-
PDF
- Split View
-
Views
-
Cite
Cite
Federico Lombardi, Ezio Calosso, Giosuè Mascioli, Egidio Marangoni, Andrea Donato, Stefano Rossi, Massimo Pala, Francesco Foti, Maurizio Lunati, Utility of implantable loop recorder (Reveal Plus®) in the diagnosis of unexplained syncope, EP Europace, Volume 7, Issue 1, 2005, Pages 19–24, https://doi.org/10.1016/j.eupc.2004.09.003
Close - Share Icon Share
Abstract
In about 30% of patients with syncope, the responsible mechanisms remain unrecognised. Nevertheless, the possibility of an arrhythmic aetiology remains, however, difficult to rule out.
We therefore monitored with an implantable loop recorder (ILR, Reveal Plus®, Medtronic) 34 subjects (60 ± 15 years) with at least two unexplained syncopal episodes and negative neurological and cardiovascular work-up.
During a follow-up of 7 ± 4 months, syncope occurred in 11 subjects. In nine of them the mechanisms responsible for these events were identified by ILR monitoring: marked bradycardia or asystole (n = 6), atrial fibrillation with wide QRS tachycardia (n = 1) and sinus rhythm with fine artifacts likely to be due to muscle contractions (n = 2). Pre-syncope occurred in seven patients: advanced atrioventricular block (n = 3), sinus tachycardia (n = 1), and wide QRS tachycardia (n = 1) were documented. Thus, when considering all 18 patients with recurrences, a diagnosis was achieved in 53% of subjects. Recognition of the rhythm disorder in seven patients with syncope and four patients with pre-syncope guided patient management.
These data indicate that ILR monitoring facilitates the identification of mechanisms responsible for recurrences and therapeutic management in subjects with syncope or pre-syncope and negative traditional neurological and cardiovascular work-up.
Introduction
In about 30% of patients with syncope, in spite of a complete and extensive neurological and cardiac work-up it is impossible to identify the responsible mechanisms [1–7]. These patients are therefore left without diagnosis and therapy. The incidence of recurrences is, however, unpredictable for the variety and complexity of the mechanisms responsible for the transient loss of consciousness, which span from the benign neuro-mediated syncope to cerebral hypoperfusion secondary to malignant ventricular arrhythmias [1]. When considering young subjects, an additional problem may arise from the fact that transient loss of consciousness due to epileptic seizure is difficult to rule out even in the presence of negative electroencephalographic evidence [1,,2]. In these subjects, empirical therapies are occasionally prescribed.
Syncopal recurrences are difficult to manage. Indeed, where patients' prognosis does not seem to be extensively affected by their number, quality of life may be seriously impaired [1,,8–,10] and in most instances several diagnostic procedures are prescribed in an attempt to identify the underlying cause of these episodes [1,,2].
In this prospective multicentre study, we evaluated the utility of the implantable loop recorder (ILR) to facilitate the understanding of the aetiology of syncope in a group of consecutive subjects with a negative cardiac and neurological work-up who were referred to our cardiology or neurology services for recurrent syncope.
Methods
Study population
Thirty-four patients with a history of syncope and negative cardiac and neurological work-up were included in this prospective multicentre study. The subjects were selected from among patients referred to cardiac institutions participating in the study, for recurrent syncope. According to the design of the study, exclusion criteria were a cardiac diagnosis based on medical history and physical examination, on two-dimensional echocardiogram, on detection of syncope related arrhythmias or conduction defects during Holter or telemetry monitoring and on a positive normal or nitroglycerine potentiated tilt test. Subjects with a neurological aetiology of transient loss of consciousness (TLOC) according to medical history and physical examination, brain CT scan or nuclear magnetic resonance, baseline or sleep deprived electroencephalogram were also excluded.
