-
PDF
- Split View
-
Views
-
Cite
Cite
Errol W Aarnink, Hueseyin Ince, Stephan Kische, Evgeny Pokushalov, Thomas Schmitz, Boris Schmidt, Tommaso Gori, Felix Meincke, Alexey Vladimir Protopopov, Timothy Betts, Patrizio Mazzone, Marek Grygier, Horst Sievert, Tom De Potter, Elisa Vireca, Kenneth Stein, Martin W Bergmann, Lucas V A Boersma, for the EWOLUTION investigators, Incidence and predictors of 2-year mortality following percutaneous left atrial appendage occlusion in the EWOLUTION trial, EP Europace, Volume 26, Issue 7, July 2024, euae188, https://doi.org/10.1093/europace/euae188
Close - Share Icon Share
Abstract
Sufficient survival time following left atrial appendage occlusion (LAAO) is essential for ensuring the efficacy and cost-effectiveness of this strategy for stroke prevention. Understanding prognostic factors for early mortality after LAAO could optimize patient selection. In the current study, we perform an in-depth analysis of 2-year mortality after LAAO, focusing particularly on potential predictors.
The EWOLUTION registry is a real-world cohort comprising 1020 patients that underwent LAAO. Endpoint definitions were pre-specified, and death was categorized as cardiovascular, non-cardiovascular, or unknown origin. Mortality rates were calculated from Kaplan–Meier estimates. Baseline characteristics significantly associated with death in univariate Cox regression analysis were incorporated into the multivariate analysis. All multivariate predictors were included in a risk model. Two-year mortality rate was 16.4% [confidence interval (CI): 14.0–18.7%], with 50% of patients dying from a non-cardiovascular cause. Multivariate baseline predictors of 2-year mortality included age [hazard ratio (HR) 1.05, CI: 1.03–1.08, per year increase], heart failure (HR 1.73, CI: 1.24–2.41), vascular disease (HR 1.47, CI: 1.05–2.05), valvular disease (HR 1.63, CI: 1.15–2.33), abnormal liver function (HR 1.80, CI: 1.02–3.17), and abnormal renal function (HR 1.58, CI: 1.10–2.27). Mortality rate exhibited a gradual rise as the number of risk factors increased, reaching 46.1% in patients presenting with five or six risk factors.
One in six patients died within 2 years after LAAO. We identified six independent predictors of mortality. When combined, this model showed a gradual increase in mortality rate with a growing number of risk factors, which may guide appropriate patient selection for LAAO.
The original EWOLUTION registry was registered at clinicaltrials.gov under identifier NCT01972282.
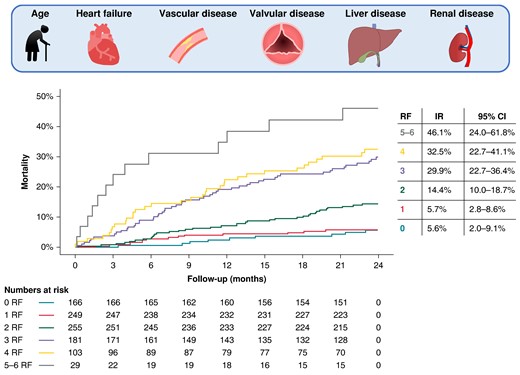
The mortality risk within EWOLUTION increased gradually with a growing number of risk factors. Risk factors included age, heart failure, vascular disease, valvular disease, abnormal liver function, and abnormal kidney function. CI, confidence interval; IR, incidence rate; RF, risk factor.
Left atrial appendage occlusion is especially efficacious and cost-effective over longer follow-up time.
Improved stratification of early mortality risk could optimize patient selection for left atrial appendage occlusion.
One in six patients died within 2 years after left atrial appendage occlusion within EWOLUTION.
Two-year mortality is associated with older age, heart failure, vascular disease, valvular disease, abnormal liver function, and abnormal renal function.
When combined in a model, a gradual increase in mortality rate with a growing number of risk factors is present, with 46% of patients presenting with five to six risk factors dying within 2 years.
Introduction
Left atrial appendage occlusion (LAAO) is an increasingly performed thromboembolic prevention strategy that may be used as an alternative for oral anticoagulation therapy (OAT) in patients with atrial fibrillation (AF) contraindicated for long-term OAT. Left atrial appendage occlusion currently may be considered for patients with AF that have an increased thromboembolic risk as quantified by the CHA2DS2-VASc score.1 Due to an increased CHA2DS2-VASc score and presence of OAT contraindications, patients indicated for LAAO generally are of older age and often present with comorbidities. This makes the LAAO population not only susceptible to thromboembolic events and bleeding but also to early occurrence of death following the procedure.
The key to benefiting from LAAO for contraindicated patients is having sufficient survival time to allow prevention of thromboembolic events. After a short period of procedural risk, LAAO may be able to provide a lifetime reduction in thromboembolic risk.2 No currently published randomized trial compares LAAO with standard of care in a population with an absolute contraindication for OAT. The unpublished ASAP-TOO3 and COMPARE LAAO4 trials could have provided insights in the efficacy and cost-effectiveness of LAAO within this specific population, but the publication of several non-randomized studies weakened the clinical equipoise, eventually causing both trials to stop due to low inclusion rate. In randomized trials comparing LAAO with standard of care in a non-contraindicated population, LAAO has proven non-inferior to warfarin for a composite endpoint of thromboembolism and death (in PROTECT-AF)5 and to direct oral anticoagulants (DOACs) for a net clinical benefit endpoint (in PRAGUE-176) with non-procedural bleeding favouring LAAO. The possibility to discontinue OAT in the LAAO group may lead to reduced bleeding rates, engendering a growing difference in bleeding hazard between LAAO and OAT populations over time. Diverging Kaplan–Meier curves can also be observed for thromboembolic outcome after (surgical) LAAO combined with OAT, as compared with OAT alone.7 Left atrial appendage occlusion, therefore, particularly seems of value as time progresses.
