-
PDF
- Split View
-
Views
-
Cite
Cite
A. Nabar, C. Timmermans, A. Medeiros, K. Polymeropoulous, H.J.G.M. Crijns, L.M. Rodriguez, Radiofrequency ablation of atrial arrhythmias after previous open-heart surgery, EP Europace, Volume 7, Issue 1, 2005, Pages 40–49, https://doi.org/10.1016/j.eupc.2004.09.009
Close - Share Icon Share
Abstract
To report the results of ablation of atrial arrhythmias (AA) after previous open-heart surgery.
Nineteen patients [50 ± 11 years, 11 women] underwent ablation of symptomatic AAs after previous open-heart surgery. In 11 patients mapping was performed using conventional multielectrode catheters. In the other eight patients CARTO electro-anatomical mapping system was used to supplement conventional mapping.
After conventional mapping, 10/11 patients (91%) were found to have typical atrial flutter (AFL). The cavotricuspid isthmus was successfully ablated in these 10 patients. CARTO combined with conventional mapping showed that 7 of 8 patients had one macro-reentry right atrial circuit. The remaining patient had two focal atrial tachycardias. CARTO-guided ablation was successful in all eight patients (100%). After follow-up of 12 ± 11 months, 2/18 patients (11%) had recurrence of either the same (n = 1) or a new (n = 1) AA.
AAs after previous open-heart surgery can be ablated successfully (>90%) with a low recurrence rate (11%) at 1-year follow-up. Typical AFL was found frequently (14/19 patients, 72%). This could be ablated successfully, often, after conventional mapping alone. CARTO helps to uncover peri-scar reentry and guide the ablation by creating a line of block connecting the scar to another landmark (unconventional isthmus).
Introduction
Various right atrial macro-reentrant circuits such as counterclockwise and clockwise atrial flutter (AFL) along the tricuspid annulus (TA) [1], lower-loop and upper-loop reentry around the vena cavae [2], and right atrial free wall flutter [3] have been mapped in the clinical electrophysiological laboratory using conventional multielectrode catheters. Reentry around an atriotomy scar, prosthetic material such as an atrial septal patch, or simply along the TA has been observed in patients developing atrial arrhythmias (AA) late after open-heart surgery [4–,12]. Double-loop reentry implies a circuit that is simultaneously around two anatomical barriers and shares a common trajectory in a part of the circuit [7–,13]. CARTO electro-anatomical mapping can precisely outline the reentrant circuit in relation to a post-surgical scar and other anatomical barriers [7–,9,,11–,13]. Akar et al. found multiple reentry circuits coexisting and concluded that the surface ECG failed to provide adequate discrimination [10]. Recent studies support that AAs can be terminated by ablating linearly (between the scar and the nearest anatomical barrier) [4,,5,,9–,12] or focally (in the “return channel” within the scar) [7,,8] across the reentrant pathway. We sought to report the results of radiofrequency ablation of AAs after previous open-heart surgery.
Methods
Patient population
The present study is a retrospective analysis of 19 patients who underwent an electrophysiological study and radiofrequency ablation of symptomatic AAs after previous open-heart surgery [14 ± 12 years (range 6 months–40 years)], see Table 1. During the study period, extending from June 1994 to November 2001, 267 other patients in our institution underwent ablation of a typical AFL. CARTO electro-anatomical mapping system was available only during the latter part of this study. The mean age of our study population was 50 ± 11 years (range 21–61 years) and included 11 women. Eight patients had undergone repair/replacement of mitral (n = 5), aortic (n = 1) or both (n = 2) heart valves. Surgical closure of the atrial septum was performed for a congenital defect in eight patients and after the excision of a left atrial myxoma in one patient. One of these latter eight patients, known to have pre-operative AFL, during the surgical closure of atrial septal defect had undergone linear cryoablation between the lower end of the right atriotomy and the inferior vena cava. One patient underwent a staged-correction of Ebstein's anomaly and Rastelli's repair was performed for d-transposition of great arteries (with ventricular septal defect and pulmonary stenosis) in one other patient. Mean age at the time of the open-heart surgery was 37 ± 16 years (range 11–56 years).