The 34 subjects enroled in this study were characterized by two syncopal episodes within 1 year of observation and lack of identification of a cardiac or neurological cause of syncope/TLOC. Their mean age was 60 ± 15 years (range 28–84), with a relative prevalence of male gender (61.7%). The co-morbidities observed are reported in Table 1.
| . | No of patients (%) . |
|---|---|
| Diabetes | 3 (8.8%) |
| Carotid or peripheral atherosclerosis | 4 (11.8%) |
| Dilated cardiomyopathy | 2 (5.9%) |
| Thyroid disease | 2 (5.9%) |
| Hypertension | 1 (3%) |
| Mild aortic stenosis | 1 (3%) |
| Epilepsy | 1 (3%) |
| . | No of patients (%) . |
|---|---|
| Diabetes | 3 (8.8%) |
| Carotid or peripheral atherosclerosis | 4 (11.8%) |
| Dilated cardiomyopathy | 2 (5.9%) |
| Thyroid disease | 2 (5.9%) |
| Hypertension | 1 (3%) |
| Mild aortic stenosis | 1 (3%) |
| Epilepsy | 1 (3%) |
| . | No of patients (%) . |
|---|---|
| Diabetes | 3 (8.8%) |
| Carotid or peripheral atherosclerosis | 4 (11.8%) |
| Dilated cardiomyopathy | 2 (5.9%) |
| Thyroid disease | 2 (5.9%) |
| Hypertension | 1 (3%) |
| Mild aortic stenosis | 1 (3%) |
| Epilepsy | 1 (3%) |
| . | No of patients (%) . |
|---|---|
| Diabetes | 3 (8.8%) |
| Carotid or peripheral atherosclerosis | 4 (11.8%) |
| Dilated cardiomyopathy | 2 (5.9%) |
| Thyroid disease | 2 (5.9%) |
| Hypertension | 1 (3%) |
| Mild aortic stenosis | 1 (3%) |
| Epilepsy | 1 (3%) |
A careful medical history and description of the last syncopal episode was collected in each subject in relation to characteristics of syncopal onset and recovery, duration of the event, family history of epilepsy (two subjects) or of migraine (three subjects). The type and number of diagnostic procedures performed in each patient to exclude a cardiac or neurological aetiology of the syncopal/TLOC events are illustrated in Table 2.
Number and percentage of patients who performed tests to complete cardiac and neurological work-up
| Neurological work-up . | Cardiac work-up . | ||
|---|---|---|---|
| . | No of patients (%) . | . | No of patients (%) . |
| Medical examination | 34 (100%) | Medical examination | 34 (100%) |
| Basal EEG | 30 (88%) | Two-dimensional echo | 34 (100%) |
| Sleep deprived EEG | 12 (35%) | 24 h Holter or >24 h telemetry | 34 (100%) |
| Brain NMR or CT scan | 25 (74%) | Exercise stress test | 24 (71%) |
| Carotid doppler | 23 (68%) | Tilt test | 34 (100%) |
| EP testing | 10 (29%) | ||
| Neurological work-up . | Cardiac work-up . | ||
|---|---|---|---|
| . | No of patients (%) . | . | No of patients (%) . |
| Medical examination | 34 (100%) | Medical examination | 34 (100%) |
| Basal EEG | 30 (88%) | Two-dimensional echo | 34 (100%) |
| Sleep deprived EEG | 12 (35%) | 24 h Holter or >24 h telemetry | 34 (100%) |
| Brain NMR or CT scan | 25 (74%) | Exercise stress test | 24 (71%) |
| Carotid doppler | 23 (68%) | Tilt test | 34 (100%) |
| EP testing | 10 (29%) | ||
EEG: electroencephalogram; EP testing: electrophysiological testing.
Number and percentage of patients who performed tests to complete cardiac and neurological work-up
| Neurological work-up . | Cardiac work-up . | ||
|---|---|---|---|
| . | No of patients (%) . | . | No of patients (%) . |
| Medical examination | 34 (100%) | Medical examination | 34 (100%) |
| Basal EEG | 30 (88%) | Two-dimensional echo | 34 (100%) |
| Sleep deprived EEG | 12 (35%) | 24 h Holter or >24 h telemetry | 34 (100%) |
| Brain NMR or CT scan | 25 (74%) | Exercise stress test | 24 (71%) |
| Carotid doppler | 23 (68%) | Tilt test | 34 (100%) |
| EP testing | 10 (29%) | ||
| Neurological work-up . | Cardiac work-up . | ||
|---|---|---|---|
| . | No of patients (%) . | . | No of patients (%) . |
| Medical examination | 34 (100%) | Medical examination | 34 (100%) |
| Basal EEG | 30 (88%) | Two-dimensional echo | 34 (100%) |
| Sleep deprived EEG | 12 (35%) | 24 h Holter or >24 h telemetry | 34 (100%) |
| Brain NMR or CT scan | 25 (74%) | Exercise stress test | 24 (71%) |
| Carotid doppler | 23 (68%) | Tilt test | 34 (100%) |
| EP testing | 10 (29%) | ||
EEG: electroencephalogram; EP testing: electrophysiological testing.