Sufficient survival time after LAAO is also essential for achieving cost-effectiveness. Using PROTECT-AF and PREVAIL data, LAAO in patients eligible for OAT became cost-effective after 7 years when compared with warfarin and after 5 years when compared with DOACs.8 In a Swedish meta-analysis focusing on patients contraindicated to OAT, first-year healthcare cost of LAAO was 14 984€, with a total lifetime healthcare cost of 19 032€, also suggesting more economic benefit from LAAO over longer follow-up. Of note, LAAO yielded an extra quality-adjusted life year (at the cost of 4047€) and was cost-effective by 10 252€ compared with standard of care from a public health perspective.9
For LAAO to be both efficacious and cost-effective, early occurrence of death should be avoided. It is, therefore, essential to carefully consider what patients could benefit from LAAO, and more importantly, what patients probably would not. To improve patient selection, identification of risk factors for early mortality after LAAO is pivotal. In the current study, we aim to identify risk factors associated with 2-year mortality after LAAO in the EWOLUTION registry.
Methods
Study design
The EWOLUTION registry comprises 1020 patients planned for Watchman device implantation, mainly because of a long-term contraindication to OAT. All included patients provided informed consent prior to LAAO. A total of 47 centres participated in the registry. Standardized definitions for adverse events and endpoints were used. Clinical events were entered in the database and adjudicated by local investigators. The Sponsor Medical Safety Group, consisting of physicians and healthcare professionals with expertise in the field, additionally reviewed relevant source documents. Data on study design, procedural outcomes, and clinical event rates have been previously published.10,11 The EWOLUTION registry was registered at clinicaltrials.gov under identifier NCT01972282. The data that support the current analysis are available from the corresponding author on reasonable request.
Outcomes
In the current study, we perform an in-depth analysis of the outcome of 2-year mortality. All deaths were classified as cardiovascular (CV death), non-cardiovascular (non-CV death), or death of unknown origin. Fatal intracranial bleeding events were classified as CV death and fatal gastrointestinal bleeding events were classified as non-CV death.
Variables included in the CHA2DS2-VASc (i.e. Congestive heart failure, Hypertension, Age, Diabetes mellitus, Stroke or thromboembolism, Vascular disease, or Sex category) or HAS-BLED score (uncontrolled Hypertension, Abnormal liver function/Abnormal renal function, Stroke, Bleeding, Labile INRs, Elderly (Age > 65), or Drugs/alcohol use) were defined according to the original publications of these risk scores.12,13 The presence of valvular disease and eligibility for OAT were determined at the physician’s discretion without strict pre-specified definitions. Definitions for valvular disease, CV death, and non-CV death are provided in Supplementary material online, Table S1, along with definitions used for clinical events [i.e. ischaemic stroke, transient ischaemic attack (TIA), and major bleeding].
Statistical analysis
Baseline characteristics, procedural characteristics, and clinical event rates during 2-year follow-up were compared between alive and deceased patients by means of unpaired t-tests or χ2 tests, as appropriate. Proportions were compared using Fisher’s exact test when any of the values in the contingency tables was lower than 20.
The association of baseline characteristics with the occurrence of death within 2 years after LAAO was investigated using multivariate Cox regression analysis. Possible predictors were selected for regression analysis based on data availability and absence of substantial collinearity between model covariates. Significant predictors (P < 0.05) within the univariate analysis were included in the multivariate model. Thirty-day, 1-year, and 2-year incidence rates were calculated accounting for censored patients. Separate incidence rates were calculated for subgroups that showed a significant association with all-cause mortality. All variables independently associated with 2-year mortality were included in a risk model. In this risk model, continuous variables were dichotomized based on the median Youden’s index over 1000 bootstrapped samples to give an estimate of the optimal cut-off point for the general LAAO population.
Results
Patient characteristics
A total of 1020 patients were included in the analysis, of whom 1005 (98.5%) successfully received LAAO. Mean age was 73 ± 9 years, and patients demonstrated an increased thromboembolic and bleeding risk (CHA2DS2-VASc score: 4.5 ± 1.6; HAS-BLED score: 2.3 ± 1.2).
Median follow-up duration was 733 days (interquartile range: 702–760 days). All-cause death was observed in 157/1020 patients within the first 2 years after their LAAO procedure [incidence rate: 16.4%; 95% confidence interval (CI): 14.0–18.7%; see Figure 1]. Incidence rates for 30-day and 1-year mortality were 0.8% (95% CI: 0.3–1.4%) and 10.3% (95% CI: 8.4–12.2%), respectively. The cause of death after 2 years was mainly non-CV (50%). Fifty patients died from a CV cause (32%). The cause of death was not known in 28 patients (18%). All fatal serious adverse events within 1 and 2 years after LAAO are further specified in Supplementary material online, Table S2.

Kaplan–Meier estimates for all-cause death and subcategories. (A) Kaplan–Meier estimates for cumulative incidence within subcategories of mortality. (B) Proportional distribution of mortality classification within the first 2 years after LAAO. The x-axis is identical to the x-axis in A. CI, confidence interval.
Various baseline characteristics of patients deceasing in the first 2 years following LAAO differed from alive patients (Table 1). Deceased patients were older, had higher CHA2DS2-VASc and HAS-BLED scores, and more often had diabetes mellitus, vascular disease, abnormal renal function, abnormal liver function, and valvular disease. Congestive heart failure was more prevalent in deceased patients, and when present, New York Heart Association class was higher. In terms of echo parameters, no statistically significant differences were present.
