-
PDF
- Split View
-
Views
-
Cite
Cite
Lien Desteghe, Michiel Delesie, Lieselotte Knaepen, Rana Önder, Johan Verbeeck, Paul Dendale, Thomas Phlips, Peter Haemers, Johan Saenen, Joris Ector, Johan Vijgen, Hein Heidbuchel, Effect of targeted education of patients with atrial fibrillation on unplanned cardiovascular outcomes: results of the multicentre randomized AF-EduCare trial, EP Europace, Volume 27, Issue 1, January 2025, euae211, https://doi.org/10.1093/europace/euae211
Close - Share Icon Share
Abstract
Trials on integrated care for atrial fibrillation (AF) showed mixed results in different AF populations using various approaches. The multicentre, randomized AF-EduCare trial evaluated the effect of targeted patient education on unplanned cardiovascular outcomes.
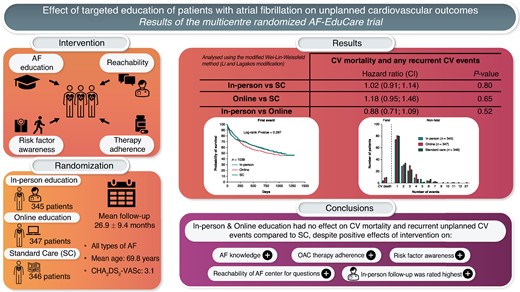
Patients willing to participate were randomly assigned to in-person education, online education, or standard care (SC) and followed for minimum 18 months. Education focused on four aspects of integrated AF care: (i) knowledge on AF and oral anticoagulation; (ii) reinforcement of medication adherence; (iii) awareness about risk factors; and (iv) reachability for AF-related questions. The primary endpoint was the composite of cumulative events of unplanned cardiovascular hospitalizations and consultations, emergency department visits for cardiovascular reasons, and cardiovascular death. A total of 1038 patients (69.8 ± 9.2 years) were followed up for 26.9 ± 9.4 months. Education (both in-person and online) significantly improved AF-related knowledge compared to SC (P < 0.001), increased patient awareness about risk factors, led to high medication adherence, and encouraged patients to ask health-related questions. However, in-person education did not show an effect on the primary outcome compared to SC [HR 1.02 (0.91–1.14); P = 0.80] that was also not the case when comparing online education vs. SC [HR 1.18 (0.95–1.46), P = 0.65]. Exploratory subgroup analyses showed a heterogeneous effect over the centres, but a positive impact of in-person education in patients with asymptomatic AF, being 70 years old or younger, and without a history of heart failure.
AF-EduCare showed that intensive targeted patient education did not lead to less unplanned cardiovascular events in the AF patient population as a whole, although subgroups might benefit.
Targeted education of patients with AF (i.e. focusing on individual knowledge gaps and aiming to improve self-care capabilities), delivered in-person or online, significantly improved AF-related knowledge compared to standard care, increased patient awareness about risk factors, and led to high medication adherence.
Nevertheless, such education did not lead to less unplanned cardiovascular events, although there might be an effect on cardiovascular and total mortality and certain subgroups may benefit.
Guideline-directed therapy, personal contact with a healthcare provider, and fast medical actionability may be important components of integrated care and not patient education and empowerment alone.
Introduction
Given the complexity of the management of patients with atrial fibrillation (AF), a holistic, integrated care approach is recommended in international guidelines.1,2 Integrated AF care is advocated in order to impact the 1.5–3.5-fold increased risk for mortality and 10–40% annual hospitalization rate of patients with AF. Moreover, more than 60% of patients with AF have an impaired quality of life.1 Patient education and empowerment have been proposed as important pillars of such integrated care.1,2
Over the past years, various trials have been performed delivering integrated care for AF with the aim to improve various outcomes (e.g. all-cause or cardiovascular mortality, hospitalization rates, and emergency department visits).3–10 Integrated care is a complex interplay of many key components, including patient-centred care, delivering treatment conform the ABC approach, patient education and engagement, promotion of medication adherence, a multidisciplinary team approach, and a structured follow-up.11 It goes beyond AF management alone and all trials translated and implemented this concept in a different way. Most of these trials were nurse-led, and used heterogeneous approaches to deliver integrated care (e.g. home-based visits, outpatient care, app-based follow-up, and making use of decision support systems). Moreover, they often included specific AF subpopulations (e.g. chronic AF patients and patients newly diagnosed with AF) that were recruited in specific settings (e.g. primary care, outpatient clinic, and emergency department).1 Patient education was part of most of the interventions, but not in a reinforced patient-targeted way (i.e. without assessment of the individual education needs of the patient). Various educational trials aimed to improve the knowledge of AF patients using diverse methods including brochures,12–15 educational videos,13,15 apps,16 group education sessions,15 and general face-to-face education,14,17 all showing diverse results. In previous research, we proved that the Jessa Atrial fibrillation Knowledge Questionnaire (JAKQ) is a suitable tool to provide individualized education for AF patients on a regular basis to significantly improve and maintain patients’ knowledge level over time.18
The results of the nurse-led integrated care trials were mixed. Hendriks et al.,3 Carter et al.,5 and van den Dries et al.,7 showed a positive effect of nurse-led follow-up on (cardiovascular) mortality and/or cardiovascular first hospitalization and/or AF-related emergency department visits, but in three totally different populations. Other studies did not show a statistically significant impact on various primary endpoints, like all-cause mortality and/or unplanned readmissions, a composite of cardiovascular death and cardiovascular hospital admissions, a composite of AF-related emergency department visits, and unplanned cardiovascular hospitalizations. When education was part of the integrated care intervention, it sometimes focused on education of the caregivers rather than patients: IMPACT-AF19 showed that this led to a higher implementation of anticoagulation, with a resultant decrease in stroke, while STEEER-AF20 and EHRA-PATHS,21 both ESC- and EHRA-led trials, and AFFIRMO22 will report later.
Therefore, it remains unknown in how far patient education contributes to any beneficial results, and whether any effectiveness is generalizable to the entire AF population. These two aspects formed the focus of the AF-EduCare trial. The main hypothesis of the AF-EduCare trial was that structured and targeted education of unselected patients with AF (delivered in two different ways, i.e. in-person or via an online platform), and empowering the patients in their self-care, would lead to a reduction of unplanned cardiovascular events during follow-up.
Methods
Study design and population
The study rationale and its design have been described previously.23 The AF-EduCare study was an open label, prospective, multicentre, randomized clinical trial with blinded endpoint adjudication, conducted in three large Flemish tertiary care centres (Antwerp University Hospital, Jessa Hospital Hasselt, and University Hospital Leuven). Consecutive patients with AF were assessed for eligibility when hospitalized at the Department of Cardiology or when presenting for an outpatient visit in one of the participating centres. Patients > 18 years of age were eligible if they had AF or atrial flutter diagnosed with an electrocardiogram. Exclusion criteria consisted of (1) the inability to speak and read Dutch, (2) cognitive impairment (e.g. severe dementia), (3) a life expectancy estimated <1 year, (4) participation in another randomized clinical trial, and (5) being pregnant. Patients willing to participate were randomly assigned to one of three study arms (see below) and were followed for a minimum of 18 months (i.e. all patients were followed until the last included patient had completed 18 months follow-up). Based on a common study end for all patients, a mean estimated follow-up of ∼27 months was anticipated.23 This duration and type of follow-up were chosen based on various aspects: (i) it was similar to prior trials on integrated AF care, (ii) the number of events needed to accumulate for the primary endpoint, and (iii) education and empowerment of patients would require some time before it might translate into real behavioural change and outcomes. The study was conducted in compliance with the Declaration of Helsinki and approved by the ethics committees of the participating centres. AF-EduCare was registered on ClinicalTrials.gov (NCT03707873) and was funded by the Research Foundation - Flanders (grant T002917N).
