 (14) | 38 (12) | 37 (13) | 38 (13) |
| Years with an ICD | 2.5 (1.7) | 2.3 (1.5) | 2.3 (1.0) | 2.3 (1.6) | 2.4 (1.7) | 2.4 (1.5) |
| Previous MI ( n ) | 18 | 19 | 18 | 18 | 19 | 92 |
| CABG ( n ) | 7 | 8 | 5 | 9 | 7 | 36 |
| Medication | ||||||
| β-blockers ( n ) | 15 | 18 | 13 | 15 | 16 | 77 |
| ACE-inhibitors ( n ) | 15 | 12 | 13 | 15 | 16 | 71 |
| Spironolactone ( n ) | 4 | 5 | 2 | 4 | 4 | 19 |
| Amiodarone ( n ) | 5 | 2 | 8 | 7 | 5 | 27 |
| Statins ( n ) | 10 | 13 | 10 | 14 | 13 | 60 |
| Arrhythmias | ||||||
| Mean number of VT | 0.55 (1.3) | 0.95 (2.9) | 0.22 (0.9) | 0.11 (0.3) | 0.16 (0.5) | 0.35 (1.1) |
| Mean number of VF | 0.75 (1.5) | 0.40 (1.6) | 0.22 (0.7) | 0.22 (0.7) | 0.05 (0.2) * | 0.41 (1.5) |
| Patients with arrhythmias ( n ) | 7 | 6 ** | 2 | 4 | 3 | 22 a |
*P < 0.05 (first quintile vs fifth).
**P < 0.05 (first quintile + second quintile vs the other quintiles, Fishers exact test).
Abbreviations: PUFA: Polyunsaturated fatty acids; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; VT: ventricular tachycardia; VF: ventricular fibrillation.
The arrhythmias given are those treated by the ICD. Values are means (SD) or exact numbers.
a Some patients had more than one episode of ventricular arrhythmias.
A highly significant positive association was found between the reported fish intake and the n-3 PUFA level in serum phospholipids (data not shown).
Ventricular arrhythmias and marine n-3 PUFA
There was a total of 32 treated VF events and 39 treated VT events in 22 patients (25%) during the 12 months of observation. Thus, 76 patients had no serious arrhythmic event.
The mean concentration of the marine n-3 PUFA, EPA, and DHA, and the total marine n-3 PUFA levels in serum phospholipids are given in Table 1 .
Patients with one or more treated events of either VF or VT tended to have lower levels of n-3 PUFA in their phospholipids compared with patients without an arrhythmic event ( Table 2 ). From Table 2 it is also seen that patients with more than one episode ( n = 14) of treated VF or VT had significantly lower levels of n-3 PUFA than patients without ventricular arrhythmias. The difference in terms of significance level seemed to be most pronounced for DHA (the highest fraction of total marine n-3 PUFA) and total n-3 PUFA.
The 98 patients characterized according to the number of ventricular events (VF and/or VT) and serum phospholipid levels of marine n-3 PUFA (% of total fatty acids)
| . | No ventricular arrhythmia . | One or more episodes of ventricular arrhythmias . | More than one episode of ventricular arrhythmias . |
|---|---|---|---|
| N | 76 | 22 | 14 |
| n-3 Fatty acids | |||
| EPA (%) | (2.4 (1.3) | (2.0 (1.0) | (1.7 (0.9) * |
| DHA level (%) | (5.8 (1.6) | (5.2 (1.8) | (4.5 (1.5) ** |
| Total n-3 PUFA (%) | (9.2 (2.7) | (8.2 (2.8) | (7.1 (2.4) ** |
| . | No ventricular arrhythmia . | One or more episodes of ventricular arrhythmias . | More than one episode of ventricular arrhythmias . |
|---|---|---|---|
| N | 76 | 22 | 14 |
| n-3 Fatty acids | |||
| EPA (%) | (2.4 (1.3) | (2.0 (1.0) | (1.7 (0.9) * |
| DHA level (%) | (5.8 (1.6) | (5.2 (1.8) | (4.5 (1.5) ** |
| Total n-3 PUFA (%) | (9.2 (2.7) | (8.2 (2.8) | (7.1 (2.4) ** |
*P < 0.04 (compared with patients with no arrhythmias).
**P < 0.01 (compared with patients with no arrhythmias).
Abbreviations as in Table 1 .
Values are means (SD).
The 98 patients characterized according to the number of ventricular events (VF and/or VT) and serum phospholipid levels of marine n-3 PUFA (% of total fatty acids)
| . | No ventricular arrhythmia . | One or more episodes of ventricular arrhythmias . | More than one episode of ventricular arrhythmias . |
|---|---|---|---|
| N | 76 | 22 | 14 |
| n-3 Fatty acids | |||
| EPA (%) | (2.4 (1.3) | (2.0 (1.0) | (1.7 (0.9) * |
| DHA level (%) | (5.8 (1.6) | (5.2 (1.8) | (4.5 (1.5) ** |
| Total n-3 PUFA (%) | (9.2 (2.7) | (8.2 (2.8) | (7.1 (2.4) ** |
| . | No ventricular arrhythmia . | One or more episodes of ventricular arrhythmias . | More than one episode of ventricular arrhythmias . |
|---|---|---|---|
| N | 76 | 22 | 14 |
| n-3 Fatty acids | |||
| EPA (%) | (2.4 (1.3) | (2.0 (1.0) | (1.7 (0.9) * |
| DHA level (%) | (5.8 (1.6) | (5.2 (1.8) | (4.5 (1.5) ** |
| Total n-3 PUFA (%) | (9.2 (2.7) | (8.2 (2.8) | (7.1 (2.4) ** |
*P < 0.04 (compared with patients with no arrhythmias).
