-
PDF
- Split View
-
Views
-
Cite
Cite
David Keane, Moussa Mansour, Jagmeet Singh, Detection by intracardiac echocardiography of early formation of left atrial thrombus during pulmonary vein isolation, EP Europace, Volume 6, Issue 2, January 2004, Pages 109–110, https://doi.org/10.1016/j.eupc.2003.11.001
Close - Share Icon Share
A 50-year-old man with no history of thromboembolism was brought to the electrophysiology laboratory for a pulmonary vein isolation procedure for atrial fibrillation. After femoral sheath access was obtained, a bolus of 5000 I.U. heparin was given intravenously. Transseptal catheterization was then performed with separate punctures and sheaths for a circumferential pulmonary vein mapping catheter (Lasso—Biosense Webster) and a radiofrequency ablating catheter (4 mm Carto—Biosense Webster). Immediately following transseptal catheterization the two transseptal sheaths were continuously flushed at 60 ml/h of heparinized saline and an another 3000 I.U. of heparin was given as a bolus followed by a continuous infusion of 1500 I.U. of heparin per hour. Baseline anatomical pull-backs of the pulmonary veins on Carto mapping were then performed under intracardiac echocardiographic and fluoroscopic guidance.
Prior to ablation and 15 min after transseptal catheterization, the activated clotting time (ACT) was found to be 211 s—significantly below our target ACT of 300 s. At this point, a thin, mobile, freshly formed thrombus attached at its base to the shaft of the mapping catheter (Lasso) was detected on intracardiac echo (Fig. 1). Additional boluses of heparin were given. A total of 15,000 I.U. of heparin boluses were required before the ACT reached its target of 300 s and a maintenance infusion of 2500 I.U. of heparin per hour was required to maintain the ACT at this level throughout the procedure.
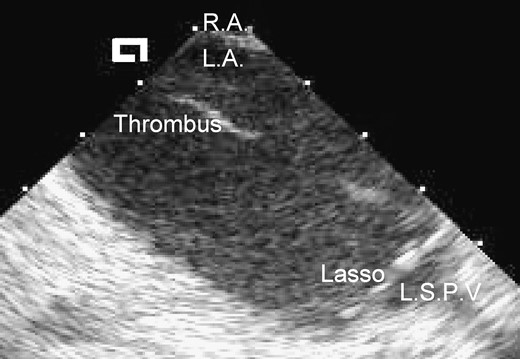
Phased-array intracardiac echo of left atrial thrombus. The intracardiac echo probe was positioned in the right atrium and the two-dimensional echo image is of the left atrium seen across the fossa ovalis (the echogenic linear structure at the top of the image between the labels R.A. and L.A.). R.A., right atrium; L.A., left atrium; L.S.P.V., left superior pulmonary vein; Thrombus, mobile strand of freshly formed thrombus which was attached to the shaft of a mapping catheter in the left atrium; Lasso, decapolar circumferential mapping catheter positioned at the ostium of the left superior pulmonary vein—four of the ten recording electrodes aligned orthogonally to the pulmonary vein ostium are seen in this two-dimensional echocardiogram.
After administration of the additional heparin boluses as above, the transseptal sheath containing the mapping catheter was aspirated as the mapping catheter was simultaneously withdrawn back into the sheath and removed. The mapping catheter was carefully inspected and no loss of integrity of its coating was detected. After the target ACT of 300 s was achieved, no further thrombus formation occurred and the procedure was resumed and all four pulmonary veins were isolated. Intraprocedural aspirin and clopidogrel were also given per our routine protocol. The procedure was well tolerated and without any complication or evidence of embolization and the patient went home after two days of intravenous anticoagulation in sinus rhythm.
This finding supports a policy of ensuring that the ACT target range is achieved rapidly after transseptal catheterization [1]. This clinical finding is consistent with the observations by Dorbala and colleagues that surrogate markers of thrombus formation (TAT and F1+2) and fibrinolysis (d-dimers) are increased significantly by placement of electrophysiology recording catheters alone prior to radiofrequency ablation [2]. These findings also lend support to consideration of pretreating patients with inhibitors of platelet aggregation prior to radiofrequency catheter ablation procedures as reported by Manolis et al. [3]. The possible advantages gained in so doing must be balanced by an anticipated greater risk of cardiac tamponade.



