-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Ribbing, Gerold Mönnig, Kristina Wasmer, Günter Breithardt, Lars Eckardt, Catheter ablation of atrial tachycardia due to recipient-to-donor transatrial conduction after orthotopic heart transplantation, EP Europace, Volume 6, Issue 3, 2004, Pages 215–219, https://doi.org/10.1016/j.eupc.2004.02.003
Close - Share Icon Share
Abstract
Atrial arrhythmias occur frequently after orthotopic heart transplantation. In some cases, they may depend on atrio-atrial conduction across the surgical suture line. We report an unusual case of a heart transplant recipient who had recipient atypical atrial flutter with 2:1 transatrial conduction and a resulting tachycardia in the donor atrium. Electroanatomical mapping identified the site of transatrial conduction and guided successful catheter ablation.
Introduction
Atrial arrhythmias, such as atrial flutter or focal atrial tachycardia, are a common finding after orthotopic heart transplantation [1–,3]. The incidence of these arrhythmias is estimated to be as high as 44% in long-term survivors [2]. In the donor atria, atrial flutter seems more frequent than atrial fibrillation or focal atrial tachycardia [2,,3]. In the recipient atria, both atrial fibrillation and atrial flutter have been reported [4]. It is generally assumed that the recipient atrial remnant does not cause an atrial tachycardia in the donor heart because they are electrically isolated from each other, even though a few reports have described transatrial conduction and catheter ablation of recipient-to-donor atrial tachycardia [5–,9].
We report a patient with recipient-to-donor atrial tachycardia in whom the location of the transatrial conduction and the tachycardia mechanism of each atrium was mapped using electroanatomic mapping (CARTO™). Guided by CARTO™, a successful interruption of the transatrial conduction was achieved by radiofrequency application.
Case report
A 70-year-old man had undergone orthotopic heart transplantation 9 years before due to ischaemic cardiomyopathy resulting in end stage heart failure. Before cardiac transplantation, he had recurrent episodes of atrial fibrillation. For the last 3 years after transplantation, the patient experienced recurrent episodes of supraventricular tachycardia that were refractory to β-blockers, sotalol and verapamil. The post-transplant course was otherwise uncomplicated. Acute or chronic rejection was excluded by coronary angiography, echocardiography and myocardial biopsy on a regular basis. After informed written consent an electrophysiological study was performed to elucidate further the mechanisms of his recent supraventricular tachycardia.
At baseline, a permanent tachycardia was present. The surface ECG showed a positive P wave in the inferior leads (Fig. 1). Recipient atrial electrograms were recorded at the posterior and lateral part of the right atrium. Donor atrial activity was recorded at the anterior and septal proportion of the right atrium. A fast atrial tachycardia (CL 180 ms) was present in the recipient atrium with a 2:1 relation to the donor atrium and a resulting cycle length of 360 ms (Fig. 2). Mapping the atrial anastomosis confirmed a fixed 2:1 conduction between the two atria (Fig. 2). A CARTO™-activation map of each atrium was created. The map from the recipient atrium confirmed atypical atrial flutter with a propagation wave in the superior region of the atrium ending at the suture line. Electroanatomic mapping from the donor atrium confirmed an origin of the atrial tachycardia at the suture line in the region where the propagation wavefront of the recipient atrium ended (Fig. 3). At this site, radiofrequency current was applied. Within 4 s of radiofrequency energy delivery, the donor atrial rhythm was dissociated from the recipient atrial tachycardia, and the patient's rhythm slowed to a cycle length of 850 ms (Fig. 4). The atrial tachycardia in the recipient atrium was unaltered, but the two atria were now dissociated. During a follow-up of 6 months, there was no re-appearance of any tachycardia.
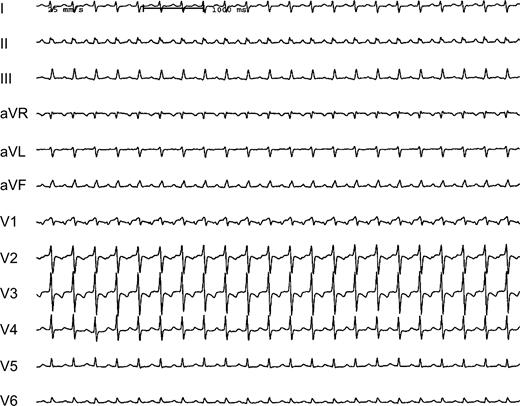
Twelve-lead ECG (25 mm/s) of a 70-year-old heart transplant recipient showing a supraventricular tachycardia (CL 360 ms). P waves appear positive in the inferior leads.
