-
PDF
- Split View
-
Views
-
Cite
Cite
Julia C. Senges-Becker, Martina Klostermann, Ruediger Becker, Alexander Bauer, Karl E. Siegler, Hugo A. Katus, Wolfgang Schoels, What is the “Optimal” follow-up schedule for ICD patients?, EP Europace, Volume 7, Issue 4, 2005, Pages 319–326, https://doi.org/10.1016/j.eupc.2005.02.117
Close - Share Icon Share
Abstract
In the absence of comparative studies, recommended routine follow-up (FU) intervals for implantable cardioverter defibrillator (ICD) patients range from 1 to 6 months; most patients are followed at 3 month intervals.
Six hundred and eighteen ICD patients were routinely seen 4 weeks after implant and then every 3 months. Unplanned visits (UPV) were either patient initiated or due to manufacturer recalls. FU visits included patient history/examination, ICD interrogation, pacing/sensing threshold and pacing/shock impedance. Chest X-rays were performed every 6 months. To validate FU interval recommendations, a comparative analysis on the detection of complications was performed, relying either on the information of every, or of every other FU visit, i.e., on 3 or 6 month intervals.
During 3.3 ± 2.8 years, 137 complications occurred in 110 patients (17%). However, identification of only 34% was dependent on the FU schedule, since the mode of detection was ICD interrogation in 38 and history/physical examination in nine patients. The remainder was diagnosed by UPV in 47, manufacturer recall in seven, accidental discovery during device replacement in two, and routine X-ray in 34 patients. Complication free survival at 2 years was 86.4% for patients implanted before 1999, and 89.2% thereafter ( P = 0.003). Regarding 6 rather than 3 month FU intervals, a theoretical maximum delay of 3 months in the detection of potentially life-threatening complications would have occurred in 1.7% of all patients. For those implanted after 1999, this related to only 0.9%.
ICD-related complications detected during routine FU visits are relatively rare, particularly with newer generation ICD systems. Thus, 6 month FU intervals appear to be safe. With new developments such as patient alert features and telemedical data transmission, FU intervals in ICD clinics might even be further extended.
Introduction
Routine follow-up (FU) of patients with implantable cardioverter defibrillators (ICDs) is routinely performed at short intervals due to safety concerns. Since the first ICD implantation in 1980, surgical approaches as well as device and electrode design have continuously been improved, resulting in a lower incidence of system-related complications accompanied by reduced perioperative mortality [1–7] . The majority of complications encountered during FU is lead-related, although the introduction of single electrode systems has apparently reduced their overall incidence [2,,5,,8–12] . Intensive FU during the first 3 months after ICD implantation seems indispensable, because complications such as lead dislodgment and system infection have been demonstrated to occur most commonly in this period [4,,13–16] . However, FU intervals employed thereafter tend to vary between centres, based on personal experience and intuition. So far, there are no comparative studies to substantiate the recommendation of any particular FU interval. Furthermore, the relative contribution of various FU modalities, such as history and physical examination, device interrogation, or chest X-ray, to the detection of device-related complications, is undetermined. Thus, the present study was designed to analyze whether 6 compared with 3 month FU intervals would have delayed the detection of system-related complications, and to determine the mode by which this detection occurred.
Methods
Study population
Data from all patients implanted with an ICD system between April 1986 and October 2001 and followed for at least 6 months at our institution were analyzed.
Follow-up schedule
After ICD implantation, all patients underwent a prehospital discharge test (PHD) including measurement of standard lead parameters (P/R wave amplitude, pacing/shocking lead impedance) and induction of ventricular fibrillation (VF). Routine outpatient FU was scheduled at 1 and 3 months postoperatively, and every 3 months thereafter ( Fig. 1 ). FU visits were scheduled every 4–6 weeks (intensive FU) once the battery voltage approached the elective replacement indicator (ERI). After device replacement and/or electrode revision, the FU schedule was restarted with the PHD. Unplanned visits (UPV) were patient initiated or due to manufacturer recall.