**P < 0.01 (compared with patients with no arrhythmias).
Abbreviations as in Table 1 .
Values are means (SD).
The patients were divided into quintiles according to their total marine n-3 PUFA concentration in serum phospholipids. Table 1 shows that the patients in each n-3 PUFA quintile were comparable with respect to age and factors related to their IHD including cardiovascular medications. The number of patients treated with amiodarone was identical in the first and the fifth quintile whereas there was a tendency towards more patients being treated with amiodarone in the upper three quintiles compared with the lower quintiles ( P = 0.08). However, only three of the patients from the two lowest quintiles treated with amiodarone experienced ventricular arrhythmias and this figure was also three for the three upper quintiles. There was a tendency towards a higher number of treated ventricular arrhythmic events among the patients in the two lowest n-3 PUFA quintiles, and comparing the first n-3 PUFA quintile with the fifth, there were significantly more VF events in the first quintile. Also, significantly more patients (33%) in the two lowest n-3 PUFA quintiles experienced ventricular arrhythmias compared with patients (18%) in the other quintiles ( Table 1 ).
Fig. 1 depicts the mean sum of treated VF + VT in the n-3 PUFA quintiles and, as seen, patients with the lowest n-3 PUFA content (in the first quintile) had six times higher mean number of treated VF and VT compared with the upper quintile (1.3 vs 0.2, respectively).
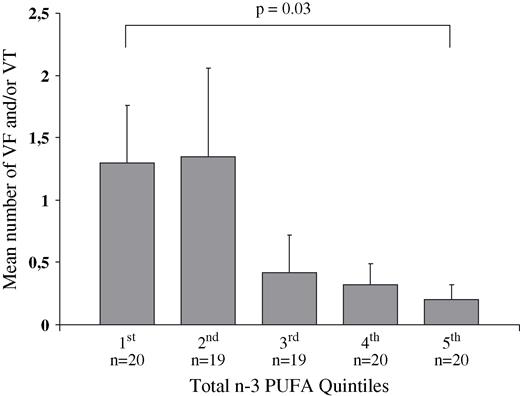
The mean number of treated ventricular tachyarrhythmic events (fibrillation and/or tachycardia) in the 98 patients after they have been divided into quintiles of total marine n-3 PUFA level in serum phospholipids.
In Fig. 2 the accumulated exact number of treated VF and VT events is given for each n-3 PUFA quintile with 26 events in the first quintile and four events in the fifth quintile. This figure reveals a significant trend (dose–response) towards a higher frequency of ventricular arrhythmic events among patients with lower levels of n-3 PUFA ( P = 0.02). n-3 PUFA levels were similar in those patients taking statins vs those not taking this medication.
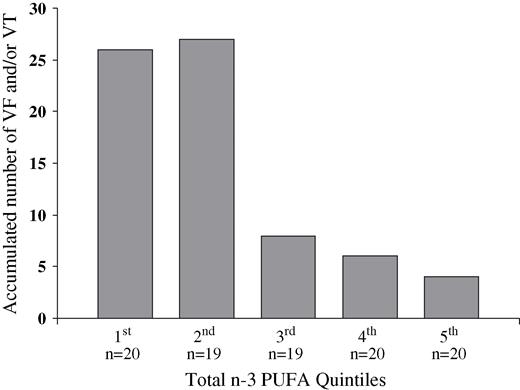
The exact accumulated number of treated ventricular tachyarrhythmic events (fibrillation and/or tachycardia) in the 98 patients after they have been divided into quintiles of total marine n-3 PUFA level in serum phospholipids. A significant trend (dose–response) towards a higher frequency of ventricular arrhythmic events among patients with lower levels of n-3 PUFA is depicted.
Discussion
The present study suggests that the concentration of n-3 PUFA in blood is inversely related to risk of development of serious ventricular arrhythmias in patients with IHD. Thus, patients with lower levels of marine n-3 PUFA in their serum phospholipids had a significantly increased risk of developing ventricular arrhythmias compared with patients with higher levels. The results are in line with previous reports showing that n-3 PUFA increase heart rate variability (HRV) in different patient groups [11 – 14] , and especially DHA is positively associated with HRV [15 – 19] . HRV reflects the cardiac autonomic balance which is associated with the development of ventricular arrhythmias [20] . Thus, an attenuated HRV indicates low vagal activity and sympathetic predominance thereby increasing the risk of arrhythmias, whereas a high HRV reflects vagal predominance and protection against arrhythmias and SCD [21] .