Pre-implant cardiac evaluation revealed the presence of premature atrial or ventricular beats in six subjects; two bouts of non-sustained ventricular tachycardia were documented in two patients. A left ventricular ejection fraction <40% was present in two patients with dilated cardiomyopathy. All these findings were not considered related to the syncopal episodes. Non-diagnostic electroencephalographic alterations were observed, at baseline and after sleep deprivation, in six and four subjects, respectively.
Details related to pre-implant events before loop recorder implant were available. Loss of consciousness was abrupt in onset in 29 and gradual in the remaining five subjects. It was preceded by a lack of well being in 18 patients. The mean duration of syncopal event was <10 s in 10 subjects and >10 s in 19 patients. This information was not available in five cases. Recovery of consciousness was sudden in 68% of subjects.
All the subjects gave their consent to participate in the study, which was approved by the Ethic Committee of San Paolo Hospital (site of the principal investigator, F.L.) and complied with the Helsinki declaration.
All the decisions concerning patients' management were not part of the study and left to the individual physicians.
Implantable loop recorder implantation
In all the subjects, ILR (Reveal Plus®, Medtronic, Minneapolis, USA) was implanted during a short hospital admission (1–3 days) according to the following procedure. After identification of the most appropriate pectoral position to record an electrocardiographic lead with a prominent QRS wave, the loop recorder was implanted subcutaneously in the selected left pectoral region using local anaesthetic to create a pocket similar to that of a pacemaker. After suture of the skin, the quality of electrocardiographic recording was tested in supine and standing positions and during movements of both the arms.
No complications including bleeding or implant site infection were observed. The device was explanted after a diagnosis was obtained or if syncope did not recur after 14 months. In two patients the explant procedure was performed after 18 months.
In all the subjects, the device was programmed with the following settings: 42 min of total electrocardiographic recording consisting of a patient activated 14 min recording and of 14 automatic activation recordings of 2 min duration. The following parameters were set for automatic activation: heart rate <40 beats per minute (measured on four consecutive intervals); heart rate >160 beats per minute (measured on 16 consecutive intervals) and asystole lasting more than 3 s.
A follow-up visit was programmed after symptomatic events or every 3 months in asymptomatic subjects to retrieve from the memory of the ILR the time and date of episodes of bradycardia or tachycardia and the corresponding electrocardiographic tracing.
Data analysis
All data are expressed as mean ± SD. Appropriate t-test was used for the comparison of continuous variables and Chi-square test was applied for the comparison of discrete variables between groups of patients. P-values less than 5% were considered statistically significant.
Results
During a follow-up of 7 ± 4 months (range 1–14), 20 patients presented 23 events classified as true syncope with loss of consciousness (13 episodes) or pre-syncope (10 episodes) due to marked asthenia, palpitations, vertigo or impending loss of consciousness. Syncope and pre-syncope occurred in, respectively, 11 and nine patients.
From the memory of the ILR it was possible to retrieve the electrocardiographic recordings of 20 of 23 episodes. The modality of recording was automatic in 14 cases and manually activated in six cases. Failure of recordings of three events was due to a lack of manual activation in one case and to memory saturation due to oversensing in two cases.
Syncopal episodes
Thirteen syncopal episodes with sudden onset occurred in 11 subjects. In nine of them, the mechanism responsible for syncope was identified. As indicated in the example presented in Fig. 1, marked bradycardia or asystole occurred in six patients, atrial fibrillation with wide QRS tachycardia was present in one subject. In two patients, with regular sinus rhythm during the syncopal episode, epilepsy was suspected because of the presence of fine artifacts on the electrocardiographic tracing likely to be due to muscle contractions. No diagnosis was reached in two patients.