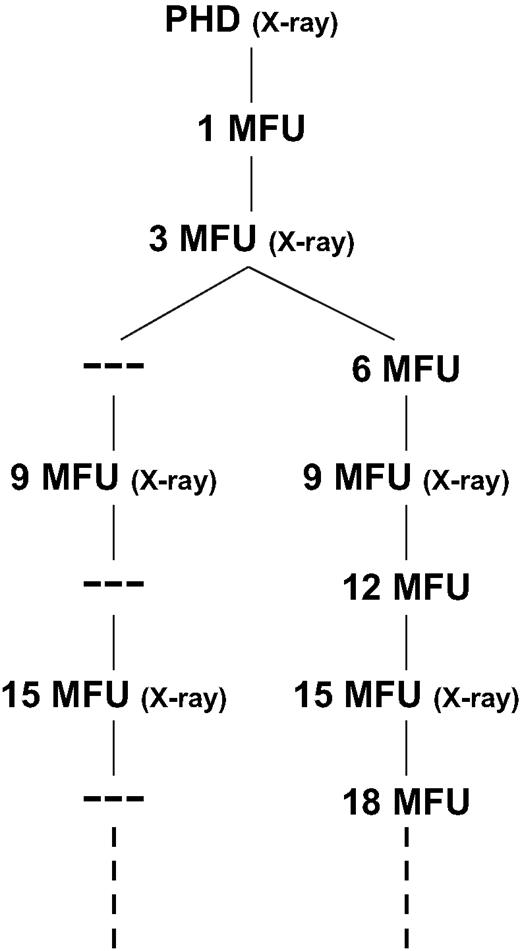
Actual 3 month and virtual 6 month follow-up schedules. PHD = prehospital discharge test.
Data acquired during routine 3 monthly follow-ups and during unplanned visits
At each visit, the following data were acquired: patient history (focusing on arrhythmia-related symptoms, ICD discharges and device-related problems), physical examination, 12-lead ECG, lead impedance test (pacing impedance, shock impedance if available), device interrogation/testing (episode data, battery voltage/charge time, P/R wave measurement, pacing threshold test), oversensing tests. Routine chest/abdominal X-rays were performed at 6 month intervals ( Fig. 1 ).
Data evaluation
Data obtained from routine 3 monthly FUs and from UPVs were analyzed with respect to incidence, timing and nature of ICD-related complications. All ICD-related complications were classified as to the mode of their detection. To assess the potential risk of 6 month FU intervals, data were reanalyzed based on the information provided by every other routine visit, i.e., 6 month intervals, and by UPVs ( Fig. 1 ). Complications detected at PHD, 1 and 3 month FU were excluded from the comparative analysis since close FU intervals in the early postoperative period are thought to be mandatory. The most recent device or lead implantation/revision served as a reference point to determine the timing of respective complications. A decrease in pacing impedance of 30% or more or a pacing impedance below 200–250 Ω was considered an insulation failure, a sudden increase in pacing impedance > 2000 Ω was categorized as lead fracture.
Statistics
Continuous values are given as mean ± SD. All data were analyzed with SAS for Windows (software release 8.2, Cary, North Carolina, USA). Comparisons between groups were performed using a Wilcoxon rank sum test for continuous variables and a χ 2 test for categorical data. A P value < 0.05 was considered statistically significant. Parameters associated with the occurrence of complications in univariate analysis were subjected to multivariate testing using a logistic regression model. To identify temporal variations in complication rates, the Cochran Armitage Trend Test was applied.
Results
Study population and follow-up
Clinical characteristics of the 618 study patients are detailed in Table 1 . Of 512 patients presenting with sinus rhythm, 160 had a history of paroxysmal atrial fibrillation. At hospital discharge, antiarrhythmic drugs were prescribed to 161 patients (26%). These were class I drugs in 20 patients, class III drugs in 134 patients and a combination of both in seven patients. During FU, another 101 patients received antiarrhythmic agents, whereas the initial regimen was changed in 44 patients. In total, 42% of all patients received concomitant antiarrhythmic medication at some time during FU. Mean FU duration was 3.3 ± 2.8 years (0.5–14.4, median 2.3 years).