Randomization and interventions
Patients were randomly assigned to in-person education, online education, or standard care (SC). Later, a fourth group for randomization was added for a substudy (app-driven education; AF-EduApp Study—NCT03788044), with shorter follow-up, reported earlier.24 Randomization was stratified for age, highest educational degree, duration of AF, and place of inclusion (i.e. cardiology ward or outpatient clinic). Patients in the in-person group were provided education by a trained AF study team member (a physician, nurse, or master in biomedical sciences with specific training and ≥1 year experience in AF) at the outpatient clinic and/or ward. Patients in the online and app groups had access to an online education platform via a browser or through an in-house developed AF-EduApp smartphone application.24,25
All included patients were given a general brochure about AF, which was the SC in all centres at the outset of AF-EduCare. In this way, all groups received at least the same standardized information at the start of the study. Education of the intervention groups (at baseline, 1, 3, 6, 12, and every 6 months until study end) focused on four aspects of integrated AF care: (i) knowledge on AF and oral anticoagulation (OAC), directed by the JAKQ26 that is a suitable instrument to provide targeted education and increase knowledge18; (ii) risk factor awareness (e.g. overweight, hypertension, and smoking) and discussions with the patient on how risk factors could be improved, guided by a newly developed self-care questionnaire (SCQ); (iii) further optimization of medication adherence by assessing intake via an electronic Medication Event Monitoring System (MEMS; Aardex Group, Belgium) both at baseline and after 12 months (each for a period of three months), and initiating additional telemonitoring-based feedback in those with an adherence < 80%27; and (iv) education of patients on how they could easily reach the AF team for AF- or management-related questions (by telephone or online). The AF team was in close contact with the treating cardiologists and implemented their suggested diagnostic or therapeutic actions. Education was given in a comprehensive and targeted18 manner focused on the knowledge gaps concerning the arrhythmia, patients’ self-care capabilities, and adherence behaviour.
Although online intervention patients visited the hospital less frequently, they received notifications via email and via the online platform for targeted education sessions. Patients who were randomly assigned to the online (or app) group but lacked access to a computer or tablet with an internet connection (or were unable to use it) were considered ‘unsuitable’ for these interventions. Unsuitable patients were followed as an extra control group with the same study follow-up as the SC group. Nevertheless, all analyses were primarily performed as intention-to-treat (ITT) analyses between the three study groups [in-person, online (suitable and unsuitable combined), and SC]. We performed secondary on-treatment (OT) analyses where appropriate to get a better understanding into the effectiveness of the interventions for impact on knowledge and adherence.
Outcomes and data collection
The primary outcome parameter of the AF-EduCare study was the cumulative occurrence of cardiovascular events including cardiovascular death, unplanned cardiovascular hospitalizations (first and recurrent), unplanned cardiovascular outpatient visits (first and recurrent), and emergency department (ED) visits for cardiovascular reasons (first and recurrent). An overview of all outcome parameters has been published previously.23
Data collection and stratified randomization were performed in an encoded electronic Case Report Form (eCRF). AF patients who were not eligible or not willing to participate were also logged in the eCRF to avoid readdressing these candidates twice during the inclusion period. Baseline demographic data and medical history of the included patients were retrieved from the patients’ hospital files. All study contacts and questions asked by patients, together with all presented and answered questionnaires, were logged in the eCRF. Details about the used questionnaires can be found in the published design paper.23 After 12 or 18 months, a study-specific Patient Reported Outcome Measure (PROM) questionnaire was used to assess patients’ satisfaction with the educational follow-up they received.
Outcome parameters were recorded during every 6-month follow-up visit in the in-person education group or via telephone follow-up in the online education and SC groups. Patients were asked to keep a diary of all their physician contacts. Additionally, the patients’ electronic health file and the Belgian interhospital exchange platform for medical reports (CoZo) were double-checked during these follow-up moments and at the end of the study. CoZo is an online medical data exchange system, only accessible by the patient and physicians with a therapeutic relationship (if consent is given by the patient, which was a requirement for study participation). CoZo allowed to trace events outside the including centres. A blinded endpoint adjudication committee reviewed primary outcome events, especially evaluating whether these were cardiovascular or not, and planned or not.
Statistical analysis
Power calculations indicated the need for study groups of 346 patients, based on the hypothesis that targeted in-person education would reduce the primary outcome event rate by 20% (60.8–48.6%), resulting in a total of 1038 AF patients, as previously described.23 Statistics were performed using SPSS 29.0 and SAS 9.4. Differences in demographic data were analysed using the chi-square test, one-way analysis of variance (ANOVA) and Kruskal–Wallis test, depending on the type and the normality of the data. Normal distribution was tested by means of the Shapiro–Wilk test. The primary analysis of the AF-EduCare study was based on the Li–Lagakos modification of the Wei–Lin–Weissfeld method evaluating fatal and recurrent events, comparing pairwise the three treatment groups.28 The Bonferroni correction was applied to correct for multiple testing (i.e. alpha-level of 0.0167 for significance). The Wald test was used to calculate the P-values in the primary and subgroup analyses, given its robustness in case of differences in the effects for each event. The average effect test was used to calculate the hazard ratios and accompanying confidence intervals. The number of patients in each group experiencing at least one primary endpoint event was analysed by a chi-square test. A Kaplan–Meier survival analysis for first, second, or third event was also generated for the three study groups with an evaluation making use of the Log-rank test and Cox regression analyses.
Differences in impact of education on knowledge, self-care, adherence, and satisfaction were tested between the three groups with chi-square tests, one-way ANOVA analyses, and Kruskal–Wallis tests, as appropriate.
Results
Patient demographics
Starting from September 2018, a total of 1979 patients were evaluated for eligibility (Figure 1) of which 128 (6.5%) were excluded, mostly due to cognitive impairment (50.0% of the exclusions) or inability to speak Dutch (34.4%). Of the eligible patients, one out of three (33.4%) was not willing to participate, with the two most commonly cited reasons ‘transportation problems’ (42.0%) and ‘no interest’ (38.4%). Eventually, 1232 patients were included, of which 345 were randomized to the in-person education group, 347 to the online education group, 346 to SC, and 194 to the later added app-based education group. As indicated above, this last group was not included in any of the analyses described below given its shorter follow-up and different primary endpoint. The demographics of the patient cohorts for all initially evaluated patients, included those not randomized, has been described before.29

Flowchart showing enrolment and follow-up of patients in the AF-EduCare trial.
The last patient completed the study in September 2022. After blinded endpoint adjudication and data cleaning, the database with 1038 patients was locked in December 2023. Patients had a mean age of 69.8 ± 9.2 years, 69.3% were male, and had various cardiovascular and non-cardiovascular comorbidities (see Supplementary material online, Table S1). New-onset AF was present in 13.6% and the average time since first AF diagnosis was 6.0 ± 7.3 years. Mean CHA2DS2-VASc score was 3.1 ± 1.7, and 41.4% of the patients were completely asymptomatic (mEHRA = 1). The three groups were overall well matched, except that some more patients in the SC group were included while attending the outpatient clinic for a non-AF reason, were slightly more physically inactive, and were less frequently taking ACE inhibitors.
Patients had a mean follow-up of 26.9 ± 9.4 months (in-person: 26.7 ± 10.4 months; online ITT: 27.9 ± 9.6 months; SC: 28.8 ± 8.6 months; P = 0.086). During the study, 47 patients (4.5%) died, 14 were lost to follow-up (1.3%), and 88 dropped out (8.5%) (Figure 1). Adherence to the protocol-mandated education moments with the JAKQ (i.e. six out of six) was 76.8% in the in-person education group and 82.9% in the online education group (P = 0.066).
Impact on cardiovascular events and mortality
In the total population, the primary endpoint of cardiovascular death, unplanned cardiovascular hospitalizations, unplanned cardiovascular outpatient visits, or cardiovascular emergency department visits occurred 1062 times during the study, in 477 patients (46.0%). No significant difference between the groups was seen for the number of patients experiencing at least one primary endpoint event (43.2% in-person, 49.0% online, and 45.7% SC; P = 0.307). According to the main analysis using the Li and Lagakos modification of the Wei–Lin–Weissfeld method evaluating recurrent events, in-person education did not show an effect on the cumulative composite primary outcome compared to SC [hazard ratio (HR) 1.02 (0.91; 1.14); P = 0.80] (Figure 2 and Supplementary material online, Table S2). A similar conclusion is reached when comparing online education vs. SC [HR 1.18 (0.95; 1.46), P = 0.65] and in-person vs. online education [HR 0.88 (0.71; 1.09); P = 0.52]. Evaluating only the non-fatal events provides the same HRs as in the main analyses without any significant effect (P = 0.76 between in-person and SC, P = 0.66 between online and SC, P = 0.45 between in-person and online). Fatal cardiovascular events were numerically lower in the in-person education group (4 vs. 9 in online and 10 in SC), but this did not reach statistical significance [HR 0.67 (0.37; 1.18); P = 0.17 for in-person vs. SC].