| Cardiac surgery . | Patients (n) . | Remarks . |
|---|---|---|
| Valvular | 8 | |
| (1) Mitral VP | 1 | With tricuspid VP |
| (2) Mitral VR | 4 | With tricuspid VP (1), prior CMC (3) |
| (3) Aortic VR | 1 | |
| (4) OMC + aortic VP | 1 | |
| (5) Mitral VR + aortic VP | 1 | |
| Atrial septal defect closure | 9 | |
| (1) Direct | 5 | With cryoablation, from atriotomy to IVC (1) |
| (2) Patch | 2 | With left atrial myxoma excision (1) |
| (3) Not known | 2 | |
| Corrective repair of CCHD | 2 | |
| (1) Ebstein's anomaly | 1 | Staged: first atrial septal defect closure, second tricuspid VP |
| (2) d-TGA, VSD, PS | 1 | Rastelli's repair. Prior Blalock-Taussig shunt |
| Cardiac surgery . | Patients (n) . | Remarks . |
|---|---|---|
| Valvular | 8 | |
| (1) Mitral VP | 1 | With tricuspid VP |
| (2) Mitral VR | 4 | With tricuspid VP (1), prior CMC (3) |
| (3) Aortic VR | 1 | |
| (4) OMC + aortic VP | 1 | |
| (5) Mitral VR + aortic VP | 1 | |
| Atrial septal defect closure | 9 | |
| (1) Direct | 5 | With cryoablation, from atriotomy to IVC (1) |
| (2) Patch | 2 | With left atrial myxoma excision (1) |
| (3) Not known | 2 | |
| Corrective repair of CCHD | 2 | |
| (1) Ebstein's anomaly | 1 | Staged: first atrial septal defect closure, second tricuspid VP |
| (2) d-TGA, VSD, PS | 1 | Rastelli's repair. Prior Blalock-Taussig shunt |
CMC, closed mitral commissurotomy; CCHD, complex congenital heart disease; d-TGA, VSD, PS, d-transposition of great arteries with ventricular septal defect and pulmonary stenosis; OMC, open mitral commissurotomy, VP, valvuloplasty; VR, valve replacement.
| Cardiac surgery . | Patients (n) . | Remarks . |
|---|---|---|
| Valvular | 8 | |
| (1) Mitral VP | 1 | With tricuspid VP |
| (2) Mitral VR | 4 | With tricuspid VP (1), prior CMC (3) |
| (3) Aortic VR | 1 | |
| (4) OMC + aortic VP | 1 | |
| (5) Mitral VR + aortic VP | 1 | |
| Atrial septal defect closure | 9 | |
| (1) Direct | 5 | With cryoablation, from atriotomy to IVC (1) |
| (2) Patch | 2 | With left atrial myxoma excision (1) |
| (3) Not known | 2 | |
| Corrective repair of CCHD | 2 | |
| (1) Ebstein's anomaly | 1 | Staged: first atrial septal defect closure, second tricuspid VP |
| (2) d-TGA, VSD, PS | 1 | Rastelli's repair. Prior Blalock-Taussig shunt |
| Cardiac surgery . | Patients (n) . | Remarks . |
|---|---|---|
| Valvular | 8 | |
| (1) Mitral VP | 1 | With tricuspid VP |
| (2) Mitral VR | 4 | With tricuspid VP (1), prior CMC (3) |
| (3) Aortic VR | 1 | |
| (4) OMC + aortic VP | 1 | |
| (5) Mitral VR + aortic VP | 1 | |
| Atrial septal defect closure | 9 | |
| (1) Direct | 5 | With cryoablation, from atriotomy to IVC (1) |
| (2) Patch | 2 | With left atrial myxoma excision (1) |
| (3) Not known | 2 | |
| Corrective repair of CCHD | 2 | |
| (1) Ebstein's anomaly | 1 | Staged: first atrial septal defect closure, second tricuspid VP |
| (2) d-TGA, VSD, PS | 1 | Rastelli's repair. Prior Blalock-Taussig shunt |
CMC, closed mitral commissurotomy; CCHD, complex congenital heart disease; d-TGA, VSD, PS, d-transposition of great arteries with ventricular septal defect and pulmonary stenosis; OMC, open mitral commissurotomy, VP, valvuloplasty; VR, valve replacement.