Clinical and device-related characteristics of the study population and of patients with complications occurring after the 3 month FU
| . | All patients ( n = 618) . | Patients with complications > 3 month FU ( n = 110) . | P -value . |
|---|---|---|---|
| Age (years) | 60 ± 12.5 | 58 ± 12.9 | NS |
| Male | 505 (82%) | 88 (80%) | NS |
| EF (%) | 37.2 ± 13.2 | 38.3 ± 13.1 | NS |
| Underlying heart disease | NS | ||
| Coronary artery disease | 370 (59.9%) | 58 (52.7%) | |
| Idiopathic dilated cardiomyopathy | 133 (21.5%) | 26 (23.6%) | |
| Others | 69 (11.3%) | 18 (16.4%) | |
| No structural heart disease | 45 (7.3%) | 8 (7.3%) | |
| Prior myocardial infarction | 289 (47%) | 48 (43.6%) | NS |
| NYHA class | NS | ||
| I + II | 397 (64%) | 71 (64.5%) | |
| III + IV | 221 (36%) | 39 (35.5%) | |
| LV aneurysm | 97 (15.7%) | 14 (12.7%) | NS |
| Prior coronary bypass surgery | 107 (17.3%) | 17 (15.5%) | NS |
| Underlying rhythm | 0.004 | ||
| Sinus rhythm | 512 (83%) | 83 (75.6%) | |
| Atrial fibrillation | 50 (8%) | 8 (7.3%) | |
| Paced rhythm | 56 (9%) | 19 (17.3%) | |
| Concomitant antiarrhythmic drugs | |||
| At implantation | 161 (26%) | 43 (39%) | 0.001 |
| During FU a | 101 (16%) | 18 (16%) | NS |
| Indication for ICD implantation | 0.014 | ||
| Primary prevention | 132 (21.4%) | 14 (12.7%) | |
| Secondary prevention | 483 (78.6%) | 96 (87.3%) | |
| Unplanned visitsa | 258 (41.7%) | 63 (57.3%) | <0.001 |
| Device location | <0.001 | ||
| Pectoral | 481 (77.8%) | 59 (53.6%) | |
| Abdominal | 137 (22.2%) | 51 (46.4%) | |
| “Cold can” system | 126 (20.4%) | 47 (42.7%) | <0.001 |
| Date of implant | <0.001 | ||
| 1986–1995 | 217 (35.1%) | 67 (61%) | |
| 1996–1998 | 181 (29.3%) | 32 (29%) | |
| 1999–2002 | 220 (35.6%) | 11 (10%) |
| . | All patients ( n = 618) . | Patients with complications > 3 month FU ( n = 110) . | P -value . |
|---|---|---|---|
| Age (years) | 60 ± 12.5 | 58 ± 12.9 | NS |
| Male | 505 (82%) | 88 (80%) | NS |
| EF (%) | 37.2 ± 13.2 | 38.3 ± 13.1 | NS |
| Underlying heart disease | NS | ||
| Coronary artery disease | 370 (59.9%) | 58 (52.7%) | |
| Idiopathic dilated cardiomyopathy | 133 (21.5%) | 26 (23.6%) | |
| Others | 69 (11.3%) | 18 (16.4%) | |
| No structural heart disease | 45 (7.3%) | 8 (7.3%) | |
| Prior myocardial infarction | 289 (47%) | 48 (43.6%) | NS |
| NYHA class | NS | ||
| I + II | 397 (64%) | 71 (64.5%) | |
| III + IV | 221 (36%) | 39 (35.5%) | |
| LV aneurysm | 97 (15.7%) | 14 (12.7%) | NS |
| Prior coronary bypass surgery | 107 (17.3%) | 17 (15.5%) | NS |
| Underlying rhythm | 0.004 | ||
| Sinus rhythm | 512 (83%) | 83 (75.6%) | |
| Atrial fibrillation | 50 (8%) | 8 (7.3%) | |
| Paced rhythm | 56 (9%) | 19 (17.3%) | |
| Concomitant antiarrhythmic drugs | |||
| At implantation | 161 (26%) | 43 (39%) | 0.001 |
| During FU a | 101 (16%) | 18 (16%) | NS |
| Indication for ICD implantation | 0.014 | ||
| Primary prevention | 132 (21.4%) | 14 (12.7%) | |
| Secondary prevention | 483 (78.6%) | 96 (87.3%) | |
| Unplanned visitsa | 258 (41.7%) | 63 (57.3%) | <0.001 |
| Device location | <0.001 | ||
| Pectoral | 481 (77.8%) | 59 (53.6%) | |
| Abdominal | 137 (22.2%) | 51 (46.4%) | |
| “Cold can” system | 126 (20.4%) | 47 (42.7%) | <0.001 |
| Date of implant | <0.001 | ||
| 1986–1995 | 217 (35.1%) | 67 (61%) | |
| 1996–1998 | 181 (29.3%) | 32 (29%) | |
| 1999–2002 | 220 (35.6%) | 11 (10%) |
a Only unplanned visits/antiarrhythmic prescriptions prior to complications were evaluated.