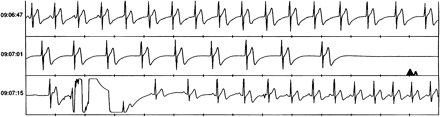
Event recorder tracing showing a 4.8 s asystole period in a 31 year old subject. Time is indicated on the right scale. After a few sinus beats, there is a progressive slowing of a junctional rhythm ending into asystole. Recovery of sinus rhythm is evident in the bottom panel.
Pre-syncopal events
Ten pre-syncopal events occurred in nine patients. The analysis of the ILR memory revealed bradycardia due to advanced atrioventricular block in three patients, symptomatic sinus tachycardia in two subjects and wide QRS tachycardia in another subject (Fig. 2). No rhythm disturbances were recorded in two patients in whom diagnoses of postural hypotension and a transient ischaemic attack were then made. No diagnosis was reached in one patient.
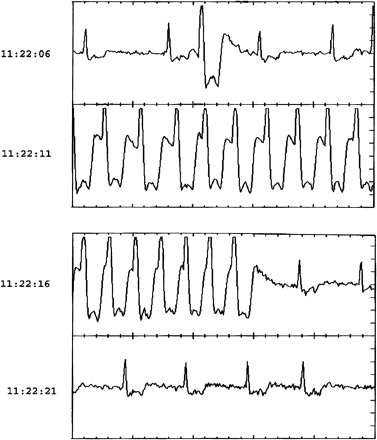
Event recorder tracing showing a bout of wide QRS tachycardia during a syncopal episode.
Thus when considering all 20 patients together, ILR monitoring made it possible to detect a rhythm disturbance in 13 subjects and to achieve a diagnosis in 18 patients corresponding to 90% of subjects with syncopal or pre-syncopal events during the follow-up period or to 53% of the whole study population. Marked bradycardia or asystole were detected in 55% and 33% of patients with, respectively, syncope and pre-syncope.
As to the factors likely to be associated with an appropriate diagnosis, duration of episode >10 s was the only significant one (P = 0.024, in comparison with episodes lasting <10 s). Neither the type of onset (sudden versus gradual) nor the presence or absence of prodromal symptoms or the modalities of loop recorder activation was correlated with a specific arrhythmic pattern.
The recognition of significant rhythm disorder in seven patients with syncope and in four with pre-syncope had important influence on patient management. In two patients a single chamber and in six patients a dual chamber pacemaker was implanted, while in one subject radiofrequency ablation of the slow atrioventricular nodal pathway was performed after an electrophysiological study that revealed the presence of AV-nodal reentrant tachycardia. In one of the two patients with wide complex tachycardia, monomorphic ventricular tachycardia was induced during programmed electrical stimulation. A cardioverter defibrillator was implanted. In two patients, antiepileptic therapy was started.
Discussion
The results of this study indicate that in patients with a negative cardiac and neurological work-up, ILR monitoring facilitates the identification of the mechanisms responsible for syncope/TLOC in more than 80% of patients with recurrences during the follow-up period.
Three aspects of the study appear of interest: (i) the usefulness of ILR monitoring to recognize rhythm disorder causing syncope or sensation of faintness; (ii) the documentation of a regular cardiac rhythm in a small number of syncopal episodes; (iii) the indication of an appropriate patient management strategy as a result of documentation of a cardiac rhythm disorder during syncopal or pre-syncopal episodes.
Identification of arrhythmias as a cause of syncope
Rhythm disorders are a common cause of syncope [1,,3,,4,,7–,10]. In many instances, however, they occur in the presence of structural heart disease. Even in subjects with no evidence of organic heart disease, syncope may, however, carry a negative prognosis and its occurrence may represent a determining factor to justify in a selected subgroup of patients, the implant of an automatic cardioverter defibrillator [11,,12].