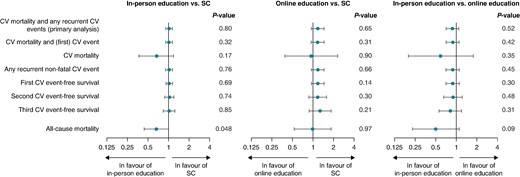
Forest plots showing hazard ratios (and 95% confidence intervals) to evaluate the impact of the educational intervention on unplanned cardiovascular events. Li and Lagakos tests of the Wei–Lin–Weissfeld method comparing pairwise the three treatment groups (in-person education group, online education group, and standard care group). The Bonferroni correction is applied to correct for multiple testing, i.e. each null hypothesis is tested with an alpha-level of 0.0167. In all analyses, the subject with the outlier of 27 events has been excluded for stability reasons. Events up to a maximum of 12 per patient during the study were taken into account.
Recurrent non-fatal events ranged up to a maximum of 12, 27, and 9 events in subjects in the in-person, online, and SC groups, respectively. Given the uncertainty for analysis with outliers, the main analysis was calculated with those with ≤3 events. Also, when restricting to subjects with a maximum of three events, there was no effect of in-person education compared to SC [HR 1.02 (0.91; 1.14); P = 0.65], nor on any of the other analyses (see Supplementary material online, Table S2).
For the first, second, and third events separately, similar HRs were observed as for the cumulative event analysis and did not reach statistical significance (Figure 2 and Supplementary material online, Table S2). Concordantly, a Kaplan–Meier analysis only looking at the first occurrence of the composite endpoint did not show any significant difference between the three groups [Log-rank P = 0.297 and Cox regression HR: 0.98 (0.88; 1.09); P = 0.674; Figure 3A]. The same was true for the second event [Log-rank P = 0.807 and Cox regression HR: 0.96 (0.82; 1.13); P = 0.633; Figure 3B] and the third event [Log-rank P = 0.331 and Cox regression HR: 0.98 (0.80; 1.19); P = 0.829; Figure 3C].
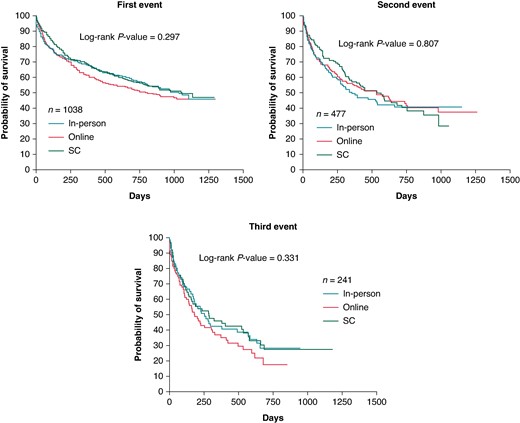
Kaplan–Meier curves showing the time to first, second, or third event of any component of the primary composite endpoint of unplanned cardiovascular events. The time to second or third event was taken as the interval in days after the first or second event, respectively. The Log-rank test was used to evaluate the equality of survival distributions between groups. For the first and second event curves, the last event-time was removed for the SC group. SC, standard care.
Figure 4A shows a visual distribution of the primary endpoint events and all-cause mortality by group. The relative frequencies of the patients with at least one event are shown in Figure 4B.
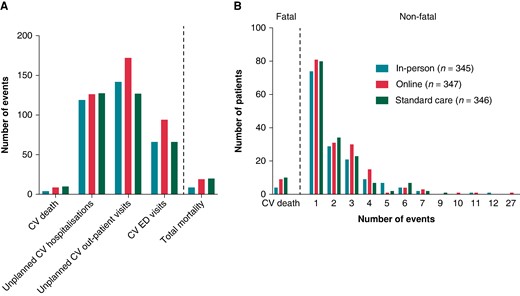
Contribution of components of the primary endpoint (A), and number of events throughout the study in each subgroup (B). Components of the primary endpoint and all-cause mortality by group. (A) The total number of the various components; (B) distribution of patients with at least one event.
Forty-seven patients (4.5%) died during the study (8 in-person, 19 online, 20 SC). Using the Li and Lagakos modification of the Wei–Lin–Weissfeld method, there was a HR of 0.66 (0.44; 0.99) in favour of in-person education vs. SC, with a P-value of 0.048. Given the multiple testing and not being part of the primary endpoint, this has to be considered as exploratory. There was no difference in total mortality between online education vs. SC [HR 0.99 (0.53; 1.84) P = 0.97], nor between in-person vs. online education [0.50 (0.23; 1.10); P = 0.09].
Subgroup analyses
The main analysis restricted to three events was used to assess, in an exploratory way, whether or not the intervention could have impacted specific subpopulations (Table 1). A first subanalysis showed that in AF patients who are 70 years old or younger (n = 507), the in-person education intervention had a significant beneficial effect compared to SC [0.88 (0.75; 1.03); P < 0.001] and to online education [0.63 (0.46; 0.86); P < 0.001], which was not seen in the more elderly patients with a HR of 1.01 (0.86; 1.17, P = 0.88) and of 0.96 (0.71; 1.30, P = 0.75), respectively. In patients without a history of congestive heart failure (n = 662), the in-person intervention was significantly more beneficial compared to SC [HR 0.82 (0.71; 0.95); P < 0.001] and to online education [HR 0.58 (0.43; 0.77); P < 0.001]. Also, in asymptomatic AF patients (mEHRA = 1, n = 430), the in-person education intervention had a beneficial effect compared to SC [HR 0.43 (0.36; 0.52); P < 0.001] and to online education [HR 0.34 (0.23; 0.49); P < 0.001], which was not seen in the symptomatic AF population (mEHRA ≥ 2a, n = 608) with a HR of 1.08 (0.94; 1.24, P = 0.68) and of 0.98 (0.76; 1.28, P = 0.71), respectively. In contrast, in-person and online education had a significantly negative impact compared to SC in patients who had undergone a catheter ablation in the past [n = 354; HR of 1.24 (1.02; 1.50) P < 0.001, and 1.67 (1.15; 2.42), P < 0.001, respectively]. The treating physician being an electrophysiologist or general cardiologist, the educational degree of the patient (high or low), the number of cardiovascular risk factors, recent AF diagnosis or not (<1 vs. ≥1 year), or CHA2DS2-VASc score could not distinguish the impact of in-person education vs. SC. The most remarkable result was the centre effect, with one hospital in which in-person education did not impact the primary outcome [HR 0.98 (0.81; 1.19); P = 0.99], another where there was a pronounced positive effect of in-person education compared to SC and online education [HR 0.31 (0.26; 0.38); P < 0.001 and 0.25 (0.18; 0.36); P < 0.001, respectively], while in the last one, a complete opposite result was noted with in-person education being significantly worse than SC [1.64 (1.35; 1.99); P < 0.001]. Supplementary material online, Table S3 shows that there were many demographic differences between the patient groups of the three hospitals, although it remains unclear what may have contributed to the different outcomes. Moreover, also the structure of the study team, and its integration within the clinical department, may have played a role in these differences.