Clinical profile of the documented AAs
In the 19 patients included, 25 AAs were clinically documented. Six patients had two AAs. Electrocardiographically, atrial tachycardia (AT) is recognized by discrete P waves separated by a clearly defined isoelectric baseline, while an ECG of AFL shows a characteristic “saw tooth” pattern or broad positive deflections depending on the direction of rotation (respectively, counterclockwise or clockwise along the TA) of the typical AFL circuit [1,,14,,15]. Based on these ECG criteria, 18 of the 25 clinically documented AAs were classified as AFL and the remaining seven as AT. In 13 patients, AAs were documented for the first time, 6 years (median, range 3–33 years) after the index surgery. AAs were known pre-operatively (n = 4) or were treated since the early post-operative period (n = 2) in the remaining six patients. More than half of the patients (12/19 patients, 63%) had associated atrial fibrillation. AAs were resistant to 3 ± 1.3 (range 1–5) anti-arrhythmic drugs, including amiodarone in 11 patients. In 13 patients, prior to considering ablation, various non-pharmacological options, including external and/or internal cardioversion, Atrioverter device (one patient), or AV-node ablation and dual chamber pacemaker implantation (one patient), were tried.
Electrophysiological study
Informed consent was obtained and all procedures were performed in a post-absorptive state. Anti-arrhythmic drugs were not stopped prior to the study. AAs that were incessant at the time of the study or those that were induced during the study and electrocardiographically found to be identical to the one previously documented were mapped and targeted for ablation. AAs were mapped using conventional techniques in 11 patients. Conventional mapping involved the use of a duodecapolar Halo catheter placed along the TA, a quadripolar catheter in the His bundle position and a decapolar catheter introduced in the coronary sinus and placed so that its proximal bipole was located at the ostium. CARTO electro-anatomical mapping (Biosense-Cordis-Webster) [7–,9,,11,,12,,15,,16] was used to supplement conventional mapping in the remaining eight patients. The right atrium was mapped by acquiring a mean of 148 ± 85 (59–190) points. The mean volume of the right atrial map was 129 ± 42 (42–175) ml. Macro-reentry was diagnosed when atrial activation times were sequentially distributed over the arrhythmia cycle length in a circular manner. The slow conducting part of the circuit was identified retrospectively. A focal mechanism was diagnosed when atrial activation spread from the site of earliest activity in a radial manner. Atrial sites recording double potentials, fractionated electrograms and/or a bipolar voltage ≤0.1–0.3 mV were empirically designated as scar [8,,11]. The left atrium was not mapped.
Radiofrequency ablation
“Conventional” cavotricuspid isthmus (posterior; TA-inferior vena cava or septal; TA-coronary sinus) was ablated in patients with a typical AFL. Bi-directional isthmus conduction block and noninduciblity during isoprenaline infusion (1–3 μg/min) was judged as a procedural success [17]. Linear ablation across the peri-scar reentrant pathway, connecting the scar to an anatomical barrier – “unconventional” isthmus, was performed using the CARTO system [5]. Such an unconventional isthmus was targeted, in some patients with typical AFL, if the flutter had recurred after the ablation of conventional cavotricuspid isthmus during a previous procedure. Each radiofrequency pulse was delivered for 90 s with the temperature cut-off preset at 70 °C. After ablation, if the targeted AA was not inducible, CARTO mapping was repeated to verify conduction block across the ablation line.
Follow-up
Follow-up was conducted at the arrhythmia clinic, 8 weeks after the procedure and at 3-monthly intervals thereafter, or was obtained from the referring cardiologist at the end of the study. Eight patients who had coexisting atrial fibrillation continued to take anti-arrhythmic drugs. Additional ECGs and Holter studies were obtained when patients had complaints suggestive of arrhythmia recurrence.
Statistical analysis
All data were expressed as mean ± SD, median and range.
Results
Electrophysiological study and radiofrequency ablation using conventional mapping alone (n = 11)
The ECG suggested AFL in 10 patients, while an AT was considered in the remaining one patient (Table 2). The mean cycle length was 288 ± 44 ms (range 210–340 ms). Conventional mapping in the former 10 patients showed sequential activation along the TA spanning a large part (>80%) of the flutter cycle length. Activation along the TA was counterclockwise in eight patients and was associated with negative flutter waves in the inferior leads; and clockwise in two patients with positive flutter waves in the same leads. Ablation of the cavotricuspid isthmus was successful in all the 10 patients. In the remaining one patient, in whom the ECG suggested an AT, the polarity of the P wave was positive in the inferior leads and in lead V1 and negative in leads I and aVL. Further, based on conventional mapping, the estimated biatrial activation time was 70 ms. This was only 21% of the tachycardia cycle length (330 ms). By inference, these findings suggest that, this arrhythmia could have been a focal AT arising from the left atrium. Ablation was not successful in this patient.