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Age, in years | 73.4 ± 8.8 | 72.6 ± 8.8 | 77.2 ± 7.5 | <0.001 |
| Age ≥ 65 | 861 (84.4) | 678 (82.1) | 148 (94.3) | <0.001 |
| Age ≥ 75 | 517 (50.7) | 388 (47.0) | 108 (68.8) | <0.001 |
| Sex (male) | 612 (60.0) | 486 (58.8) | 101 (64.3) | 0.23 |
| Non-paroxysmal AF | 559 (55.3) | 449 (54.9) | 87 (55.8) | 0.91 |
| Contraindicated for OAT | 737 (72.3) | 590 (71.4) | 121 (77.1) | 0.18 |
| CHA2DS2-VASc score | 4.5 ± 1.6 | 4.4 ± 1.6 | 5.0 ± 1.5 | <0.001 |
| ≤1 | 18 (1.8) | 17 (2.1) | 1 (0.6) | |
| 2–3 | 258 (25.3) | 230 (27.8) | 19 (12.1) | |
| ≥4 | 744 (72.9) | 579 (70.1) | 137 (87.3) | |
| HAS-BLED score | 2.3 ± 1.2 | 2.2 ± 1.2 | 2.8 ± 1.3 | <0.001 |
| <3 | 612 (60.0) | 513 (62.1) | 73 (46.5) | |
| ≥3 | 408 (40.0) | 313 (37.9) | 84 (53.5) | |
| Congestive heart failure | 349 (34.2) | 264 (32.0) | 80 (51.0) | <0.001 |
| NYHA I | 35 (10.1) | 25 (9.5) | 10 (12.5) | |
| NYHA II | 193 (55.6) | 159 (60.7) | 33 (41.3) | |
| NYHA III | 112 (32.3) | 73 (27.9) | 35 (43.8) | |
| NYHA IV | 7 (2.0) | 5 (1.9) | 2 (2.5) | |
| Hypertension | 885 (86.8) | 717 (86.8) | 137 (87.3) | 0.98 |
| Diabetes mellitus | 304 (29.8) | 235 (28.5) | 59 (37.6) | 0.028 |
| History of stroke/thromboembolism | 403 (39.5) | 335 (40.6) | 51 (32.5) | 0.07 |
| History of intracranial haemorrhage | 153 (15.0) | 132 (16.0) | 12 (7.6) | 0.010 |
| Vascular disease | 463 (45.4) | 354 (42.9) | 97 (61.8) | <0.001 |
| Abnormal renal function | 162 (15.9) | 111 (13.4) | 47 (29.9) | <0.001 |
| Abnormal liver function | 44 (4.3) | 27 (3.3) | 15 (9.6) | <0.001 |
| Prior major bleeding or predisposition to bleeding | 393 (38.5) | 293 (35.5) | 89 (56.7) | <0.001 |
| Recurrent anaemia | 203 (19.9) | 138 (16.7) | 60 (38.2) | <0.001 |
| History of PCI | 262 (25.7) | 195 (23.6) | 58 (36.9) | <0.001 |
| Valvular disease | 450 (44.1) | 329 (39.8) | 103 (65.6) | <0.001 |
| Echocardiographic parameters | ||||
| SEC at baseline | 129 (12.8) | 101 (12.2) | 24 (15.3) | 0.32 |
| LAA ostium diameter (mm) | 21.3 ± 3.5 | 21.2 ± 3.4 | 21.7 ± 3.6 | 0.07 |
| LAA length (mm) | 28.1 ± 5.9 | 28.1 ± 5.7 | 28.8 ± 6.5 | 0.18 |
| LVEF >50% | 545 (73.5) | 445 (74.7) | 78 (66.1) | 0.12 |
| LVEF 30–50% | 168 (22.7) | 132 (22.1) | 33 (28.0) | 0.28 |
| LVEF <30% | 28 (3.8) | 19 (3.2) | 7 (5.9) | 0.25 |
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Age, in years | 73.4 ± 8.8 | 72.6 ± 8.8 | 77.2 ± 7.5 | <0.001 |
| Age ≥ 65 | 861 (84.4) | 678 (82.1) | 148 (94.3) | <0.001 |
| Age ≥ 75 | 517 (50.7) | 388 (47.0) | 108 (68.8) | <0.001 |
| Sex (male) | 612 (60.0) | 486 (58.8) | 101 (64.3) | 0.23 |
| Non-paroxysmal AF | 559 (55.3) | 449 (54.9) | 87 (55.8) | 0.91 |
| Contraindicated for OAT | 737 (72.3) | 590 (71.4) | 121 (77.1) | 0.18 |
| CHA2DS2-VASc score | 4.5 ± 1.6 | 4.4 ± 1.6 | 5.0 ± 1.5 | <0.001 |
| ≤1 | 18 (1.8) | 17 (2.1) | 1 (0.6) | |
| 2–3 | 258 (25.3) | 230 (27.8) | 19 (12.1) | |
| ≥4 | 744 (72.9) | 579 (70.1) | 137 (87.3) | |
| HAS-BLED score | 2.3 ± 1.2 | 2.2 ± 1.2 | 2.8 ± 1.3 | <0.001 |
| <3 | 612 (60.0) | 513 (62.1) | 73 (46.5) | |
| ≥3 | 408 (40.0) | 313 (37.9) | 84 (53.5) | |
| Congestive heart failure | 349 (34.2) | 264 (32.0) | 80 (51.0) | <0.001 |
| NYHA I | 35 (10.1) | 25 (9.5) | 10 (12.5) | |
| NYHA II | 193 (55.6) | 159 (60.7) | 33 (41.3) | |
| NYHA III | 112 (32.3) | 73 (27.9) | 35 (43.8) | |
| NYHA IV | 7 (2.0) | 5 (1.9) | 2 (2.5) | |
| Hypertension | 885 (86.8) | 717 (86.8) | 137 (87.3) | 0.98 |
| Diabetes mellitus | 304 (29.8) | 235 (28.5) | 59 (37.6) | 0.028 |
| History of stroke/thromboembolism | 403 (39.5) | 335 (40.6) | 51 (32.5) | 0.07 |
| History of intracranial haemorrhage | 153 (15.0) | 132 (16.0) | 12 (7.6) | 0.010 |
| Vascular disease | 463 (45.4) | 354 (42.9) | 97 (61.8) | <0.001 |
| Abnormal renal function | 162 (15.9) | 111 (13.4) | 47 (29.9) | <0.001 |
| Abnormal liver function | 44 (4.3) | 27 (3.3) | 15 (9.6) | <0.001 |
| Prior major bleeding or predisposition to bleeding | 393 (38.5) | 293 (35.5) | 89 (56.7) | <0.001 |
| Recurrent anaemia | 203 (19.9) | 138 (16.7) | 60 (38.2) | <0.001 |
| History of PCI | 262 (25.7) | 195 (23.6) | 58 (36.9) | <0.001 |
| Valvular disease | 450 (44.1) | 329 (39.8) | 103 (65.6) | <0.001 |
| Echocardiographic parameters | ||||
| SEC at baseline | 129 (12.8) | 101 (12.2) | 24 (15.3) | 0.32 |
| LAA ostium diameter (mm) | 21.3 ± 3.5 | 21.2 ± 3.4 | 21.7 ± 3.6 | 0.07 |
| LAA length (mm) | 28.1 ± 5.9 | 28.1 ± 5.7 | 28.8 ± 6.5 | 0.18 |
| LVEF >50% | 545 (73.5) | 445 (74.7) | 78 (66.1) | 0.12 |
| LVEF 30–50% | 168 (22.7) | 132 (22.1) | 33 (28.0) | 0.28 |
| LVEF <30% | 28 (3.8) | 19 (3.2) | 7 (5.9) | 0.25 |
Continuous variables are expressed as mean ± SD and compared using unpaired t-tests when normally distributed. Proportions are presented as number of patients (%) and compared using χ2 tests or Fisher’s exact test.