Average hazard ratios (and 95% confidence intervals) evaluating the composite endpoint of unplanned cardiovascular events in various subgroups
| . | In-person vs. SC . | Online vs. SC . | In-person vs. online . | |||
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Treating cardiologist | ||||||
| Non-electrophysiologist (n = 427) | 0.94 (0.79; 1.11) | 0.70 | 1.07 (0.78; 1.46) | 0.44 | 0.82 (0.58; 1.15) | 0.32 |
| Electrophysiologist (n = 611) | 1.10 (0.95; 1.27) | 0.76 | 1.31 (0.98; 1.76) | 0.05 | 0.94 (0.71; 1.25) | 0.18 |
| Highest degree | ||||||
| Primary/secondary school (n = 613) | 1.04 (0.90; 1.21) | 0.65 | 1.34 (1.01; 1.77) | 0.17 | 0.82 (0.62; 1.09) | 0.49 |
| College/university (n = 425) | 0.99 (0.84; 1.18) | 0.46 | 0.99 (0.71; 1.38) | 0.78 | 0.99 (0.71; 1.39) | 0.93 |
| Age ≤ 70 years old | ||||||
| Yes (n = 507) | 0.88 (0.75; 1.03) | <0.001 | 1.33 (0.98; 1.82) | 0.15 | 0.63 (0.46; 0.86) | <0.001 |
| No (n = 531) | 1.01 (0.86; 1.17) | 0.88 | 1.06 (0.79; 1.43) | 0.98 | 0.96 (0.71; 1.30) | 0.75 |
| History of congestive heart failure | ||||||
| Yes (n = 376) | 0.95 (0.81; 1.13) | 0.88 | 1.10 (0.80; 1.51) | 0.74 | 0.81 (0.59; 1.13) | 0.45 |
| No (n = 662) | 0.82 (0.71; 0.95) | <0.001 | 1.18 (0.87; 1.61) | 0.28 | 0.58 (0.43; 0.77) | <0.001 |
| At least two cardiovascular risk factors | ||||||
| Yes (n = 728) | 1.07 (0.94; 1.22) | 0.26 | 1.28 (0.99; 1.64) | 0.41 | 0.90 (0.69; 1.16) | 0.30 |
| No (n = 310) | 0.94 (0.76; 1.15) | 0.39 | 1.00 (0.67; 1.49) | 0.91 | 0.87 (0.58; 1.33) | 0.62 |
| At least three cardiovascular risk factors | ||||||
| Yes (n = 369) | 1.02 (0.85; 1.22) | 0.42 | 1.44 (1.03; 2.02) | 0.11 | 0.72 (0.51; 1.02) | 0.24 |
| No (n = 642) | 1.02 (0.89; 1.18) | 0.78 | 1.03 (0.78; 1.36) | 0.81 | 1.02 (0.77; 1.35) | 0.98 |
| Catheter ablation in history | ||||||
| Yes (n = 354) | 1.24 (1.02; 1.50) | <0.001 | 1.67 (1.15; 2.42) | <0.001 | 0.75 (0.51; 1.10) | 0.36 |
| No (n = 684) | 1.05 (0.92; 1.20) | 0.24 | 1.14 (0.87; 1.48) | 0.77 | 0.96 (0.74; 1.25) | 0.63 |
| AF diagnosis < 1 year | ||||||
| Yes (n = 327) | 1.12 (0.91; 1.36) | 0.37 | 1.42 (0.97; 2.06) | 0.20 | 0.90 (0.62; 1.31) | 0.21 |
| No (n = 711) | 0.98 (0.86; 1.12) | 0.73 | 1.09 (0.84; 1.42) | 0.93 | 0.89 (0.68; 1.17) | 0.50 |
| mEHRA score ≥ 2a | ||||||
| Yes (n = 608) | 1.08 (0.94; 1.24) | 0.68 | 1.20 (0.92; 1.57) | 0.70 | 0.98 (0.76; 1.28) | 0.71 |
| No (n = 430) | 0.43 (0.36; 0.52) | <0.001 | 1.12 (0.78; 1.61) | 0.53 | 0.34 (0.23; 0.49) | <0.001 |
| CHA₂DS₂-VASc score ≥ 2 (men) or ≥3 (women) | ||||||
| Yes (n = 801) | 1.04 (0.92; 1.18) | 0.48 | 1.21 (0.96; 1.54) | 0.50 | 0.90 (0.71; 1.15) | 0.68 |
| No (n = 237) | – | – | – | – | – | – |
| CHA₂DS₂-VASc score ≥ 3 (men) or ≥4 (women) | ||||||
| Yes (n = 574) | 1.04 (0.90; 1.21) | 0.50 | 1.27 (0.96; 1.67) | 0.52 | 0.86 (0.65; 1.14) | 0.61 |
| No (n = 464) | 0.89 (0.75; 1.06) | <0.001 | 0.88 (0.63; 1.23) | <0.001 | – | – |
| Patient contacted team with at least one clinical question throughout the study | ||||||
| Yes (n = 160) | 1.35 (1.03; 1.76) | <0.001 | 3.84 (2.23; 6.63) | <0.001 | 1.05 (0.71; 1.55) | 0.34 |
| No (n = 878) | 0.94 (0.82; 1.07) | 0.80 | 1.16 (0.91; 1.46) | 0.79 | 0.76 (0.58; 1.00) | 0.39 |
| Centre | ||||||
| Hospital 1 | 0.98 (0.81; 1.19) | 0.99 | 1.16 (0.82; 1.64) | 0.87 | 0.83 (0.56; 1.21) | 0.77 |
| Hospital 2 | 0.31 (0.26; 0.38) | <0.001 | 1.19 (0.84; 1.69) | 0.57 | 0.25 (0.18; 0.36) | <0.001 |
| Hospital 3 | 1.64 (1.35; 1.99) | <0.001 | 2.20 (1.43; 3.37) | <0.001 | 1.40 (0.93; 2.10) | 0.56 |
| . | In-person vs. SC . | Online vs. SC . | In-person vs. online . | |||
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Treating cardiologist | ||||||
| Non-electrophysiologist (n = 427) | 0.94 (0.79; 1.11) | 0.70 | 1.07 (0.78; 1.46) | 0.44 | 0.82 (0.58; 1.15) | 0.32 |
| Electrophysiologist (n = 611) | 1.10 (0.95; 1.27) | 0.76 | 1.31 (0.98; 1.76) | 0.05 | 0.94 (0.71; 1.25) | 0.18 |
| Highest degree | ||||||
| Primary/secondary school (n = 613) | 1.04 (0.90; 1.21) | 0.65 | 1.34 (1.01; 1.77) | 0.17 | 0.82 (0.62; 1.09) | 0.49 |
| College/university (n = 425) | 0.99 (0.84; 1.18) | 0.46 | 0.99 (0.71; 1.38) | 0.78 | 0.99 (0.71; 1.39) | 0.93 |
| Age ≤ 70 years old | ||||||
| Yes (n = 507) | 0.88 (0.75; 1.03) | <0.001 | 1.33 (0.98; 1.82) | 0.15 | 0.63 (0.46; 0.86) | <0.001 |
| No (n = 531) | 1.01 (0.86; 1.17) | 0.88 | 1.06 (0.79; 1.43) | 0.98 | 0.96 (0.71; 1.30) | 0.75 |
| History of congestive heart failure | ||||||
| Yes (n = 376) | 0.95 (0.81; 1.13) | 0.88 | 1.10 (0.80; 1.51) | 0.74 | 0.81 (0.59; 1.13) | 0.45 |
| No (n = 662) | 0.82 (0.71; 0.95) | <0.001 | 1.18 (0.87; 1.61) | 0.28 | 0.58 (0.43; 0.77) | <0.001 |
| At least two cardiovascular risk factors | ||||||
| Yes (n = 728) | 1.07 (0.94; 1.22) | 0.26 | 1.28 (0.99; 1.64) | 0.41 | 0.90 (0.69; 1.16) | 0.30 |
| No (n = 310) | 0.94 (0.76; 1.15) | 0.39 | 1.00 (0.67; 1.49) | 0.91 | 0.87 (0.58; 1.33) | 0.62 |
| At least three cardiovascular risk factors | ||||||
| Yes (n = 369) | 1.02 (0.85; 1.22) | 0.42 | 1.44 (1.03; 2.02) | 0.11 | 0.72 (0.51; 1.02) | 0.24 |
| No (n = 642) | 1.02 (0.89; 1.18) | 0.78 | 1.03 (0.78; 1.36) | 0.81 | 1.02 (0.77; 1.35) | 0.98 |
| Catheter ablation in history | ||||||
| Yes (n = 354) | 1.24 (1.02; 1.50) | <0.001 | 1.67 (1.15; 2.42) | <0.001 | 0.75 (0.51; 1.10) | 0.36 |
| No (n = 684) | 1.05 (0.92; 1.20) | 0.24 | 1.14 (0.87; 1.48) | 0.77 | 0.96 (0.74; 1.25) | 0.63 |
| AF diagnosis < 1 year | ||||||