Results of radiofrequency ablation after conventional mapping in 11 patients
| Radiofrequency ablation during | |
| AFL | 3 |
| Coronary sinus pacing | 2 |
| Both | 6 |
| Ablated cavotricuspid isthmus | |
| Posterior | 8 |
| Septal | 1 |
| Both | 2 |
| Ablation catheter used | |
| 4 mm-tip | 2 |
| 8 mm-tip | 6 |
| Both | 3 |
| Radiofrequency pulses delivered | 20 (8) (range 6–35) |
| Procedural success | 10/11 (91%) |
| Radiofrequency ablation during | |
| AFL | 3 |
| Coronary sinus pacing | 2 |
| Both | 6 |
| Ablated cavotricuspid isthmus | |
| Posterior | 8 |
| Septal | 1 |
| Both | 2 |
| Ablation catheter used | |
| 4 mm-tip | 2 |
| 8 mm-tip | 6 |
| Both | 3 |
| Radiofrequency pulses delivered | 20 (8) (range 6–35) |
| Procedural success | 10/11 (91%) |
Results of radiofrequency ablation after conventional mapping in 11 patients
| Radiofrequency ablation during | |
| AFL | 3 |
| Coronary sinus pacing | 2 |
| Both | 6 |
| Ablated cavotricuspid isthmus | |
| Posterior | 8 |
| Septal | 1 |
| Both | 2 |
| Ablation catheter used | |
| 4 mm-tip | 2 |
| 8 mm-tip | 6 |
| Both | 3 |
| Radiofrequency pulses delivered | 20 (8) (range 6–35) |
| Procedural success | 10/11 (91%) |
| Radiofrequency ablation during | |
| AFL | 3 |
| Coronary sinus pacing | 2 |
| Both | 6 |
| Ablated cavotricuspid isthmus | |
| Posterior | 8 |
| Septal | 1 |
| Both | 2 |
| Ablation catheter used | |
| 4 mm-tip | 2 |
| 8 mm-tip | 6 |
| Both | 3 |
| Radiofrequency pulses delivered | 20 (8) (range 6–35) |
| Procedural success | 10/11 (91%) |
Electrophysiological study and RF ablation using conventional and CARTO electro-anatomical mapping (n = 8)
The 12-lead ECG suggested AT in five patients and AFL in the remaining three patients (Table 3). The mean cycle length was longer, 351 ± 87 ms (range 240–550 ms). In patients 1–4, the CARTO system was used for mapping an AA that had recurred after the ablation of cavotricuspid isthmus during a previous procedure. In two of these patients, the ECG morphology of the recurrent AA was different from the one previously ablated. Previous cavotricuspid isthmus ablation being successful in only one of these two patients. In the remaining two patients, the ECG morphology of the recurrent AA was identical to the one previously ablated. One patient had recurrence despite a previously successful cavotricuspid isthmus ablation, while ablation had failed to terminate the tachycardia in the second patient. Inadequate arrhythmia localization with conventional multielectrode catheters was the cause of failed ablation. CARTO mapping system was used de novo in patients 5–8.
Results of radiofrequency ablation after conventional and CARTO electro-anatomical mapping in eight patients
| No . | ECG diagnosis . | P or F waves in leads II, III, aVF . | Cycle length (ms) . | Conventional mapping – along the Halo catheter . | CARTO electro-anatomical mapping of the right atrium . | |||
|---|---|---|---|---|---|---|---|---|
| Location of the scar . | Location of the reentry circuit . | Slowest part of the circuit (velocity, m/s) . | Length of the ablated isthmus (length, cm) . | |||||
| 1 | AT | Positive | 350 | Clockwise | Superior–anterior–inferior | Clockwise – along the TA | TA–IVC (0.4) | Atriotomy–IVC (2.6) |
| 2 | AT | Positive | 335 | Counterclockwise | Atriotomy, septal | Only voltage map | – | Atriotomy–TA (4.3) |
| 3 | AT | Positive | 330 | Counterclockwise | Septal | Septal | Inferior to the scar (0.2) | SVC–scar (3.7) |
| 4a | AFL | II (Positive), III (negative) | 240 | Counterclockwise | Septal | Septal | Scar–TA (0.3) | SVC–scar (3.4) |