Clinical and device-related characteristics of the study population and of patients with complications occurring after the 3 month FU
| . | All patients ( n = 618) . | Patients with complications > 3 month FU ( n = 110) . | P -value . |
|---|---|---|---|
| Age (years) | 60 ± 12.5 | 58 ± 12.9 | NS |
| Male | 505 (82%) | 88 (80%) | NS |
| EF (%) | 37.2 ± 13.2 | 38.3 ± 13.1 | NS |
| Underlying heart disease | NS | ||
| Coronary artery disease | 370 (59.9%) | 58 (52.7%) | |
| Idiopathic dilated cardiomyopathy | 133 (21.5%) | 26 (23.6%) | |
| Others | 69 (11.3%) | 18 (16.4%) | |
| No structural heart disease | 45 (7.3%) | 8 (7.3%) | |
| Prior myocardial infarction | 289 (47%) | 48 (43.6%) | NS |
| NYHA class | NS | ||
| I + II | 397 (64%) | 71 (64.5%) | |
| III + IV | 221 (36%) | 39 (35.5%) | |
| LV aneurysm | 97 (15.7%) | 14 (12.7%) | NS |
| Prior coronary bypass surgery | 107 (17.3%) | 17 (15.5%) | NS |
| Underlying rhythm | 0.004 | ||
| Sinus rhythm | 512 (83%) | 83 (75.6%) | |
| Atrial fibrillation | 50 (8%) | 8 (7.3%) | |
| Paced rhythm | 56 (9%) | 19 (17.3%) | |
| Concomitant antiarrhythmic drugs | |||
| At implantation | 161 (26%) | 43 (39%) | 0.001 |
| During FU a | 101 (16%) | 18 (16%) | NS |
| Indication for ICD implantation | 0.014 | ||
| Primary prevention | 132 (21.4%) | 14 (12.7%) | |
| Secondary prevention | 483 (78.6%) | 96 (87.3%) | |
| Unplanned visitsa | 258 (41.7%) | 63 (57.3%) | <0.001 |
| Device location | <0.001 | ||
| Pectoral | 481 (77.8%) | 59 (53.6%) | |
| Abdominal | 137 (22.2%) | 51 (46.4%) | |
| “Cold can” system | 126 (20.4%) | 47 (42.7%) | <0.001 |
| Date of implant | <0.001 | ||
| 1986–1995 | 217 (35.1%) | 67 (61%) | |
| 1996–1998 | 181 (29.3%) | 32 (29%) | |
| 1999–2002 | 220 (35.6%) | 11 (10%) |
| . | All patients ( n = 618) . | Patients with complications > 3 month FU ( n = 110) . | P -value . |
|---|---|---|---|
| Age (years) | 60 ± 12.5 | 58 ± 12.9 | NS |
| Male | 505 (82%) | 88 (80%) | NS |
| EF (%) | 37.2 ± 13.2 | 38.3 ± 13.1 | NS |
| Underlying heart disease | NS | ||
| Coronary artery disease | 370 (59.9%) | 58 (52.7%) | |
| Idiopathic dilated cardiomyopathy | 133 (21.5%) | 26 (23.6%) | |
| Others | 69 (11.3%) | 18 (16.4%) | |
| No structural heart disease | 45 (7.3%) | 8 (7.3%) | |
| Prior myocardial infarction | 289 (47%) | 48 (43.6%) | NS |
| NYHA class | NS | ||
| I + II | 397 (64%) | 71 (64.5%) | |
| III + IV | 221 (36%) | 39 (35.5%) | |
| LV aneurysm | 97 (15.7%) | 14 (12.7%) | NS |
| Prior coronary bypass surgery | 107 (17.3%) | 17 (15.5%) | NS |
| Underlying rhythm | 0.004 | ||
| Sinus rhythm | 512 (83%) | 83 (75.6%) | |
| Atrial fibrillation | 50 (8%) | 8 (7.3%) | |