DHA is abundant in both the heart and the neuronal tissue [22,,23] which may be important for its modulation of cardiac autonomic function. Also, DHA increases the membrane fluidity to a larger extent than other PUFA when it is incorporated into the phopholipid bilayer of the cell membrane [24,,25] probably because of its high degree of unsaturation and long chain length with 22 carbon atoms. This may be one of the underlying mechanisms behind the antiarrhythmic effect of n-3 PUFA because most receptor complexes alter their affinities for their ligands when the membrane fluidity changes [26] . However, it must be emphasized, that the other two essential n-3 PUFA, α-linolenic acid (18 carbon atoms) and EPA (20 carbon atoms) to some extent can be metabolised to DHA in the human body [27] . A more comprehensive review on possible antiarrhythmic mechanisms induced by n-3 PUFA has recently been published [28] .
We examined patients with an ICD and documented IHD because this group of patients has a high incidence of ventricular arrhythmias, also reflected in our data. The inverse relationship between events and the plasma level of marine n-3 PUFA suggest that intake of n-3 PUFA may reduce arrhythmic events in these patients. However, this hypothesis can only be validated in a clinically controlled trial, and such trials are certainly warranted.
Since the results from the Diet And Reinfarction Trial (DART) were published in 1989 increased attention has been paid to an effect of marine n-3 PUFA on the risk of SCD [29] . In the DART study, men with a recent MI advised to eat fatty fish twice weekly had a significant 29% reduction in total mortality and deaths from IHD compared with controls. However, the number of non-fatal MI's tended to increase which made the underlying mechanism of protection in the fish group unlikely to be antithrombotic. Based on results from animal studies [30] the authors suggested an antiarrhythmic effect of n-3 PUFA as an explanation for their findings. Ten years later the large GISSI-Prevenzione trial was published [31] . It enrolled 11 324 patients with a recent MI and half of the patients received one capsule of n-3 PUFA daily. The capsules had a high concentration of marine n-3 PUFA with an average ratio of EPA/DHA 1:2. After 3.5 years of follow-up, a 20% relative reduction in the primary endpoint (cardiovascular death + non-fatal MI + non-fatal stroke) was observed in patients randomized to n-3 PUFA compared with controls. Much of this reduction could be explained by a highly significant 45% reduction in cases of SCD which was obvious within 4 months of supplementation [2] .
A case–control study from 1995 showed that eating fatty fish once a week was associated with a 50% reduction in the risk of SCD [5] and a similar large reduction in SCD was also reported from the Physicians Health Study [3] . In both of these studies the risk reduction in SCD was further validated by measuring the level of n-3 PUFA in blood cells or in blood [5,,32] . Thus, the higher the concentration of n-3 PUFA the greater is the protection against SCD, which is in line with our results. Other recent studies have also found noticeable reductions in mortality by marine n-3 PUFA which could be related to SCD. Thus, in a prospective cohort study adults aged ≥65 years who ate fish at least three times a week had a 58% lower risk of arrhythmic death compared with subjects seldom eating fish [33] . Another nested case–control study showed that a high concentration of EPA + DHA measured in phospholipids 2 years prior to a cardiovascular event was associated with a significantly (68%) lower risk of fatal IHD [34] .
A small non-blinded study on ICD patients very recently suggested an antiarrhythmic effect of n-3 PUFA [35] . Thus, in ICD patients with inducible ventricular tachycardia, it was not possible to induce arrhythmias in five of seven patients if n-3 PUFA had been given intravenously just prior to the induction attempt.
Our study has limitations. The use of n-3 PUFA in serum phospholipids is one. The concentration of these fatty acids in the phospholipids reflects the recent intake of fatty acids before blood sampling [36] , but the content of fatty acids in serum phospholipids and in phospholipids of cell membranes are closely related [37] . Also, we cannot rule out the possibility that the patients dietary habits changed during the 12 months of observation. There might have been more clarity if measurement of n-3 PUFA levels at the beginning of the study had also been made. A comparison of the two n-3 PUFA values (start and 12 months data) would have revealed changes in the intake of n-3 PUFA. Furthermore, the concentration of n-3 PUFA could not be used to discriminate between zero and one or more episodes of ventricular arrhythmias probably due to a type 2 error, because only six patients had only one ventricular event. Another major limitation is the cross-sectional design, which precludes causal inference. Only 8% of the patients were women making it impossible to draw any conclusions regarding women.
In conclusion, we found that patients with a low content of n-3 PUFA in plasma had a significantly higher incidence of malignant ventricular arrhythmias compared with patients with high levels of n-3 PUFA. Thus, our data on patients with IHD and an ICD suggest that the protection offered by marine n-3 PUFA on SCD observed in previous observational studies and randomised trials may be caused by an antiarrhythmic effect of marine n-3 PUFA. Intervention trials with n-3 PUFA in ICD patients and in other patients at a high risk of SCD are warranted to confirm the present data.
The study was supported by a grant from Obels Family Foundation.