| Yes (n = 327) | 1.12 (0.91; 1.36) | 0.37 | 1.42 (0.97; 2.06) | 0.20 | 0.90 (0.62; 1.31) | 0.21 |
| No (n = 711) | 0.98 (0.86; 1.12) | 0.73 | 1.09 (0.84; 1.42) | 0.93 | 0.89 (0.68; 1.17) | 0.50 |
| mEHRA score ≥ 2a | ||||||
| Yes (n = 608) | 1.08 (0.94; 1.24) | 0.68 | 1.20 (0.92; 1.57) | 0.70 | 0.98 (0.76; 1.28) | 0.71 |
| No (n = 430) | 0.43 (0.36; 0.52) | <0.001 | 1.12 (0.78; 1.61) | 0.53 | 0.34 (0.23; 0.49) | <0.001 |
| CHA₂DS₂-VASc score ≥ 2 (men) or ≥3 (women) | ||||||
| Yes (n = 801) | 1.04 (0.92; 1.18) | 0.48 | 1.21 (0.96; 1.54) | 0.50 | 0.90 (0.71; 1.15) | 0.68 |
| No (n = 237) | – | – | – | – | – | – |
| CHA₂DS₂-VASc score ≥ 3 (men) or ≥4 (women) | ||||||
| Yes (n = 574) | 1.04 (0.90; 1.21) | 0.50 | 1.27 (0.96; 1.67) | 0.52 | 0.86 (0.65; 1.14) | 0.61 |
| No (n = 464) | 0.89 (0.75; 1.06) | <0.001 | 0.88 (0.63; 1.23) | <0.001 | – | – |
| Patient contacted team with at least one clinical question throughout the study | ||||||
| Yes (n = 160) | 1.35 (1.03; 1.76) | <0.001 | 3.84 (2.23; 6.63) | <0.001 | 1.05 (0.71; 1.55) | 0.34 |
| No (n = 878) | 0.94 (0.82; 1.07) | 0.80 | 1.16 (0.91; 1.46) | 0.79 | 0.76 (0.58; 1.00) | 0.39 |
| Centre | ||||||
| Hospital 1 | 0.98 (0.81; 1.19) | 0.99 | 1.16 (0.82; 1.64) | 0.87 | 0.83 (0.56; 1.21) | 0.77 |
| Hospital 2 | 0.31 (0.26; 0.38) | <0.001 | 1.19 (0.84; 1.69) | 0.57 | 0.25 (0.18; 0.36) | <0.001 |
| Hospital 3 | 1.64 (1.35; 1.99) | <0.001 | 2.20 (1.43; 3.37) | <0.001 | 1.40 (0.93; 2.10) | 0.56 |
Li and Lagakos tests of the Wei–Lin–Weissfeld method restricting the number of events to three per subject given that the minority of the subjects had four events or more. The Bonferroni correction is applied to correct for multiple testing, i.e. each null hypothesis is tested with an alpha-level of 0.0167. The Wald test was used to calculate the P-values, given its robustness in case of differences in the effects for each event. The average effect test was used to calculate the hazard ratios and accompanying confidence intervals. Significant P-values are depicted in bold. Only the global test of the effect over all recurrent events is shown (thus including both fatal and non-fatal unplanned cardiovascular events). If no values are shown, this means that no fatal events occurred.
Average hazard ratios (and 95% confidence intervals) evaluating the composite endpoint of unplanned cardiovascular events in various subgroups
| . | In-person vs. SC . | Online vs. SC . | In-person vs. online . | |||
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Treating cardiologist | ||||||
| Non-electrophysiologist (n = 427) | 0.94 (0.79; 1.11) | 0.70 | 1.07 (0.78; 1.46) | 0.44 | 0.82 (0.58; 1.15) | 0.32 |
| Electrophysiologist (n = 611) | 1.10 (0.95; 1.27) | 0.76 | 1.31 (0.98; 1.76) | 0.05 | 0.94 (0.71; 1.25) | 0.18 |
| Highest degree | ||||||
| Primary/secondary school (n = 613) | 1.04 (0.90; 1.21) | 0.65 | 1.34 (1.01; 1.77) | 0.17 | 0.82 (0.62; 1.09) | 0.49 |
| College/university (n = 425) | 0.99 (0.84; 1.18) | 0.46 | 0.99 (0.71; 1.38) | 0.78 | 0.99 (0.71; 1.39) | 0.93 |
| Age ≤ 70 years old | ||||||
| Yes (n = 507) | 0.88 (0.75; 1.03) | <0.001 | 1.33 (0.98; 1.82) | 0.15 | 0.63 (0.46; 0.86) | <0.001 |
| No (n = 531) | 1.01 (0.86; 1.17) | 0.88 | 1.06 (0.79; 1.43) | 0.98 | 0.96 (0.71; 1.30) | 0.75 |
| History of congestive heart failure | ||||||
| Yes (n = 376) | 0.95 (0.81; 1.13) | 0.88 | 1.10 (0.80; 1.51) | 0.74 | 0.81 (0.59; 1.13) | 0.45 |
| No (n = 662) | 0.82 (0.71; 0.95) | <0.001 | 1.18 (0.87; 1.61) | 0.28 | 0.58 (0.43; 0.77) | <0.001 |
| At least two cardiovascular risk factors | ||||||
| Yes (n = 728) | 1.07 (0.94; 1.22) | 0.26 | 1.28 (0.99; 1.64) | 0.41 | 0.90 (0.69; 1.16) | 0.30 |
| No (n = 310) | 0.94 (0.76; 1.15) | 0.39 | 1.00 (0.67; 1.49) | 0.91 | 0.87 (0.58; 1.33) | 0.62 |
| At least three cardiovascular risk factors | ||||||
| Yes (n = 369) | 1.02 (0.85; 1.22) | 0.42 | 1.44 (1.03; 2.02) | 0.11 | 0.72 (0.51; 1.02) | 0.24 |
| No (n = 642) | 1.02 (0.89; 1.18) | 0.78 | 1.03 (0.78; 1.36) | 0.81 | 1.02 (0.77; 1.35) | 0.98 |
| Catheter ablation in history | ||||||
| Yes (n = 354) | 1.24 (1.02; 1.50) | <0.001 | 1.67 (1.15; 2.42) | <0.001 | 0.75 (0.51; 1.10) | 0.36 |
| No (n = 684) | 1.05 (0.92; 1.20) | 0.24 | 1.14 (0.87; 1.48) | 0.77 | 0.96 (0.74; 1.25) | 0.63 |
| AF diagnosis < 1 year | ||||||
| Yes (n = 327) | 1.12 (0.91; 1.36) | 0.37 | 1.42 (0.97; 2.06) | 0.20 | 0.90 (0.62; 1.31) | 0.21 |
| No (n = 711) | 0.98 (0.86; 1.12) | 0.73 | 1.09 (0.84; 1.42) | 0.93 | 0.89 (0.68; 1.17) | 0.50 |
| mEHRA score ≥ 2a | ||||||
| Yes (n = 608) | 1.08 (0.94; 1.24) | 0.68 | 1.20 (0.92; 1.57) | 0.70 | 0.98 (0.76; 1.28) | 0.71 |
| No (n = 430) | 0.43 (0.36; 0.52) | <0.001 | 1.12 (0.78; 1.61) | 0.53 | 0.34 (0.23; 0.49) | <0.001 |
| CHA₂DS₂-VASc score ≥ 2 (men) or ≥3 (women) | ||||||
| Yes (n = 801) | 1.04 (0.92; 1.18) | 0.48 | 1.21 (0.96; 1.54) | 0.50 | 0.90 (0.71; 1.15) | 0.68 |
| No (n = 237) | – | – | – | – | – | – |
| CHA₂DS₂-VASc score ≥ 3 (men) or ≥4 (women) | ||||||
| Yes (n = 574) | 1.04 (0.90; 1.21) | 0.50 | 1.27 (0.96; 1.67) | 0.52 | 0.86 (0.65; 1.14) | 0.61 |
| No (n = 464) | 0.89 (0.75; 1.06) | <0.001 | 0.88 (0.63; 1.23) | <0.001 | – | – |
| Patient contacted team with at least one clinical question throughout the study | ||||||
| Yes (n = 160) | 1.35 (1.03; 1.76) | <0.001 | 3.84 (2.23; 6.63) | <0.001 | 1.05 (0.71; 1.55) | 0.34 |
| No (n = 878) | 0.94 (0.82; 1.07) | 0.80 | 1.16 (0.91; 1.46) | 0.79 | 0.76 (0.58; 1.00) | 0.39 |
| Centre | ||||||