In the majority of young subjects with no evidence of structural heart disease, bradycardia is the predominant rhythm disorder associated with neuro-mediated syncope. In most of these patients [4,,5] a careful description of the event and a positive tilt test are often diagnostic. More difficult is the interpretation of episodes that may occur in subjects with no evidence of structural heart disease, negative tilt test and no specific symptoms before the syncopal episodes. In these subjects, the presence of an undetected rhythm disorder or of epileptic seizures is usually considered.
Our results indicate that also in subjects with a negative cardiac and neurologic work-up according to international guidelines [1], asystole or marked bradyarrhythmias may cause syncope more commonly than expected. Indeed, in 55% of the reported syncopal episodes, a bradyarrhythmia was recorded in patients who at the time of initial evaluation had a negative 24 h Holter recording and carotid sinus massage response.
A wide QRS tachycardia was observed in two patients. In these subjects, it was possible to induce, respectively, an AV-nodal reentrant tachycardia and a ventricular tachycardia during the electrophysiological study performed after the loop recorder explantation.
Of clinical interest was also the finding that in two patients, recordings of sinus rhythm with low voltage high frequency artifacts during the syncopal episodes was the motivation to request a new neurological evaluation for an epileptic cause of the loss of consciousness.
Role of electrocardiographic monitoring
Twenty-four hour Holter monitoring is of limited value in the diagnosis of syncope [1,,2,,13]. The possibility of performing a Holter recording during a recurrence of a syncopal episode is very low and unpredictable. According to the age and the presence or absence of structural heart disease, different supraventricular or ventricular arrhythmia can be detected although the correlation between symptoms and electrocardiographic finding is poor. In an overview [13] of the results of ambulatory monitoring in syncope, only 4% of patients had a correlation of symptoms with arrhythmia. To overcome these limitations, event recorders [1,,14] have been applied to patients with syncope. These external devices may be particularly useful in patients with a high recurrence rate. Their major limitations are the necessity of a manual activation and the poor quality of recording when the electrodes are repositioned by the patient.
The ILR represents a relatively new and useful tool in the management of selected group of patients with syncope [1,,15–,18]. The solid-state loop memory allows the retrieval of events stored according to predefined rate thresholds or activated by the patients after recovery of consciousness. In a report of Krahn and coworkers [15] it was possible to establish a correlation between symptoms and electrocardiographic findings in 59% of 85 patients within 10 months from implantation. In particular, these authors observed that a symptom/rhythm correlation was present in about one quarter of subjects who experienced syncopal episodes during the follow-up, whereas it was less frequent in patients with pre-syncopal symptoms. Our findings suggest that in a heterogeneous population with high rate of syncope and pre-syncope recurrences, unexplained after a careful diagnostic work-up, ILR monitoring may detect rhythm disturbances and facilitate an appropriate diagnosis in about 80% of subjects with recurrences during the follow-up period or in 50% of the whole study population. These figures as well as the finding that most relevant rhythm disturbances were detectable mostly in syncopal episodes are well in keeping with the result of the ISSUE trial where patients with isolated syncope [16] or with syncope associated with heart disease and negative electrophysiological testing [17] were studied using ILR monitoring. Bradycardia with an asystolic pause was the most frequent finding recorded in 46% of patients with isolated syncope [16] and in three of the six patients with syncopal recurrences in the heart disease group [17]. When considering, instead, pre-syncopal episodes, no major rhythm disturbances were observed in three of the seven patients who did not present any significant rhythm disturbance in six events. Thus we have confirmed the previous data of the ISSUE study [16] and the recent report of Boersma et al. [18].
Finally, it must be recalled that no recording at the time of the event was available in three patients thus confirming that in spite of device programming, patient cooperation remains important. An open question is related to the effects of transient loss of signal on autodetection sensing that may generate false flat baseline tracing. Even after multiple refinements of programming to achieve adequate sensing, this may still be a clinical problem [19].
Study limitations and conclusions
The small number and the heterogeneity of patients enroled in the study is, in our opinion, the major limitation of the study, which from another point of view reflects common clinical practice: the patients enroled in the study were characterized by a relatively high frequency of syncope, a completely negative cardiac and neurological work-up and low incidence of structural heart disease. Nevertheless, we were able to unmask rhythm alterations and to achieve a clinical diagnosis in about 50% of our patients leaving us in no doubt as to their future management.