| 5 | AT | Positive | 330 | Clockwise | Septal | Clockwise – along the TA | Anterior (0.4) | TA–IVC (2.4) |
| 6 | AFL | Positive | 320 | Clockwise | Atriotomy | 2 – loops | Septal (0.3) | Scar–TA (2.1) |
| 7b | AFL | Negative | 280 | Both | Septal, posterolateral | Only voltage map | Intra-scar (0.2) | TA– IVC(1.5) |
| 8 | AT1, AT2 | Positive, undetermined | 550, 400 | Bi-directional, bi-directional | Septal, posterolateral | Focal, focal | – | High anterior, low posterior |
| No . | ECG diagnosis . | P or F waves in leads II, III, aVF . | Cycle length (ms) . | Conventional mapping – along the Halo catheter . | CARTO electro-anatomical mapping of the right atrium . | |||
|---|---|---|---|---|---|---|---|---|
| Location of the scar . | Location of the reentry circuit . | Slowest part of the circuit (velocity, m/s) . | Length of the ablated isthmus (length, cm) . | |||||
| 1 | AT | Positive | 350 | Clockwise | Superior–anterior–inferior | Clockwise – along the TA | TA–IVC (0.4) | Atriotomy–IVC (2.6) |
| 2 | AT | Positive | 335 | Counterclockwise | Atriotomy, septal | Only voltage map | – | Atriotomy–TA (4.3) |
| 3 | AT | Positive | 330 | Counterclockwise | Septal | Septal | Inferior to the scar (0.2) | SVC–scar (3.7) |
| 4a | AFL | II (Positive), III (negative) | 240 | Counterclockwise | Septal | Septal | Scar–TA (0.3) | SVC–scar (3.4) |
| 5 | AT | Positive | 330 | Clockwise | Septal | Clockwise – along the TA | Anterior (0.4) | TA–IVC (2.4) |
| 6 | AFL | Positive | 320 | Clockwise | Atriotomy | 2 – loops | Septal (0.3) | Scar–TA (2.1) |
| 7b | AFL | Negative | 280 | Both | Septal, posterolateral | Only voltage map | Intra-scar (0.2) | TA– IVC(1.5) |
| 8 | AT1, AT2 | Positive, undetermined | 550, 400 | Bi-directional, bi-directional | Septal, posterolateral | Focal, focal | – | High anterior, low posterior |
IVC, inferior vena cava; SVC, superior vena cava. All ablation procedures were successful.
aPatient 4 underwent two CARTO procedures.
bIn patient 7, during the electrophysiological study, the rotation of para-tricuspid AFL altered between CW and CCW.
Results of radiofrequency ablation after conventional and CARTO electro-anatomical mapping in eight patients
| No . | ECG diagnosis . | P or F waves in leads II, III, aVF . | Cycle length (ms) . | Conventional mapping – along the Halo catheter . | CARTO electro-anatomical mapping of the right atrium . | |||
|---|---|---|---|---|---|---|---|---|
| Location of the scar . | Location of the reentry circuit . | Slowest part of the circuit (velocity, m/s) . | Length of the ablated isthmus (length, cm) . | |||||
| 1 | AT | Positive | 350 | Clockwise | Superior–anterior–inferior | Clockwise – along the TA | TA–IVC (0.4) | Atriotomy–IVC (2.6) |
| 2 | AT | Positive | 335 | Counterclockwise | Atriotomy, septal | Only voltage map | – | Atriotomy–TA (4.3) |
| 3 | AT | Positive | 330 | Counterclockwise | Septal | Septal | Inferior to the scar (0.2) | SVC–scar (3.7) |
| 4a | AFL | II (Positive), III (negative) | 240 | Counterclockwise | Septal | Septal | Scar–TA (0.3) | SVC–scar (3.4) |
| 5 | AT | Positive | 330 | Clockwise | Septal | Clockwise – along the TA | Anterior (0.4) | TA–IVC (2.4) |
| 6 | AFL | Positive | 320 | Clockwise | Atriotomy | 2 – loops | Septal (0.3) | Scar–TA (2.1) |
| 7b | AFL | Negative | 280 | Both | Septal, posterolateral | Only voltage map | Intra-scar (0.2) | TA– IVC(1.5) |
| 8 | AT1, AT2 | Positive, undetermined | 550, 400 | Bi-directional, bi-directional | Septal, posterolateral | Focal, focal | – | High anterior, low posterior |