| Paced rhythm | 56 (9%) | 19 (17.3%) | |
| Concomitant antiarrhythmic drugs | |||
| At implantation | 161 (26%) | 43 (39%) | 0.001 |
| During FU a | 101 (16%) | 18 (16%) | NS |
| Indication for ICD implantation | 0.014 | ||
| Primary prevention | 132 (21.4%) | 14 (12.7%) | |
| Secondary prevention | 483 (78.6%) | 96 (87.3%) | |
| Unplanned visitsa | 258 (41.7%) | 63 (57.3%) | <0.001 |
| Device location | <0.001 | ||
| Pectoral | 481 (77.8%) | 59 (53.6%) | |
| Abdominal | 137 (22.2%) | 51 (46.4%) | |
| “Cold can” system | 126 (20.4%) | 47 (42.7%) | <0.001 |
| Date of implant | <0.001 | ||
| 1986–1995 | 217 (35.1%) | 67 (61%) | |
| 1996–1998 | 181 (29.3%) | 32 (29%) | |
| 1999–2002 | 220 (35.6%) | 11 (10%) |
a Only unplanned visits/antiarrhythmic prescriptions prior to complications were evaluated.
Implanted devices
Due to 265 device replacements in 198 patients, the total number of implanted devices amounted to 883. ICDs were provided by the following manufacturers: Medtronic ( n = 672), ELA Medical ( n = 60), Biotronik ( n = 52), Guidant ( n = 53), Telectronics ( n = 40), St. Jude Medical ( n = 6). Seventeen percent of the patients received a dual chamber device ( n = 105), the proportion of “active/hot can” systems was 81% ( n = 713).
Occurrence, type and consequences of complications
After the 3 month FU, 137 complications occurred in 110 (17.8%) patients. The types of complications are specified in Table 2 . A single complication was evident in 88 patients, while two and three complications were observed in 17 and five patients, respectively. There was a 14.4% incidence of lead-related complications and a 5% incidence of device-related complications. The proportion of complications resulting in invasive measures was 80%. Complications defined as potentially life-threatening were lead fractures/dislocations/insulation defects, sudden pacing and/or sensing defects, rise in defibrillation threshold (with a safety margin <10 J), device dislodgments (only with “active/hot can” devices), device dysfunctions, pocket/system infections and right ventricular thrombi. Of all complications, 69% ( n = 94) were classified as potentially life-threatening.
Lead-related complications were detected after 38.2 ± 30.2 (3.6–130.4) months, device-related complications after 17.5 (3.2–50.0) months. The temporal distribution of complications ( n = 137) was as follows: 29.2% occurred within the first year, 54% within two, 65% within three and 96.4% within 8 years after implantation. Of note, most complications observed >24 months after implantation (47/63 = 74.6%) were the first to be detected in respective patients.