| Hospital 1 | 0.98 (0.81; 1.19) | 0.99 | 1.16 (0.82; 1.64) | 0.87 | 0.83 (0.56; 1.21) | 0.77 |
| Hospital 2 | 0.31 (0.26; 0.38) | <0.001 | 1.19 (0.84; 1.69) | 0.57 | 0.25 (0.18; 0.36) | <0.001 |
| Hospital 3 | 1.64 (1.35; 1.99) | <0.001 | 2.20 (1.43; 3.37) | <0.001 | 1.40 (0.93; 2.10) | 0.56 |
| . | In-person vs. SC . | Online vs. SC . | In-person vs. online . | |||
|---|---|---|---|---|---|---|
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Treating cardiologist | ||||||
| Non-electrophysiologist (n = 427) | 0.94 (0.79; 1.11) | 0.70 | 1.07 (0.78; 1.46) | 0.44 | 0.82 (0.58; 1.15) | 0.32 |
| Electrophysiologist (n = 611) | 1.10 (0.95; 1.27) | 0.76 | 1.31 (0.98; 1.76) | 0.05 | 0.94 (0.71; 1.25) | 0.18 |
| Highest degree | ||||||
| Primary/secondary school (n = 613) | 1.04 (0.90; 1.21) | 0.65 | 1.34 (1.01; 1.77) | 0.17 | 0.82 (0.62; 1.09) | 0.49 |
| College/university (n = 425) | 0.99 (0.84; 1.18) | 0.46 | 0.99 (0.71; 1.38) | 0.78 | 0.99 (0.71; 1.39) | 0.93 |
| Age ≤ 70 years old | ||||||
| Yes (n = 507) | 0.88 (0.75; 1.03) | <0.001 | 1.33 (0.98; 1.82) | 0.15 | 0.63 (0.46; 0.86) | <0.001 |
| No (n = 531) | 1.01 (0.86; 1.17) | 0.88 | 1.06 (0.79; 1.43) | 0.98 | 0.96 (0.71; 1.30) | 0.75 |
| History of congestive heart failure | ||||||
| Yes (n = 376) | 0.95 (0.81; 1.13) | 0.88 | 1.10 (0.80; 1.51) | 0.74 | 0.81 (0.59; 1.13) | 0.45 |
| No (n = 662) | 0.82 (0.71; 0.95) | <0.001 | 1.18 (0.87; 1.61) | 0.28 | 0.58 (0.43; 0.77) | <0.001 |
| At least two cardiovascular risk factors | ||||||
| Yes (n = 728) | 1.07 (0.94; 1.22) | 0.26 | 1.28 (0.99; 1.64) | 0.41 | 0.90 (0.69; 1.16) | 0.30 |
| No (n = 310) | 0.94 (0.76; 1.15) | 0.39 | 1.00 (0.67; 1.49) | 0.91 | 0.87 (0.58; 1.33) | 0.62 |
| At least three cardiovascular risk factors | ||||||
| Yes (n = 369) | 1.02 (0.85; 1.22) | 0.42 | 1.44 (1.03; 2.02) | 0.11 | 0.72 (0.51; 1.02) | 0.24 |
| No (n = 642) | 1.02 (0.89; 1.18) | 0.78 | 1.03 (0.78; 1.36) | 0.81 | 1.02 (0.77; 1.35) | 0.98 |
| Catheter ablation in history | ||||||
| Yes (n = 354) | 1.24 (1.02; 1.50) | <0.001 | 1.67 (1.15; 2.42) | <0.001 | 0.75 (0.51; 1.10) | 0.36 |
| No (n = 684) | 1.05 (0.92; 1.20) | 0.24 | 1.14 (0.87; 1.48) | 0.77 | 0.96 (0.74; 1.25) | 0.63 |
| AF diagnosis < 1 year | ||||||
| Yes (n = 327) | 1.12 (0.91; 1.36) | 0.37 | 1.42 (0.97; 2.06) | 0.20 | 0.90 (0.62; 1.31) | 0.21 |
| No (n = 711) | 0.98 (0.86; 1.12) | 0.73 | 1.09 (0.84; 1.42) | 0.93 | 0.89 (0.68; 1.17) | 0.50 |
| mEHRA score ≥ 2a | ||||||
| Yes (n = 608) | 1.08 (0.94; 1.24) | 0.68 | 1.20 (0.92; 1.57) | 0.70 | 0.98 (0.76; 1.28) | 0.71 |
| No (n = 430) | 0.43 (0.36; 0.52) | <0.001 | 1.12 (0.78; 1.61) | 0.53 | 0.34 (0.23; 0.49) | <0.001 |
| CHA₂DS₂-VASc score ≥ 2 (men) or ≥3 (women) | ||||||
| Yes (n = 801) | 1.04 (0.92; 1.18) | 0.48 | 1.21 (0.96; 1.54) | 0.50 | 0.90 (0.71; 1.15) | 0.68 |
| No (n = 237) | – | – | – | – | – | – |
| CHA₂DS₂-VASc score ≥ 3 (men) or ≥4 (women) | ||||||
| Yes (n = 574) | 1.04 (0.90; 1.21) | 0.50 | 1.27 (0.96; 1.67) | 0.52 | 0.86 (0.65; 1.14) | 0.61 |
| No (n = 464) | 0.89 (0.75; 1.06) | <0.001 | 0.88 (0.63; 1.23) | <0.001 | – | – |
| Patient contacted team with at least one clinical question throughout the study | ||||||
| Yes (n = 160) | 1.35 (1.03; 1.76) | <0.001 | 3.84 (2.23; 6.63) | <0.001 | 1.05 (0.71; 1.55) | 0.34 |
| No (n = 878) | 0.94 (0.82; 1.07) | 0.80 | 1.16 (0.91; 1.46) | 0.79 | 0.76 (0.58; 1.00) | 0.39 |
| Centre | ||||||
| Hospital 1 | 0.98 (0.81; 1.19) | 0.99 | 1.16 (0.82; 1.64) | 0.87 | 0.83 (0.56; 1.21) | 0.77 |
| Hospital 2 | 0.31 (0.26; 0.38) | <0.001 | 1.19 (0.84; 1.69) | 0.57 | 0.25 (0.18; 0.36) | <0.001 |
| Hospital 3 | 1.64 (1.35; 1.99) | <0.001 | 2.20 (1.43; 3.37) | <0.001 | 1.40 (0.93; 2.10) | 0.56 |
Li and Lagakos tests of the Wei–Lin–Weissfeld method restricting the number of events to three per subject given that the minority of the subjects had four events or more. The Bonferroni correction is applied to correct for multiple testing, i.e. each null hypothesis is tested with an alpha-level of 0.0167. The Wald test was used to calculate the P-values, given its robustness in case of differences in the effects for each event. The average effect test was used to calculate the hazard ratios and accompanying confidence intervals. Significant P-values are depicted in bold. Only the global test of the effect over all recurrent events is shown (thus including both fatal and non-fatal unplanned cardiovascular events). If no values are shown, this means that no fatal events occurred.
Impact of the intervention on knowledge, risk factor awareness, and adherence
As stated above, the educational intervention was based on four main pillars: (i) knowledge on AF and OAC, directed by the JAKQ questionnaire; (ii) awareness about risk factors and possible corrective measures; (iii) reinforcement of medication adherence to OAC; and (iv) reachability for AF- or management-related questions.
Knowledge: A total of 903 patients (in-person: 288; online ITT: 302; SC: 313) completed the JAKQ after 18 months of follow-up. Education in both the in-person and online ITT groups led to significantly higher knowledge compared to SC: scores on the JAKQ were 86.5 ± 13.2%, 82.5 ± 19.3%, and 65.3 ± 16.6%, respectively (P < 0.001 for both vs. SC), without significant difference between in-person and online education (P = 0.175) (Figure 5A). Education in both groups significantly improved knowledge over time (P < 0.001 for both) and preserved it up till the end of the study (18 months) (Figure 5B).