| No . | ECG diagnosis . | P or F waves in leads II, III, aVF . | Cycle length (ms) . | Conventional mapping – along the Halo catheter . | CARTO electro-anatomical mapping of the right atrium . | |||
|---|---|---|---|---|---|---|---|---|
| Location of the scar . | Location of the reentry circuit . | Slowest part of the circuit (velocity, m/s) . | Length of the ablated isthmus (length, cm) . | |||||
| 1 | AT | Positive | 350 | Clockwise | Superior–anterior–inferior | Clockwise – along the TA | TA–IVC (0.4) | Atriotomy–IVC (2.6) |
| 2 | AT | Positive | 335 | Counterclockwise | Atriotomy, septal | Only voltage map | – | Atriotomy–TA (4.3) |
| 3 | AT | Positive | 330 | Counterclockwise | Septal | Septal | Inferior to the scar (0.2) | SVC–scar (3.7) |
| 4a | AFL | II (Positive), III (negative) | 240 | Counterclockwise | Septal | Septal | Scar–TA (0.3) | SVC–scar (3.4) |
| 5 | AT | Positive | 330 | Clockwise | Septal | Clockwise – along the TA | Anterior (0.4) | TA–IVC (2.4) |
| 6 | AFL | Positive | 320 | Clockwise | Atriotomy | 2 – loops | Septal (0.3) | Scar–TA (2.1) |
| 7b | AFL | Negative | 280 | Both | Septal, posterolateral | Only voltage map | Intra-scar (0.2) | TA– IVC(1.5) |
| 8 | AT1, AT2 | Positive, undetermined | 550, 400 | Bi-directional, bi-directional | Septal, posterolateral | Focal, focal | – | High anterior, low posterior |
IVC, inferior vena cava; SVC, superior vena cava. All ablation procedures were successful.
aPatient 4 underwent two CARTO procedures.
bIn patient 7, during the electrophysiological study, the rotation of para-tricuspid AFL altered between CW and CCW.
After combined conventional and CARTO mapping, seven out of eight patients were found to have one macro-reentrant circuit in the right atrium. The macro-reentrant circuit was delineated almost completely (92% ± 11%) in five of the seven patients. A clockwise typical AFL and a circuit around the septal scar were found in two patients each. A double-loop reentry, simultaneously around the lateral right atriotomy scar and the TA, was recognized in one patient, see Figs. 1–3. In the remaining two patients only a voltage map, during sinus rhythm, could be constructed. In both these patients, the diagnosis of typical AFL was based on conventional mapping.
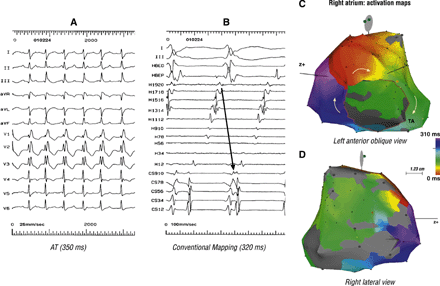
Mapping was performed using conventional and CARTO electro-anatomical mapping system in a 44 year-old woman who had undergone staged repair of Ebstein's anomaly. The ECG in panel A suggests AT. However, conventional mapping (in panel B), showed a clockwise reentry along the TA. Panel C, a solid isochronal activation map of the right atrium, confirms the clockwise typical AFL. Panel D shows an extensive scar (grey coloured) extending along the superior–anterior–inferior right atrium, forming the posterior boundary of the typical AFL circuit.
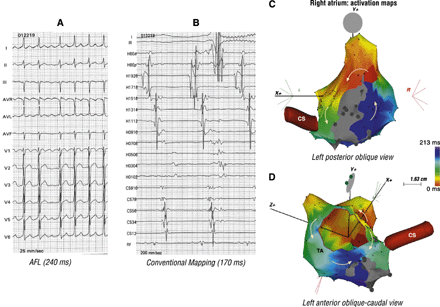
This 44 year-old man underwent patch closure of the septal defect resulting after excision of a left atrial myxoma. Panel A suggests AFL with positive flutter waves in lead II and negative in lead III. Panel B shows counterclockwise activation along the TA. The bipole CS 9–10 is not activated in tandem with the Halo catheter and double potentials, as a result of previous ablation, were recorded on the cavotricuspid isthmus (bipole RF). These findings suggest a bystander counterclockwise activation along the TA. Panel C confirms reentry around the septal scar. Activation along the TA, seen in panel D, via two wave fronts that collide in the posterior isthmus at the site of previously obtained bi-directional block is bystander.