| Lead-related complications ( n = 105) . | . | Device-related complications ( n = 32) . | . |
|---|---|---|---|
| Lead fracture | n = 35 | Device dislodgment | n = 8 |
| Lead dislocation | n = 13 | Device dysfunction | n = 14 |
| Pacing and/or sensing defect | |||
| Gradual | n = 12 | ||
| Sudden | n = 5 | ||
| Insulation defect | n = 8 | ||
| Oversensing | n = 17 | ||
| Rise in DFT | n = 4 | ||
| Lead perforation | n = 2 | Device perforation | n = 2 |
| Patch pocket infection | n = 2 | ICD pocket infection | n = 3 |
| Pain | n = 1 | Pain | n = 4 |
| Haematoma/thrombosis | n = 1 | Haematoma | n = 1 |
| RV | n = 2 | ||
| Subclavian vein | n = 3 |
| Lead-related complications ( n = 105) . | . | Device-related complications ( n = 32) . | . |
|---|---|---|---|
| Lead fracture | n = 35 | Device dislodgment | n = 8 |
| Lead dislocation | n = 13 | Device dysfunction | n = 14 |
| Pacing and/or sensing defect | |||
| Gradual | n = 12 | ||
| Sudden | n = 5 | ||
| Insulation defect | n = 8 | ||
| Oversensing | n = 17 | ||
| Rise in DFT | n = 4 | ||
| Lead perforation | n = 2 | Device perforation | n = 2 |
| Patch pocket infection | n = 2 | ICD pocket infection | n = 3 |
| Pain | n = 1 | Pain | n = 4 |
| Haematoma/thrombosis | n = 1 | Haematoma | n = 1 |
| RV | n = 2 | ||
| Subclavian vein | n = 3 |
| Lead-related complications ( n = 105) . | . | Device-related complications ( n = 32) . | . |
|---|---|---|---|
| Lead fracture | n = 35 | Device dislodgment | n = 8 |
| Lead dislocation | n = 13 | Device dysfunction | n = 14 |
| Pacing and/or sensing defect | |||
| Gradual | n = 12 | ||
| Sudden | n = 5 | ||
| Insulation defect | n = 8 | ||
| Oversensing | n = 17 | ||
| Rise in DFT | n = 4 | ||
| Lead perforation | n = 2 | Device perforation | n = 2 |
| Patch pocket infection | n = 2 | ICD pocket infection | n = 3 |
| Pain | n = 1 | Pain | n = 4 |
| Haematoma/thrombosis | n = 1 | Haematoma | n = 1 |
| RV | n = 2 | ||
| Subclavian vein | n = 3 |
| Lead-related complications ( n = 105) . | . | Device-related complications ( n = 32) . | . |
|---|---|---|---|
| Lead fracture | n = 35 | Device dislodgment | n = 8 |
| Lead dislocation | n = 13 | Device dysfunction | n = 14 |
| Pacing and/or sensing defect | |||
| Gradual | n = 12 | ||
| Sudden | n = 5 | ||
| Insulation defect | n = 8 | ||
| Oversensing | n = 17 | ||
| Rise in DFT | n = 4 | ||
| Lead perforation | n = 2 | Device perforation | n = 2 |
| Patch pocket infection | n = 2 | ICD pocket infection | n = 3 |
| Pain | n = 1 | Pain | n = 4 |
| Haematoma/thrombosis | n = 1 | Haematoma | n = 1 |
| RV | n = 2 | ||
| Subclavian vein | n = 3 |
Characteristics of patients with/without complications
Univariate analysis revealed a relation between the occurrence of complications and the following parameters: underlying paced rhythm, presence of an antiarrhythmic drug regimen at the time of ICD implantation, secondary arrhythmia prevention as an ICD indication, presence of unplanned visits prior to detection of complications, early date of ICD implantation, abdominal device location and implantation of a “cold can” system ( Table 1 ). On multivariate analysis, only paced rhythm, unplanned visits and early implantation date were identified as independent predictors of complications ( Fig. 2 ). Clinical parameters like age, EF, NYHA class and others were equally distributed in both the groups.
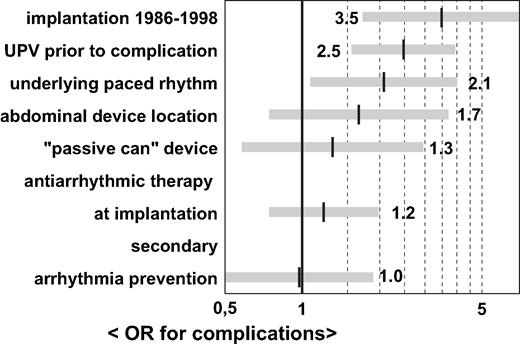
Odds ratios for the occurrence of complications. AA = antiarrhythmic medication.