Awareness and self-management of risk factors: At baseline and after 18 months of follow-up, 905 patients (Education ITT: 589; SC: 316) filled out the SCQ. For all patients (concerning medication and physical inactivity) or for those with a risk factor present from the medical evaluation at baseline as determined by the study team, awareness by the patients was evaluated. Whereas there was almost no improvement in SC patients from baseline to 18 months about the awareness and management of their risk factors, there was significant improvement for different aspects in education patients (Figure 6). Intriguingly, physical inactivity proved to be the hardest risk factor to modify, in all patients.
Adherence to anticoagulation intake: Electronic measurement of the intake adherence for OAC was measured in 513 patients at baseline, and proved to be high in all groups, likely due to a Hawthorn effect (in-person: 94.4 ± 8.3%; online: 93.9 ± 13.0%; SC: 91.3 ± 15.0%) (Figure 7A).27 After one year of follow-up, 414 patients underwent a second period of therapy monitoring, showing a further increased adherence in all groups (in-person: 95.4 ± 7.3%; online: 94.0 ± 10.8%; SC: 91.6 ± 14.0%), but now being significantly higher in the in-person or online education group than in the SC group (P = 0.006 and P = 0.042, respectively) (Figure 7B).
Contacts with the care team: Altogether, 160 patients contacted the care team with 347 clinically related questions [in-person: 192 questions (55.3%); online: 139 questions (40.1%); SC: 16 questions (4.6%) (Figure 8A); mean of 2.2 ± 2.2 questions per patient]. Significantly more patients in the education groups than SC contacted the team: in-person 88 (25.5%), online 62 (17.9%), and SC 10 (2.9%) (P < 0.001). Questions were mostly related to AF symptoms (n = 139, 40.1%), followed by medication-related questions (n = 115, 33.1%), questions about AF treatment and investigations (n = 55, 15.9%), and about self-recorded parameters during follow-up (n = 38, 11.0%). The study team spent an overall of 60.3 h answering clinically related questions during a mean follow-up of 26.9 ± 9.4 months. Most time was spent in the in-person group (36.2 h). The total duration per group and per type of clinically related questions is shown in Figure 8B
A PROM questionnaire at 12 or 18 months evaluated the patient's satisfaction with the received follow-up. In general, patients in each study arm were positive about the follow-up [median score of 8/10; interquartile range (IQR): 8–9], although the score was significantly higher in the in-person education group (9/10, IQR 8–10), both compared to online ITT (8/10, IQR: 7–9; P < 0.001) and to SC (8/10, IQR: 7–9; P < 0.001).
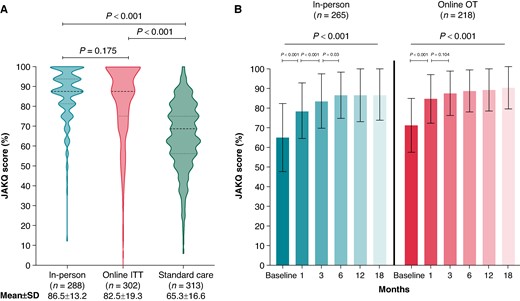
Knowledge level of the patients after 18 months of follow-up (A), and knowledge level over time for the on-treatment education groups (B). Knowledge scores based on the Jessa Atrial fibrillation Knowledge Questionnaire (JAKQ). (A) Difference in JAKQ score between the intention-to-treat groups at 18 months. (B) Mean JAKQ score over time within the different on-treatment education groups. JAKQ, Jessa Atrial fibrillation Knowledge Questionnaire; ITT, intention-to-treat; OT, on-treatment. Patients in the standard care group only received the JAKQ at 18 months (and at the end of the study if applicable) to minimally intervene and to not trigger these patients by giving them this knowledge questionnaire at baseline.
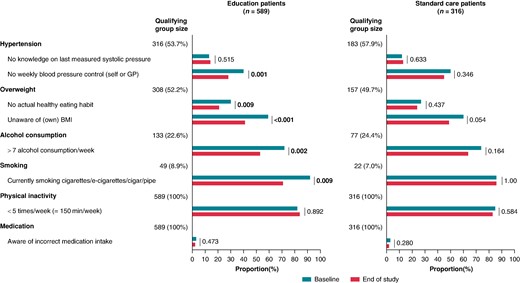
Awareness and self-management of risk factors. Analyses are on all patients with a risk factor present according to the medical file at baseline as determined by the study team (except medication and physical activity, which were not reported in the medical file and based on the baseline awareness assessment in all patients).
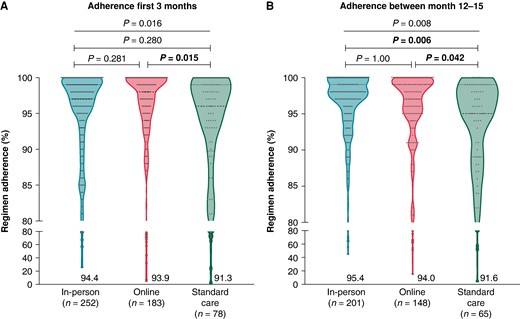
Therapy adherence to oral anticoagulation during the first (A) and second monitoring period (B). Regimen adherence to OAC between the study groups that was measured for 3 months between baseline and 3 months of follow-up (period 1; A) and between 12 months and 15 months (period 2; B) for patients who received MEMS monitoring during the study (i.e. on treatment analysis). Data are shown as violin plots with dots.
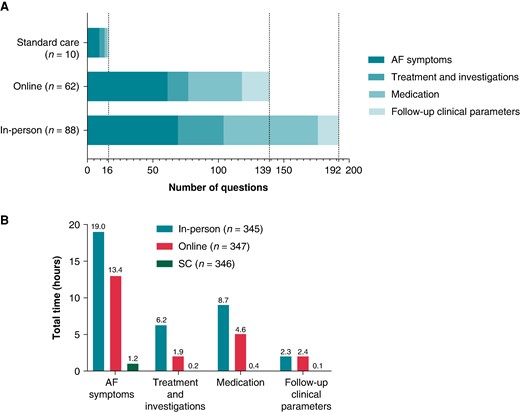
Time spent for communication in-between visits on clinically related questions. Bar charts with number of clinical related questions asked by study patients (A) and overall time needed to tackle the questions per group (B).
Discussion
The AF-EduCare study did not show a benefit of intensive, repeated, and targeted patient education on top of standard care on unplanned cardiovascular endpoints during medium-term follow-up in an unselected overall population of patients with AF. The main concept behind the AF-EduCare trial was the conviction, as expressed in AF practice guidelines worldwide, that educated patients would be more empowered to improve their self-care, which might lead to fewer complications and less need for urgent medical assistance. The intervention (both delivered during personal contacts with an AF nurse or via an online platform) indeed improved patient knowledge (based on the JAKQ), increased awareness about risk factors (steered by the SCQ), led to very high adherence values to OAC intake (also supported by electronic monitoring with feedback using MEMS), and made patients more eager to ask questions about their health. On top of this, the in-person education group was more positive about the follow-up they received compared to the other two groups. The strength of our study is that it provides data to assess the (absence of) contribution of patient education to overall outcomes, something that remained unclear after prior trials.
Prior trials with integrated and/or nurse-led care
In most other nurse-led integrated care trials, the intervention was more elaborate into different domains of care, making the assessment of the contribution of each to the overall outcome difficult. In Hendriks et al., ambulatory AF patients were included and followed up with a scheme that was similar to our study (i.e. after 3, 6, 12 months, and every 6 months thereafter). Nurse-led outpatient care was mainly based on a medical decision support software rooted in the guidelines and supervised by a cardiologist.3 It was directed more to treatment decisions and not only education. After 22 months, it led to significantly less cardiovascular hospitalizations and mortality. The nurse-led intervention by Carter et al. was evaluated in patients referred from the emergency department with new-onset AF. The intervention consisted of a clinic visit with a detailed management plan, treatment recommendations, supported by education and group teaching sessions. It too showed a positive impact on a composite of death, cardiovascular hospitalizations, and AF emergency department visits.5 In our study, new-onset AF patients only comprised 13.6% of the investigated population, and we did not note a positive impact of our intervention in those with a history of AF of <1 year. Focus on medical interventions (anticoagulation, rate and rhythm control, comorbidity management) seems to be primordial for outcomes, with recent trials showing that an ABC approach is associated to beneficial results in the mAFA-II trial, a post hoc analysis of the ENGAGE AF-TIMI 48 trial and a report of both the COOL-AF registry and the ESC-EHRA EORP-AF long-term general registry.9,10,30–33 On the other hand, the SAFETY and RACE-4 integrated care trials4,6 were also not able to show an impact of their nurse-led home visits and nurse-led outpatient care on primary outcomes of all-cause mortality and/or unplanned readmission, or of cardiovascular death and hospitalizations, respectively. Especially in RACE-4, nurse-led care was extensive, including guideline-based software decision tools, cardiologist-supervised care, pre-planned diagnostic tests, and treatments during the first visit, along with psychosocial support and personalized education.6 All these trials point to the fact that diverse included elements of both standard care and the integrated care intervention on top of the patient group included may strongly define the impact of various new care strategies on the diverse outcome parameters that were evaluated.