AF, atrial fibrillation; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; mm, millimetres; NYHA, New York Heart Association; OAT, oral anticoagulation therapy; PCI, percutaneous coronary intervention; SEC, spontaneous echocardiographic contrast.
P-values <0.05 are bold.
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Age, in years | 73.4 ± 8.8 | 72.6 ± 8.8 | 77.2 ± 7.5 | <0.001 |
| Age ≥ 65 | 861 (84.4) | 678 (82.1) | 148 (94.3) | <0.001 |
| Age ≥ 75 | 517 (50.7) | 388 (47.0) | 108 (68.8) | <0.001 |
| Sex (male) | 612 (60.0) | 486 (58.8) | 101 (64.3) | 0.23 |
| Non-paroxysmal AF | 559 (55.3) | 449 (54.9) | 87 (55.8) | 0.91 |
| Contraindicated for OAT | 737 (72.3) | 590 (71.4) | 121 (77.1) | 0.18 |
| CHA2DS2-VASc score | 4.5 ± 1.6 | 4.4 ± 1.6 | 5.0 ± 1.5 | <0.001 |
| ≤1 | 18 (1.8) | 17 (2.1) | 1 (0.6) | |
| 2–3 | 258 (25.3) | 230 (27.8) | 19 (12.1) | |
| ≥4 | 744 (72.9) | 579 (70.1) | 137 (87.3) | |
| HAS-BLED score | 2.3 ± 1.2 | 2.2 ± 1.2 | 2.8 ± 1.3 | <0.001 |
| <3 | 612 (60.0) | 513 (62.1) | 73 (46.5) | |
| ≥3 | 408 (40.0) | 313 (37.9) | 84 (53.5) | |
| Congestive heart failure | 349 (34.2) | 264 (32.0) | 80 (51.0) | <0.001 |
| NYHA I | 35 (10.1) | 25 (9.5) | 10 (12.5) | |
| NYHA II | 193 (55.6) | 159 (60.7) | 33 (41.3) | |
| NYHA III | 112 (32.3) | 73 (27.9) | 35 (43.8) | |
| NYHA IV | 7 (2.0) | 5 (1.9) | 2 (2.5) | |
| Hypertension | 885 (86.8) | 717 (86.8) | 137 (87.3) | 0.98 |
| Diabetes mellitus | 304 (29.8) | 235 (28.5) | 59 (37.6) | 0.028 |
| History of stroke/thromboembolism | 403 (39.5) | 335 (40.6) | 51 (32.5) | 0.07 |
| History of intracranial haemorrhage | 153 (15.0) | 132 (16.0) | 12 (7.6) | 0.010 |
| Vascular disease | 463 (45.4) | 354 (42.9) | 97 (61.8) | <0.001 |
| Abnormal renal function | 162 (15.9) | 111 (13.4) | 47 (29.9) | <0.001 |
| Abnormal liver function | 44 (4.3) | 27 (3.3) | 15 (9.6) | <0.001 |
| Prior major bleeding or predisposition to bleeding | 393 (38.5) | 293 (35.5) | 89 (56.7) | <0.001 |
| Recurrent anaemia | 203 (19.9) | 138 (16.7) | 60 (38.2) | <0.001 |
| History of PCI | 262 (25.7) | 195 (23.6) | 58 (36.9) | <0.001 |
| Valvular disease | 450 (44.1) | 329 (39.8) | 103 (65.6) | <0.001 |
| Echocardiographic parameters | ||||
| SEC at baseline | 129 (12.8) | 101 (12.2) | 24 (15.3) | 0.32 |
| LAA ostium diameter (mm) | 21.3 ± 3.5 | 21.2 ± 3.4 | 21.7 ± 3.6 | 0.07 |
| LAA length (mm) | 28.1 ± 5.9 | 28.1 ± 5.7 | 28.8 ± 6.5 | 0.18 |
| LVEF >50% | 545 (73.5) | 445 (74.7) | 78 (66.1) | 0.12 |
| LVEF 30–50% | 168 (22.7) | 132 (22.1) | 33 (28.0) | 0.28 |
| LVEF <30% | 28 (3.8) | 19 (3.2) | 7 (5.9) | 0.25 |
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Age, in years | 73.4 ± 8.8 | 72.6 ± 8.8 | 77.2 ± 7.5 | <0.001 |
| Age ≥ 65 | 861 (84.4) | 678 (82.1) | 148 (94.3) | <0.001 |
| Age ≥ 75 | 517 (50.7) | 388 (47.0) | 108 (68.8) | <0.001 |
| Sex (male) | 612 (60.0) | 486 (58.8) | 101 (64.3) | 0.23 |
| Non-paroxysmal AF | 559 (55.3) | 449 (54.9) | 87 (55.8) | 0.91 |
| Contraindicated for OAT | 737 (72.3) | 590 (71.4) | 121 (77.1) | 0.18 |
| CHA2DS2-VASc score | 4.5 ± 1.6 | 4.4 ± 1.6 | 5.0 ± 1.5 | <0.001 |
| ≤1 | 18 (1.8) | 17 (2.1) | 1 (0.6) | |
| 2–3 | 258 (25.3) | 230 (27.8) | 19 (12.1) | |
| ≥4 | 744 (72.9) | 579 (70.1) | 137 (87.3) | |
| HAS-BLED score | 2.3 ± 1.2 | 2.2 ± 1.2 | 2.8 ± 1.3 | <0.001 |
| <3 | 612 (60.0) | 513 (62.1) | 73 (46.5) | |
| ≥3 | 408 (40.0) | 313 (37.9) | 84 (53.5) | |
| Congestive heart failure | 349 (34.2) | 264 (32.0) | 80 (51.0) | <0.001 |
| NYHA I | 35 (10.1) | 25 (9.5) | 10 (12.5) | |
| NYHA II | 193 (55.6) | 159 (60.7) | 33 (41.3) | |
| NYHA III | 112 (32.3) | 73 (27.9) | 35 (43.8) | |
| NYHA IV | 7 (2.0) | 5 (1.9) | 2 (2.5) | |
| Hypertension | 885 (86.8) | 717 (86.8) | 137 (87.3) | 0.98 |
| Diabetes mellitus | 304 (29.8) | 235 (28.5) | 59 (37.6) | 0.028 |
| History of stroke/thromboembolism | 403 (39.5) | 335 (40.6) | 51 (32.5) | 0.07 |
| History of intracranial haemorrhage | 153 (15.0) | 132 (16.0) | 12 (7.6) | 0.010 |
| Vascular disease | 463 (45.4) | 354 (42.9) | 97 (61.8) | <0.001 |
| Abnormal renal function | 162 (15.9) | 111 (13.4) | 47 (29.9) | <0.001 |
| Abnormal liver function | 44 (4.3) | 27 (3.3) | 15 (9.6) | <0.001 |
| Prior major bleeding or predisposition to bleeding | 393 (38.5) | 293 (35.5) | 89 (56.7) | <0.001 |
| Recurrent anaemia | 203 (19.9) | 138 (16.7) | 60 (38.2) | <0.001 |
| History of PCI | 262 (25.7) | 195 (23.6) | 58 (36.9) | <0.001 |
| Valvular disease | 450 (44.1) | 329 (39.8) | 103 (65.6) | <0.001 |
| Echocardiographic parameters | ||||