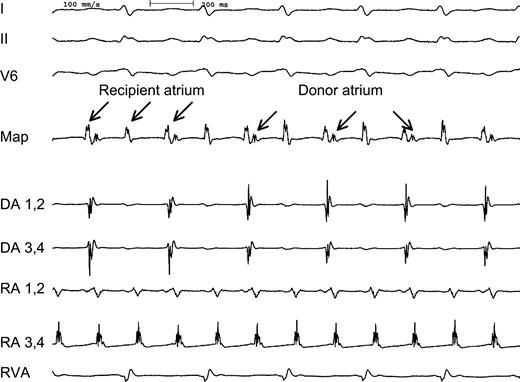
Intracardiac electrograms demonstrating recipient atrial tachycardia (RA) with 2:1 conduction to the donor atrium (DA). Three surface leads (I, II, V6), two bipolar recordings from the recipient atrium (RA, CL 180 ms) and two bipolar recordings from the donor atrium (DA, CL 360 ms) as well as a recording from the right ventricle (RVA) are shown. A bipolar recording at the mapping catheter (map) placed at the region of the suture line showed a fixed 2:1 coupling interval between the two atria during tachycardia.
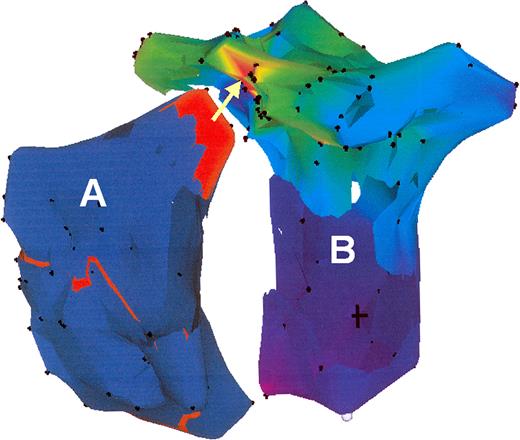
RAO view of a CARTO™ map of the tachycardia in the recipient atrium (A) and the donor atrium (B). In the recipient atrium the propagation map displayed an activation front (red) in the superior region. The activation map of the donor atrium confirmed an origin (red) of the tachycardia at the suture line at the region where the activation propagated in the recipient atrium (arrow).
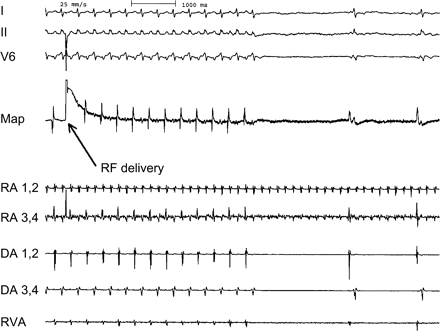
Termination of the tachycardia 4 s after RF delivery due to interruption of the transatrial conduction. This resulted in dissociated rhythms in the two atria. In the recipient atrium atrial flutter was unaltered, in the donor atrium a junctional rhythm occurred. RA, recipient atrium; DA, donor atrium; Map, mapping catheter.
Discussion
Recipient-to-donor atrial tachycardia is a rare event of unknown prevalence. In the above reported case, atrial flutter in the recipient atrium with a 2:1 conduction block to the donor atrium led to a clinically relevant tachycardia. Electroanatomical mapping verified the site of transatrial conduction and subsequent dissociation was achieved by radiofrequency application.
Several factors may contribute to arrhythmias in the recipient atrium. This includes the preexisting disease and atrial arrhythmias before transplantation, the injury from the surgical procedure or postoperative pericarditis. Two mechanisms have mainly been proposed for the electrical interaction between the recipient and the donor atria [9–,11]. First, electrotonic transmission can synchronize the recipient and donor atria, although direct transmission may not occur across the suture line. However, if the donor atrium and the recipient atrium are not completely isolated from each other, ionic current flow may occur between these closely adjacent tissues [10]. Viable myocardium entrapped within or bridging the suture line may be another possible mechanism for electrical conduction. This is supported by experimental [10] and clinical studies [9,,11,,12]. The conduction properties of transatrial conduction were reported to be decremental [9] or, as in our case, fixed [8]. Furthermore unidirectional or bi-directional conduction has been reported [7].
Only a few reported cases of catheter ablation for transatrial conduction after heart transplantation exist. To our knowledge, this is the first case reported so far of successful catheter ablation of transatrial conduction using an electroanatomic mapping system.
Summary
Recipient-to-donor transatrial conduction is an uncommon and fascinating cause of arrhythmias in heart transplant recipients. The identification of this mechanism by an electrophysiological study is of particular importance for successful treatment. Electroanatomic mapping can guide successful ablation in these patients.
References
Author notes
*This work was supported by (1) Sonderforschungsbereich 556 (SFB 556): Projekt C2 of the Deutsche Forschungsgemeinschaft, Bonn, Germany; (2) Franz-Loogen Foundation, Düsseldorf, Germany; and (3) Alfried Krupp von Bohlen and Halbach Foundation, Essen, Germany.