It is clear that patient education alone is no guarantee for positive outcomes, and that it needs to be rooted in well-established medical (and likely multidisciplinary) care delivered by personnel. If any trend can be discerned from our data, it is that the online education group fared slightly less favourable than in-person education or even standard care. Education without direct patient contact may have created more uncertainties and concerns, especially in patients, but this can also be related to healthcare providers. On top of this, it could result in less practical and personal advice on self-management aspects, which all together could have led to more unplanned contacts. Even when making use of the most recent technologies such as artificial intelligence, providing patient education stays challenging. The appropriateness of responses generated by natural language processing chatbots is only limited, and important aspects are often not mentioned.34 Personal and direct patient contact is still a prime determinant for care quality.
Factors that may have contributed to lack of impact
We can postulate different reasons why our intervention was not able to show an impact on a composite endpoint of unplanned cardiovascular events. The time horizon of our trial (2 years and 3 months on average) might have been too short to detect the impact of the positive intermediate outcomes on unplanned cardiovascular events, although prior trials on integrated care had similar (or shorter) follow-up, and also analysis of second or third events was also not impacted in our trial. The event rate was lower than anticipated (i.e. 46.0% of the patients having an event during the study compared to the estimated 60.8%). One may also wonder whether COVID-19 had a direct effect on (the difference between groups) of unplanned cardiovascular events, as fewer hospital visits and physician contacts were allowed during this period. Besides the fact that the study included a wide AF population, still one out of three invited patients was excluded or declined to participate.29 It can be postulated that these patients (being significantly older, having a higher CHA2DS2-VASc score, being more in AF at baseline, and having more cardiovascular comorbidities and risk factors)29 would have a higher event rate than the included patients, and hence, a higher potential impact from the AF-EduCare intervention. Since the trial was conducted in three tertiary care centres, each having all therapy options for AF patients, background SC may have been too solid to allow for a significant impact of patient empowerment. All patients, in each of the three centres, perceived their standard care as ‘high’. This leads back to the conclusion above that mainly the systematic application of up-to-date medical care, delivered by guideline-adherent medical personal, is the driver of outcome. To further support this conclusion, the AF-EduCare trial strengthened the fact that an AF population has various cardiovascular and non-cardiovascular comorbidities (as shown in the Supplementary material online, Table S1) driving AF and its outcomes. A structured intervention hinging on targeted education about these comorbidities and how to handle these is clearly not sufficient to show a benefit on overall events and prognosis. A more intensive follow-up including the set-up of concrete management plans with direct referrals seems needed to tackle the comorbidities. Finally, health literacy in general and digital health literacy specifically is an essential skill that people require to engage with their care. A minimum of knowledge and (technological) skills are valuable to seek, appraise, and understand health-related information and make use of digital tools to support their care.35 With an average age of ∼70 years old, the impact of this factor may not be underestimated especially in the light of the results of the online education group.
Insights from subgroups in which education improved outcomes
In the exploratory subgroup analyses, it was found that in-person education positively affected the primary outcome in AF patients without congestive heart failure, those 70 years old or younger and patients without symptomatic AF. Such patients may have less regular and strict follow-ups by their healthcare providers, a background against which increased support from an AF team, as in the trial, may have impacted unplanned cardiovascular events. We also noticed a large centre effect, with one neutral centre, one positive centre, and one negative centre. This was also the case in the RACE-4 trial, in which the heterogeneity was attributed to the ‘experience’ of the centres in delivering integrated care.6 All centres and study teams in the AF-EduCare trial received the same on-site training, used the same tools, and had even trainings together (including role-playing) and intermediate follow-up moments. There were notable differences among the patient groups in the three centres (see Supplementary material online, Table S3), although it is impossible to attribute the different outcomes to any specific imbalance. Later cluster-based analysis may reveal which patient characteristics over the three centres were associated with the best outcome of education. The guidelines have stressed the importance of optimal communication in multidisciplinary teams as a key aspect of integrated care. This could have potentially played a role in the positive outcome in one centre where the educational AF team was permanently led by a physician/PhD student, whereas other study teams had to contact their cardiologists for decisions. Possibly, the direct availability of a physician in the AF team allows for more autonomous and faster action, which may have better prevented unplanned hospitalizations and emergency department visits. Again, the subgroup findings point to medical actionability along guideline-directed therapy by a healthcare professional as being a more important determinant of outcome than patient education and empowerment per se.
Limitations and future perspectives
This study was performed in three large Flemish tertiary care centres. The generalizability of the results to other settings or geographies cannot be guaranteed. Although a strength of the study was the inclusion of a general AF population without focusing on specific AF subpopulations, one-third of eligible patients opted not to participate. During the COVID-19 outbreak, in-person visits were not possible. The education, as well as the follow-up visits, was conducted as much as possible in the recommended time window, albeit by telephone and not in person: this may have diluted the effect of the in-person educational intervention. Also, patients in the SC group in our study received a phone call every 6 months, which may have levelled care improvements with the education groups. Although 9.8% of the patients were lost to follow-up or dropped out during the study, this was an acceptable number and less than the anticipated 15% to retain the power of the trial. Additional (sub)analyses on secondary endpoints collected during this study are still underway (e.g. non-cardiovascular events, AF-related events, and general practitioner visits), together with an evaluation of other tertiary outcomes such as symptom burden and quality of life. We will also explore whether specific clusters of patient characteristics might be associated with a positive impact of additional education.
Conclusions
The AF-EduCare study showed that intensive patient education in an unselected AF patient population, targeted at knowledge gaps and aiming to improve self-care capabilities, did not lead to fewer unplanned cardiovascular events. Nevertheless, there are hints of impact of in-person education on cardiovascular and all-cause mortality. Online and in-person education had the same impact on knowledge of patients. Subgroup analyses showed a heterogeneous centre effect and a positive effect of in-person education compared to SC in patients without a history of congestive heart failure, those being 70 years or younger, and those with asymptomatic AF. Guideline-directed therapy, personal contact with a healthcare provider, and fast medical actionability may be important determinants of outcome in the scope of integrated AF care and not patient education and empowerment alone.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
We would also like to thank the study nurses and AF nurse specialists of the involved hospitals for their support in recruiting and following up the patients in this study, and Wouter Smeets (MSc) and Cécile Kremer (MSc, PhD) for their help in performing the statistical analyses.
Funding
The AF-EduCare study is a project supported by the Research Foundation - Flanders (grant number T002917N).
Data availability
The data underlying this article can be shared on reasonable request to the corresponding author.
References
Author notes
Lien Desteghe and Michiel Delesie shared first authors.
Conflict of interest: H.H. received personal lecture and consultancy fees from Bayer, Biotronik, Bristol-Myers Squibb, Centrix Healthcare Ltd, Daiichi-Sankyo, Downtown Europe, Pfizer-BMS, European Society of Cardiology, Medscape, Springer Healthcare Ltd, and Viatris Pharmaceuticals Inc. M.D. received support to attend medical meetings from Pfizer and Biosense Webster. J.E. received lecture fees and unconditional research support from Bayer and Biotronik. None of the other authors did receive any personal honoraria. H.H. and L.D. received unconditional research support through the University of Hasselt or University of Antwerp from Abbott, Bayer, Biosense Webster, Boston-Scientific, Daicchi-Sankyo, Fibricheck/Qompium, Medtronic, and Pfizer-BMS, none related to this work. All remaining authors have declared no conflicts of interest.