Mode of detection of complications and relevance of follow-up schedule
The mode of detection for all 137 complications was as follows: UPVs, n = 47; manufacturer recall, n = 7; lead defect accidentally discovered during device replacement, n = 2; routine 6 monthly X-ray, n = 34; device interrogation, n = 38 and patient history/physical examination, n = 9. Thus, the routinely scheduled physical presence of the patient at the ICD clinic was of relevance in 81 of 137 cases (59%). Given the options for transtelephonic or electronic transmission of respective data (device interrogation, X-ray), this number could be as low as nine of 137 cases (6.6%). The point in time at which complications were detected was independent of the routine FU schedule in 66% of all cases (UPVs, manufacturer recall, device replacement, routine X-ray). Only for those 47 (34%) complications revealed by routine device interrogation or patient history/physical examination, the FU schedule was crucial for the timing of their detection.
Detection of complications dependent on follow-up schedule – 3 vs. 6 month FU
Assuming 6 month FU intervals, in fact 21 of 137 complications (15%) would have been detected with a theoretical maximum delay of 3 months. This includes 11 complications in 11 patients (1.7%, 11/618) classified as potentially life-threatening ( Fig. 3 ). In Fig. 4 the actual 3 month FU schedule is compared with a virtual 6 month FU schedule referring exclusively to complications with the timing of their detection dependent on the FU intervals ( n = 47). In the graph, each step between two 6 month intervals represents delayed detection of complications.
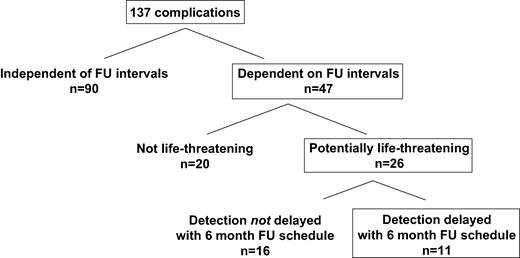
Complications with their detection dependent on the FU schedule: clinical significance and potential delay in detection with 6 month FU intervals.
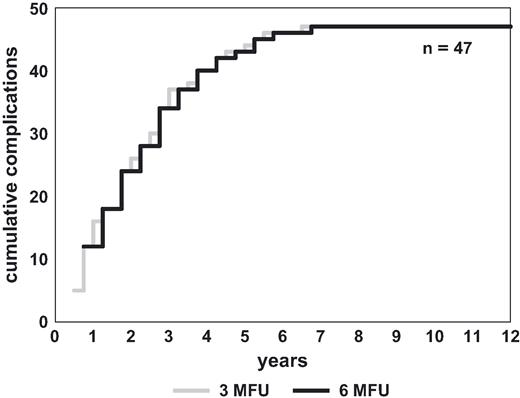
Complications (>3 month FU) with their detection dependent on FU schedule: actual detection with 3 month FU schedule and expected detection with 6 month FU schedule.
Battery depletion
Of 196 device replacements that were due to battery depletion, 125 (64%) had been preceded by an intensive FU. Mean interval between initiation of the intensified FU and actually reaching ERI was 15.8 ± 6.0 months (4.0–30.8). Assuming a 6 month FU schedule, the detection of ERI would have been delayed in four patients. To avoid a sudden loss of function, battery longevity of at least 3 months is warranted by the manufacturers after reaching ERI, assuming delivery of one shock per month at maximum energy (shock lead impedance, 50 Ω) and 100% pacing at standard output (DIN prEN 45502-2-2; Beuth Verlag GmbH 1998). Adding the shortest intensive FU duration observed (4 months) to a remaining longevity of at least 3 months after reaching ERI, none of the patients would have been affected by a potential loss of function within an FU interval of 6 months.
Discussion
To the best of our knowledge, this is the first study to systematically address the issue of routine FU intervals in ICD patients. During long-term FU (3.3 ± 2.8 years) in a large cohort ( n = 618), a 17.8% incidence of late complications (>3 months) was observed, the majority being lead-related (77%). However, detection of only 34% (47/137) of all complications was dependent on the FU schedule, whereas the remainder was detected either by routine chest X-rays (6 monthly), UPVs or manufacturer recall. With 6 instead of 3 month FU intervals, the detection of potentially life-threatening complications would have possibly been delayed in 1.7% of all patients studied (11/618; Fig. 3 ). In keeping with a reduced incidence of complications (5 vs. 25%, 1999–2001 vs. 1986–1998), this related to only 0.9% (2/220) of patients implanted with an ICD after 1998.