| SEC at baseline | 129 (12.8) | 101 (12.2) | 24 (15.3) | 0.32 |
| LAA ostium diameter (mm) | 21.3 ± 3.5 | 21.2 ± 3.4 | 21.7 ± 3.6 | 0.07 |
| LAA length (mm) | 28.1 ± 5.9 | 28.1 ± 5.7 | 28.8 ± 6.5 | 0.18 |
| LVEF >50% | 545 (73.5) | 445 (74.7) | 78 (66.1) | 0.12 |
| LVEF 30–50% | 168 (22.7) | 132 (22.1) | 33 (28.0) | 0.28 |
| LVEF <30% | 28 (3.8) | 19 (3.2) | 7 (5.9) | 0.25 |
Continuous variables are expressed as mean ± SD and compared using unpaired t-tests when normally distributed. Proportions are presented as number of patients (%) and compared using χ2 tests or Fisher’s exact test.
AF, atrial fibrillation; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; mm, millimetres; NYHA, New York Heart Association; OAT, oral anticoagulation therapy; PCI, percutaneous coronary intervention; SEC, spontaneous echocardiographic contrast.
P-values <0.05 are bold.
Furthermore, deceased patients more often had a history of recurrent anaemia, percutaneous coronary intervention, and major bleeding or a predisposition towards bleeding. Contradictory, prior intracranial haemorrhage was more frequently present in the group surviving the first 2 years after LAAO, although this proportion was low.
Procedural and follow-up characteristics
We did not observe any difference in the number of recaptures or device seal between alive and deceased patients (Table 2). Device size tended to be larger in deceased patients, coherent with LAA ostium diameter, but this observation lacked statistical significance. The majority of patients (60%) were discharged on dual antiplatelet therapy (DAPT) after LAAO. Antithrombotic medication at discharge more often included anticoagulation in patients alive after 2 years, possibly reflecting a lower bleeding risk.
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Procedural success | 1005 (98.5) | 826 (100.0) | 157 (100.0) | 1.00 |
| Device size | 0.20 | |||
| 21mm | 122 (12.2) | 106 (12.9) | 15 (9.7) | |
| 24mm | 295 (29.6) | 246 (30.0) | 45 (29.0) | |
| 27mm | 315 (31.6) | 260 (31.7) | 43 (27.7) | |
| 30mm | 164 (16.4) | 131 (16.0) | 29 (18.7) | |
| 33mm | 102 (10.2) | 78 (9.5) | 23 (14.8) | |
| Number of recaptures | 0.92 | |||
| 0 | 760 (76.2) | 625 (76.1) | 118 (76.1) | |
| 1 | 141 (14.1) | 115 (14.0) | 23 (14.8) | |
| 2 | 61 (6.1) | 50 (6.1) | 10 (6.5) | |
| 3 | 36 (3.6) | 31 (3.8) | 4 (2.6) | |
| Device seal | 0.19 | |||
| Complete seal | 913 (91.8) | 749 (91.3) | 143 (93.5) | |
| Flow ≤5mm | 80 (8.0) | 70 (8.5) | 9 (5.9) | |
| Flow >5mm | 2 (0.2) | 1 (0.1) | 1 (0.7) | |
| Discharge medication | 0.002 | |||
| None | 61 (6.1) | 52 (6.3) | 7 (4.5) | |
| SAPT | 72 (7.2) | 54 (6.5) | 18 (11.5) | |
| DAPT | 600 (60.1) | 487 (58.7) | 107 (68.6) | |
| VKA | 156 (15.6) | 141 (17.0) | 13 (8.3) | |
| DOAC | 109 (10.9) | 95 (11.5) | 11 (7.1) | |
| Event <2 years | ||||
| Ischaemic stroke | 22 (2.3) | 18 (2.2) | 4 (3.1) | 0.52 |
| TIA | 15 (1.6) | 14 (1.7) | 1 (0.8) | 0.71 |
| DRT | 33 (3.5) | 28 (3.4) | 5 (4.0) | 0.79 |
| Major bleeding | 55 (5.7) | 27 (3.3) | 27 (20.6) | <0.001 |
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Procedural success | 1005 (98.5) | 826 (100.0) | 157 (100.0) | 1.00 |
| Device size | 0.20 | |||
| 21mm | 122 (12.2) | 106 (12.9) | 15 (9.7) | |
| 24mm | 295 (29.6) | 246 (30.0) | 45 (29.0) | |
| 27mm | 315 (31.6) | 260 (31.7) | 43 (27.7) | |
| 30mm | 164 (16.4) | 131 (16.0) | 29 (18.7) | |
| 33mm | 102 (10.2) | 78 (9.5) | 23 (14.8) | |
| Number of recaptures | 0.92 | |||
| 0 | 760 (76.2) | 625 (76.1) | 118 (76.1) | |
| 1 | 141 (14.1) | 115 (14.0) | 23 (14.8) | |
| 2 | 61 (6.1) | 50 (6.1) | 10 (6.5) | |
| 3 | 36 (3.6) | 31 (3.8) | 4 (2.6) | |
| Device seal | 0.19 | |||
| Complete seal | 913 (91.8) | 749 (91.3) | 143 (93.5) | |
| Flow ≤5mm | 80 (8.0) | 70 (8.5) | 9 (5.9) | |
| Flow >5mm | 2 (0.2) | 1 (0.1) | 1 (0.7) | |
| Discharge medication | 0.002 | |||
| None | 61 (6.1) | 52 (6.3) | 7 (4.5) | |
| SAPT | 72 (7.2) | 54 (6.5) | 18 (11.5) | |
| DAPT | 600 (60.1) | 487 (58.7) | 107 (68.6) | |
| VKA | 156 (15.6) | 141 (17.0) | 13 (8.3) | |
| DOAC | 109 (10.9) | 95 (11.5) | 11 (7.1) | |
| Event <2 years | ||||
| Ischaemic stroke | 22 (2.3) | 18 (2.2) | 4 (3.1) | 0.52 |
| TIA | 15 (1.6) | 14 (1.7) | 1 (0.8) | 0.71 |
| DRT | 33 (3.5) | 28 (3.4) | 5 (4.0) | 0.79 |
| Major bleeding | 55 (5.7) | 27 (3.3) | 27 (20.6) | <0.001 |
DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant; DRT, device-related thrombus; SAPT, single antiplatelet therapy; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
P-values <0.05 are bold.