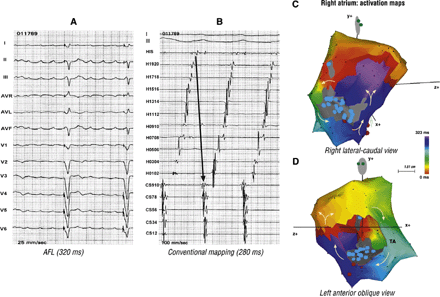
Atrial septal defect was closed directly in this 44 year-old woman. She had previously undergone His ablation followed by pacemaker implantation because of recurrent AAs. Panel A suggests AFL with positive flutter waves in the inferior leads. Conventional mapping, in panel B, might suggest clockwise typical AFL. Panel C and D, seen together, confirm double-loop reentry as the mechanism of this AA. The first loop is around the right lateral atriotomy scar and the second is along the TA in a clockwise direction. An unconventional isthmus, between the atriotomy scar and the TA is shared by both the loops.
Ablation was performed during sinus rhythm in five patients. An unconventional isthmus, between the scar and the TA/vena cava, was ablated in five patients; while in the other two patients the conventional posterior isthmus was ablated. Thus the reentry circuit could be interrupted successfully in all seven patients, albeit after two CARTO procedures in one patient. Retrospective analysis of the CARTO maps showed that the ablated isthmus did not transect the slowest conducting part of the macro-reentry circuit. The mean length of the ablated isthmus was 2.8 ± 0.9 cm (range 1.5–4.3 cm). Linear ablation required a mean of 23 ± 16 radiofrequency pulses.
The remaining one patient had two focal ATs (Figs. 4 and 5). A focal mechanism was suspected when conventional mapping suggested a short biatrial activation time, 53% (290/550 ms) and 45% (180/400 ms) of the AT1 and AT2 cycle lengths, respectively. CARTO mapping showed earliest activation in the high anterior and low posterior right atrium. Successful ablation sites recorded an electrogram 54 ms (AT1) and 46 ms (AT2) earlier than the onset of the surface P wave.
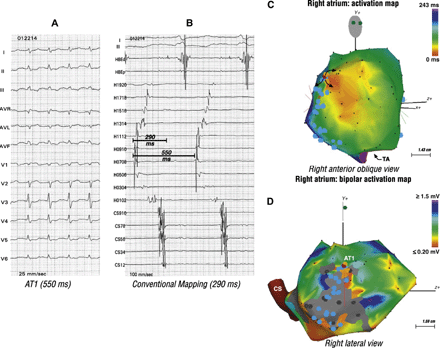
Mapping and ablation of two focal ATs in a 55 year-old woman post atrial septal defect closure. Panel A shows the first AT (AT1). Panel B showed bi-directional activation along the crista terminalis (Halo catheter) and a short biatrial activation time suggestive of a focal mechanism. Panel C shows origin of the focal AT1 in the high anterior right atrium (orange). Panel D, a bipolar voltage map, shows relation of AT1 to the atriotomy scar.
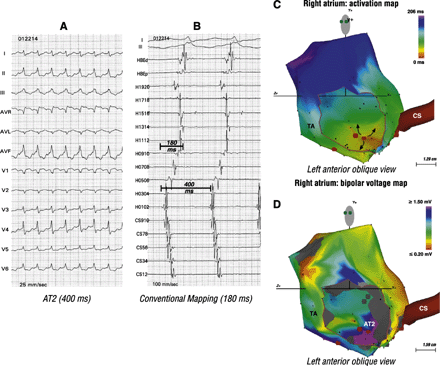
A second AT (AT2) found in the patient described in Fig. 4. Panel A shows AT2. Once again, panel B, shows bi-directional activation along the crista terminalis and a short biatrial activation time. These findings suggest a focal mechanism. Panel C shows origin of the focal AT2 from the low posterior right atrium. Panel D shows relation of AT2 to the septal scar.
Follow-up
After a mean follow-up of 12 ± 11 months (range 2–45 months), 2 out of 18 patients (11%) had recurrence of either the same or a new AA.