Comparison with previous studies
Comparison of complication rates and occurrence of complications
Overall complication rates published since 1985 range from 3.9% to 53% [2,,4,,7,,13,,17–22] , with lead-related complications varying between 2.1% and 22% [2,,7–13,,17–20] . The present study registered all complications occurring during long-term FU in a large patient cohort, documenting an overall complication rate of 29.4% during a 3.3 ± 2.8 year FU. However, for the comparative analysis of FU intervals, only those complications occurring after the 3 month FU were considered. Due to clustering of complications in the early postoperative period, a close FU schedule seems mandatory for the first 3 months after implant [4,,12,,14–16,,18] . Supporting this notion, the present study found 48.3% of all complications to occur within the first 3 months (128/265). On the other hand, more than half of all complications may occur at any time during long-term FU, as shown in this and in previous studies [8,,11,,21,,23–25] . Therefore, any extension of subsequent FU intervals carries a small but definite risk of delayed detection of late complications.
Relation between clinical/device-related parameters and complication rate
Previous studies observed higher complication rates in patients with an early implantation date, abdominal device location [4,,5,,12] and “cold can” systems [3] . However, most studies were unable to identify clinical parameters positively correlated with the occurrence of system-related complications [6,,12,,14,,18,,26,,27] . In the present study, univariate testing suggested an association between seven parameters and higher complication rates. However, with multivariate analysis this association only held true for the variables paced rhythm, unplanned visits and early implantation date, since abdominal device location, “cold can” systems, antiarrhythmic therapy at implant and secondary arrhythmia prevention were all significantly more common in patients implanted between 1986–1998.
FU schedule in previous studies
A comparison of FU schedules applied in 29 ICD complication studies published between 1985 and 2003 reveals that the majority of centres ( n = 16) chose 3 month intervals [1,,3,,4,,8,,11,,12,,13,,14,,18,,20,,22,,24,,25,,28,,29] , whereas others used either a 1–2 month FU ( n = 1) [18] , 1–4 month FU ( n = 1) [7] , 2 month FU ( n = 3) [6,,16,,27] , 2–3 month FU ( n = 5) [9,,10,,15,,19,,26] , 2–6 month FU ( n = 1) [2] or 3–6 month FU ( n = 2) [2,,26] schedule. This might reflect differences in expert opinion on the safety issue. In addition, the choice of an FU schedule may also be influenced by feasibility aspects, which depend on local circumstances and peculiarities of different health care systems.
FU schedule suggested by official guidelines and ICD manufacturers
All manufacturers of devices employed in this study recommend 3 month FU intervals. Official guidelines issued by various national cardiological societies have proposed routine intervals between 1 and 6 months [30–33] . However, in the absence of comparative studies, these recommendations are solely based on expert consensus.
Mode of detection
Apart from clinical symptoms leading to unplanned visits, the majority of complications were detected by routine X-ray or by device interrogation. Given all telemedical options of data transmission, neither X-ray nor device interrogation would require the physical presence of a patient at an ICD clinic. Thus, it is quite conceivable that with transtelephonic or other forms of home monitoring, transmission of locally performed X-ray scans, intervals for physical FU visits at an ICD clinic could be further extended.
Battery depletion
Even with a 6 month FU schedule, none of the patients in this study would have been affected by a potential loss of function due to battery depletion. Nevertheless, if routine FU is performed at 6 month intervals, intensified FU should be implemented once the battery voltage approaches ERI, depending on the projected remaining battery longevity.
Conclusions and future directions
Except for the first 3 months after implantation, 6 months FU intervals should be safe with single-lead pectoral devices, since a delay in the detection of serious complications with a 6 instead of a 3 month schedule appears to be very rare in systems implanted after 1998. Since complications may occur at any time during FU, regular ICD check-ups should be maintained at predefined intervals even in patients with an uncomplicated course over years. New safety features such as automatic measurements of lead and battery parameters in combination with a patient alert function [34] should enhance early detection of system-related complications and with telemedical options of data transmission, FU intervals might even be further extended.