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Procedural success | 1005 (98.5) | 826 (100.0) | 157 (100.0) | 1.00 |
| Device size | 0.20 | |||
| 21mm | 122 (12.2) | 106 (12.9) | 15 (9.7) | |
| 24mm | 295 (29.6) | 246 (30.0) | 45 (29.0) | |
| 27mm | 315 (31.6) | 260 (31.7) | 43 (27.7) | |
| 30mm | 164 (16.4) | 131 (16.0) | 29 (18.7) | |
| 33mm | 102 (10.2) | 78 (9.5) | 23 (14.8) | |
| Number of recaptures | 0.92 | |||
| 0 | 760 (76.2) | 625 (76.1) | 118 (76.1) | |
| 1 | 141 (14.1) | 115 (14.0) | 23 (14.8) | |
| 2 | 61 (6.1) | 50 (6.1) | 10 (6.5) | |
| 3 | 36 (3.6) | 31 (3.8) | 4 (2.6) | |
| Device seal | 0.19 | |||
| Complete seal | 913 (91.8) | 749 (91.3) | 143 (93.5) | |
| Flow ≤5mm | 80 (8.0) | 70 (8.5) | 9 (5.9) | |
| Flow >5mm | 2 (0.2) | 1 (0.1) | 1 (0.7) | |
| Discharge medication | 0.002 | |||
| None | 61 (6.1) | 52 (6.3) | 7 (4.5) | |
| SAPT | 72 (7.2) | 54 (6.5) | 18 (11.5) | |
| DAPT | 600 (60.1) | 487 (58.7) | 107 (68.6) | |
| VKA | 156 (15.6) | 141 (17.0) | 13 (8.3) | |
| DOAC | 109 (10.9) | 95 (11.5) | 11 (7.1) | |
| Event <2 years | ||||
| Ischaemic stroke | 22 (2.3) | 18 (2.2) | 4 (3.1) | 0.52 |
| TIA | 15 (1.6) | 14 (1.7) | 1 (0.8) | 0.71 |
| DRT | 33 (3.5) | 28 (3.4) | 5 (4.0) | 0.79 |
| Major bleeding | 55 (5.7) | 27 (3.3) | 27 (20.6) | <0.001 |
| . | All (n = 1020) . | Alive after 2 years (n = 826) . | Deceased within 2 years (n = 157) . | P-value . |
|---|---|---|---|---|
| Procedural success | 1005 (98.5) | 826 (100.0) | 157 (100.0) | 1.00 |
| Device size | 0.20 | |||
| 21mm | 122 (12.2) | 106 (12.9) | 15 (9.7) | |
| 24mm | 295 (29.6) | 246 (30.0) | 45 (29.0) | |
| 27mm | 315 (31.6) | 260 (31.7) | 43 (27.7) | |
| 30mm | 164 (16.4) | 131 (16.0) | 29 (18.7) | |
| 33mm | 102 (10.2) | 78 (9.5) | 23 (14.8) | |
| Number of recaptures | 0.92 | |||
| 0 | 760 (76.2) | 625 (76.1) | 118 (76.1) | |
| 1 | 141 (14.1) | 115 (14.0) | 23 (14.8) | |
| 2 | 61 (6.1) | 50 (6.1) | 10 (6.5) | |
| 3 | 36 (3.6) | 31 (3.8) | 4 (2.6) | |
| Device seal | 0.19 | |||
| Complete seal | 913 (91.8) | 749 (91.3) | 143 (93.5) | |
| Flow ≤5mm | 80 (8.0) | 70 (8.5) | 9 (5.9) | |
| Flow >5mm | 2 (0.2) | 1 (0.1) | 1 (0.7) | |
| Discharge medication | 0.002 | |||
| None | 61 (6.1) | 52 (6.3) | 7 (4.5) | |
| SAPT | 72 (7.2) | 54 (6.5) | 18 (11.5) | |
| DAPT | 600 (60.1) | 487 (58.7) | 107 (68.6) | |
| VKA | 156 (15.6) | 141 (17.0) | 13 (8.3) | |
| DOAC | 109 (10.9) | 95 (11.5) | 11 (7.1) | |
| Event <2 years | ||||
| Ischaemic stroke | 22 (2.3) | 18 (2.2) | 4 (3.1) | 0.52 |
| TIA | 15 (1.6) | 14 (1.7) | 1 (0.8) | 0.71 |
| DRT | 33 (3.5) | 28 (3.4) | 5 (4.0) | 0.79 |
| Major bleeding | 55 (5.7) | 27 (3.3) | 27 (20.6) | <0.001 |
DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulant; DRT, device-related thrombus; SAPT, single antiplatelet therapy; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
P-values <0.05 are bold.