Discussion
Our experience suggests that ablation of AAs after previous open-heart surgery, performed for valvular or a simple congenital heart disease like an atrial septal defect, can be performed successfully (18/19 patients, >90%) and with a low recurrence rate (2/18 patients, 11%) at 1-year follow-up. In conformity with Chan et al. [6], we found that, despite the presence of different atrial scars – related to atriotomy, venous cannulation or prosthetic patch material, typical AFL occurred frequently (14/19 patients, 72%). Distinctively, this study included a significant proportion of patients with previous valve surgery (eight out of 19). Yet, typical AFL remained a frequent occurrence. This could, most probably, be related to the right atriotomy scar. The latter can form an inter-caval line of block, constituting the posterior boundary of the typical AFL circuit. More often than not, typical flutter circuits (along the TA) could be mapped adequately using conventional multielectrode catheters alone, and successfully ablated. In addition, peri-scar septal and double-loop reentry circuits were mapped. CARTO mapping helped to uncover peri-scar reentry and provided a road-map for the ablation of an unconventional isthmus. In our patient cohort, focal ATs were observed in an isolated case. Recently, ablation studies in patients who had undergone valve repair/replacement [12,,18,,19], have reported AAs based on macro-reentrant circuit (right atrial free wall, between septum and right pulmonary veins and left atrium) as well as focal ATs. Using conventional [4–,6,,10,19] or three-dimensional activation mapping [7–,9,,11,12],18] different authors, in small groups of 8–20 patients, have reported acute success ranging from 80% to 100%. Considering the preponderance of typical AFL and the possibility of developing AAs based on peri-scar reentry after open-heart surgery, it may be appropriate pre-emptively to ablate the conventional isthmus intra-operatively and extend the atriotomy incision line to the closest anatomical barrier, by radiofrequency- or cryo-ablation [20,,21].
Intra-cardiac mapping revealed typical AFL along the TA in three quarters (14/19) of our patients. Importantly, the ECG had suggested AT in three of these patients. A similar experience has been documented by Kasai et al., who mapped a clockwise AFL, in a patient with surgically repaired tetralogy of Fallot, in whom the ECG suggested an AT [22]. In 12 patients, the involvement of the conventional cavotricuspid isthmus was verified by almost complete mapping of the flutter circuit along the TA, interruption of the AFL in the cavotricuspid isthmus during ablation and/or non-inducibility after obtaining bi-directional isthmus conduction block. In the remaining two patients, in whom the typical AFL had recurred, in spite of cavotricuspid isthmus ablation in the past, an unconventional isthmus was successfully targeted under the guidance of the CARTO system. Recording of low-voltage electrograms on the cavotricuspid isthmus with the consequent difficulty in selecting ablation sites lead to the choice of an unconventional isthmus in both these patients. One patient, who had undergone linear cryoablation intra-operatively (during septal defect closure), between the right atriotomy incision and the inferior vena cava, for pre-operatively known AFL, presented post-operatively with recurrent clockwise typical AFL. In such a case an alternative ablation strategy could be to extend the atriotomy to the TA [20].
We used the CARTO system to supplement conventional mapping, if (1) the ECG morphology of the clinically documented AA suggested an AT, (2) conventional mapping was inadequate or inconsistent with reentry along the TA, or (3) an AA recurred after the ablation of conventional cavotricuspid isthmus in the past. In two patients with a reentry circuit localized around the septal scar, conventional mapping could only document bystander activation along the TA. In case of the double-loop reentry-based AA, only the activation loop along the TA was recorded using conventional techniques. These cases illustrate the inadequacy of conventional mapping in the setting of peri-scar reentrant AAs. On the other hand, the CARTO system allowed complete delineation of the peri-scar reentry circuit and helped to overlap reliably the radiofrequency pulses to create a continuous ablation line across the unconventional isthmus. When we analyzed the CARTO maps retrospectively, the ablated isthmus was not found to be the slowest conducting part of the reentry circuit. This supports that the reentry pathway could be interrupted across any anatomic isthmus that is short enough and, importantly, provides adequate electrode–tissue contact.
Study limitations
The following limitations of this study should be acknowledged. Firstly, the likelihood of successful ablation, judged on the basis of: the ECG morphology of the targeted AA and the possibility of using the CARTO system, could have led to a pre-selection of patients referred to us. Secondly, although >90% of the AA circuit was accurately delineated using the CARTO system, entrainment mapping for localizing the reentry circuit and selecting appropriate ablation sites was not performed. However, the entrainment technique remains an important tool when studying macro-reentrant circuits. Thirdly, due to the presence of a left-sided prosthetic valve or a previous valve repair and the hesitation to perform transseptal puncture in patients with a surgically closed atrial spetal defect, mapping of the left atrium was not performed. Fourthly, despite regular follow-up and every attempt to document symptomatic recurrences, asymptomatic AAs could have been missed, leading to an underestimation of true recurrences. Finally, anti-arrhythmic medications were continued in patients with coexisting atrial fibrillation. This would be expected to have influenced the evaluation of recurrences during the follow-up.
References
Author notes
1Tel.: +31 43 387 7207; fax: +31 43 387 5104.