Ischaemic stroke, TIA, and device-related thrombus (DRT) did not occur more frequently in deceased patients. The proportion of patients that experienced major bleeding after LAAO was markedly higher in the group that died within 2 years (21% vs. 3%, P < 0.001). The majority of patients with a bleeding event after LAAO had a history of bleeding or predisposition to bleeding (64%), while this accounted for only 37% for patients without bleeding during follow-up (P < 0.001). The temporal relation of clinical events and death is plotted in Figure 2 for deceased patients that suffered from an ischaemic stroke, TIA, DRT, or major bleeding after LAAO. A total of 27 patients both had a major bleeding and died within 2 years after LAAO, of whom seven deceased within 2 weeks after their bleeding event. A fatal bleeding event occurred in 10 patients, of whom one patient used vitamin K antagonist, seven patients used DAPT, one patient used single antiplatelet therapy, and one patient used no antithrombotic medication.

Temporal relation of clinical events and death. Horizontal lines represent timelines for individual patients that both had a clinical event and died within 2 years after LAAO. Multiple events of the same type within individual patients were not registered and are therefore not plotted. LAAO, left atrial appendage occlusion; TIA, transient ischaemic attack.
Cox regression analysis
Univariate Cox regression analysis showed an association of all-cause death with older age, valvular disease, larger LAA ostium diameter (but not LAA length), congestive heart failure, vascular disease, abnormal liver function, abnormal renal function, diabetes mellitus, and prior major bleeding/predisposition to bleeding (see Table 3). Figure 3 shows all variables included in the multivariate analysis. Predictors comprised age [hazard ratio (HR) 1.05, 95% CI: 1.03–1.08, per year increase], valvular disease (HR 1.63, 95% CI: 1.15–2.33), congestive heart failure (HR 1.73, 95% CI: 1.24–2.41), vascular disease (HR 1.47, 95% CI: 1.05–2.05), abnormal liver function (HR 1.80, 95% CI: 1.02–3.17), and abnormal renal function (HR 1.58, 95% CI: 1.10–2.27). Statistical significance was lost in the multivariate model for LAA ostium diameter, diabetes mellitus, and prior major bleeding or predisposition. No considerable collinearity was present in the variables included in univariate and multivariate analysis (see Supplementary material online, Figure S1).

Multivariate hazard ratios for 2-year mortality. CI, confidence interval; HR, hazard ratio; LAA, left atrial appendage; mm: millimetre.
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Covariate . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Age, per year | 1.06 | 1.04–1.09 | <0.001 | 1.05 | 1.03–1.08 | <0.001 |
| Male | 1.22 | 0.88–1.68 | 0.24 | |||
| Non-paroxysmal AF | 1.05 | 0.76–1.44 | 0.77 | |||
| Eligible for OAT | 0.73 | 0.50–1.06 | 0.10 | |||
| Valvular disease | 2.67 | 1.92–3.72 | <0.001 | 1.63 | 1.15–2.33 | 0.007 |
| LAA ostium diameter, per mm | 1.05 | 1.00–1.10 | 0.047 | 1.03 | 0.99–1.08 | 0.18 |
| Congestive heart failure | 2.06 | 1.51–2.81 | <0.001 | 1.73 | 1.24–2.41 | 0.001 |
| Hypertension | 1.01 | 0.63–1.62 | 0.95 | |||
| Diabetes mellitus | 1.50 | 1.08–2.07 | 0.015 | 1.30 | 0.93–1.81 | 0.13 |
| Prior thromboembolism | 0.85 | 0.72–1.00 | 0.052 | |||
| Vascular disease | 2.06 | 1.49–2.84 | <0.001 | 1.47 | 1.05–2.05 | 0.026 |
| Abnormal liver function | 2.82 | 1.66–4.80 | <0.001 | 1.80 | 1.02–3.17 | 0.042 |
| Abnormal renal function | 2.54 | 1.80–3.57 | <0.001 | 1.58 | 1.10–2.27 | 0.014 |
| Prior major bleed or predisposition to bleeding | 1.89 | 1.38–2.58 | <0.001 | 1.27 | 0.90–1.78 | 0.17 |
| Labile INRs | 0.85 | 0.54–1.32 | 0.46 | |||
| NSAID use | 1.28 | 0.92–1.79 | 0.14 | |||
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Covariate . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Age, per year | 1.06 | 1.04–1.09 | <0.001 | 1.05 | 1.03–1.08 | <0.001 |
| Male | 1.22 | 0.88–1.68 | 0.24 | |||
| Non-paroxysmal AF | 1.05 | 0.76–1.44 | 0.77 | |||
| Eligible for OAT | 0.73 | 0.50–1.06 | 0.10 | |||
| Valvular disease | 2.67 | 1.92–3.72 | <0.001 | 1.63 | 1.15–2.33 | 0.007 |
| LAA ostium diameter, per mm | 1.05 | 1.00–1.10 | 0.047 | 1.03 | 0.99–1.08 | 0.18 |
| Congestive heart failure | 2.06 | 1.51–2.81 | <0.001 | 1.73 | 1.24–2.41 | 0.001 |
| Hypertension | 1.01 | 0.63–1.62 | 0.95 | |||
| Diabetes mellitus | 1.50 | 1.08–2.07 | 0.015 | 1.30 | 0.93–1.81 | 0.13 |
| Prior thromboembolism | 0.85 | 0.72–1.00 | 0.052 | |||
| Vascular disease | 2.06 | 1.49–2.84 | <0.001 | 1.47 | 1.05–2.05 | 0.026 |
| Abnormal liver function | 2.82 | 1.66–4.80 | <0.001 | 1.80 | 1.02–3.17 | 0.042 |
| Abnormal renal function | 2.54 | 1.80–3.57 | <0.001 | 1.58 | 1.10–2.27 | 0.014 |
| Prior major bleed or predisposition to bleeding | 1.89 | 1.38–2.58 | <0.001 | 1.27 | 0.90–1.78 | 0.17 |
| Labile INRs | 0.85 | 0.54–1.32 | 0.46 | |||
| NSAID use | 1.28 | 0.92–1.79 | 0.14 | |||
AF, atrial fibrillation; INR, international normalized ratio; LAA, left atrial appendage; NSAID, non-steroidal anti-inflammatory drug; OAT, oral anticoagulation therapy.
P-values <0.05 are bold.
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Covariate . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Age, per year | 1.06 | 1.04–1.09 | <0.001 | 1.05 | 1.03–1.08 | <0.001 |
| Male | 1.22 | 0.88–1.68 | 0.24 | |||
| Non-paroxysmal AF | 1.05 | 0.76–1.44 | 0.77 | |||
| Eligible for OAT | 0.73 | 0.50–1.06 | 0.10 | |||
| Valvular disease | 2.67 | 1.92–3.72 | <0.001 | 1.63 | 1.15–2.33 | 0.007 |
| LAA ostium diameter, per mm | 1.05 | 1.00–1.10 | 0.047 | 1.03 | 0.99–1.08 | 0.18 |
| Congestive heart failure | 2.06 | 1.51–2.81 | <0.001 | 1.73 | 1.24–2.41 | 0.001 |
| Hypertension | 1.01 | 0.63–1.62 | 0.95 | |||
| Diabetes mellitus | 1.50 | 1.08–2.07 | 0.015 | 1.30 | 0.93–1.81 | 0.13 |
| Prior thromboembolism | 0.85 | 0.72–1.00 | 0.052 | |||
| Vascular disease | 2.06 | 1.49–2.84 | <0.001 | 1.47 | 1.05–2.05 | 0.026 |
| Abnormal liver function | 2.82 | 1.66–4.80 | <0.001 | 1.80 | 1.02–3.17 | 0.042 |
| Abnormal renal function | 2.54 | 1.80–3.57 | <0.001 | 1.58 | 1.10–2.27 | 0.014 |
| Prior major bleed or predisposition to bleeding | 1.89 | 1.38–2.58 | <0.001 | 1.27 | 0.90–1.78 | 0.17 |
| Labile INRs | 0.85 | 0.54–1.32 | 0.46 | |||
| NSAID use | 1.28 | 0.92–1.79 | 0.14 | |||
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Covariate . | HR . | 95% CI . | P-value . | HR . | 95% CI . | P-value . |
| Age, per year | 1.06 | 1.04–1.09 | <0.001 | 1.05 | 1.03–1.08 | <0.001 |
| Male | 1.22 | 0.88–1.68 | 0.24 | |||
| Non-paroxysmal AF | 1.05 | 0.76–1.44 | 0.77 | |||
| Eligible for OAT | 0.73 | 0.50–1.06 | 0.10 | |||
| Valvular disease | 2.67 | 1.92–3.72 | <0.001 | 1.63 | 1.15–2.33 | 0.007 |
| LAA ostium diameter, per mm | 1.05 | 1.00–1.10 | 0.047 | 1.03 | 0.99–1.08 | 0.18 |
| Congestive heart failure | 2.06 | 1.51–2.81 | <0.001 | 1.73 | 1.24–2.41 | 0.001 |
| Hypertension | 1.01 | 0.63–1.62 | 0.95 | |||
| Diabetes mellitus | 1.50 | 1.08–2.07 | 0.015 | 1.30 | 0.93–1.81 | 0.13 |
| Prior thromboembolism | 0.85 | 0.72–1.00 | 0.052 | |||
| Vascular disease | 2.06 | 1.49–2.84 | <0.001 | 1.47 | 1.05–2.05 | 0.026 |
| Abnormal liver function | 2.82 | 1.66–4.80 | <0.001 | 1.80 | 1.02–3.17 | 0.042 |
| Abnormal renal function | 2.54 | 1.80–3.57 | <0.001 | 1.58 | 1.10–2.27 | 0.014 |
| Prior major bleed or predisposition to bleeding | 1.89 | 1.38–2.58 | <0.001 | 1.27 | 0.90–1.78 | 0.17 |
| Labile INRs | 0.85 | 0.54–1.32 | 0.46 | |||
| NSAID use | 1.28 | 0.92–1.79 | 0.14 | |||
AF, atrial fibrillation; INR, international normalized ratio; LAA, left atrial appendage; NSAID, non-steroidal anti-inflammatory drug; OAT, oral anticoagulation therapy.
P-values <0.05 are bold.
Mortality rates in subgroups
The incidence rate of 2-year death is plotted for subgroups in Figure 4. Mortality rate gradually increases with age, with almost one in three patients older than 84 dying within 2 years after LAAO. Two-year mortality rate was also substantial in patients with valvular disease (24.7%, 95% CI: 20.4–28.7%), congestive heart failure (23.6%, 95% CI: 18.9–28.0%), abnormal liver function (36.3%, 95% CI: 19.8–49.4%), abnormal renal function (30.4%, 95% CI: 22.7–37.3%), and prior bleeding or predisposition to bleeding (24.0%, 95% CI: 19.5–28.3%).

Two-year mortality rates for subgroups. Univariate predictors include LAA ostium diameter, diabetes mellitus and prior major bleed or predisposition. Multivariate predictors include age, valvular disease, congestive heart failure, vascular disease, abnormal liver function and abnormal renal function. Incidence rates are calculated per subgroup. The vertical reference line represents the mortality rate within the whole cohort. CI, confidence interval; HR, hazard ratio; LAA, left atrial appendage; mm: millimetre.
Risk stratification model
To combine multivariate predictors in a risk model, age was dichotomized at a cut-off value of 76 years. Figure 5 illustrates a stepwise increment in the mortality rate as the number of multivariate risk factors increases. Two-year mortality rate was low in patients with zero or one risk factor (respectively, 5.6% and 5.7%), increased to 14.4% in patients with two risk factors, and showed a substantial increase in patients with three or four risk factors (i.e. 29.9% and 32.5%). Patients with more than four risk factors exhibited an exceptionally high mortality risk (46.1%). No internal or external validation was carried out, as not all known predictors of mortality after LAAO were available for integration in the model. CHA2DS2-VASc scores increased with mortality risk groups, but no difference in 2-year ischaemic stroke rate between was observed between groups (log rank P = 0.82). The competing risk of death may nonetheless be substantial for ischaemic stroke in higher CHA2DS2-VASc groups. Mortality risk stratified for CHA2DS2-VASc score and chronic kidney disease are shown in Supplementary material online, Figure S2and3.

Two-year mortality rates according to number of risk factors. The table on the right side represents 2-year mortality rates with 95% CI per risk subgroup. CI, confidence interval; IR, incidence rate; RF, risk factors.
Discussion
Our study finds that approximately one in six patients in EWOLUTION died within 2 years after LAAO. Independent predictors of all-cause death included older age, valvular disease, congestive heart failure, vascular disease, and abnormal renal or liver function. Mortality rate showed a stepwise increment when more risk factors were present. Almost half of patients having five or six risk factors died within 2 years after LAAO.
The high mortality rate in EWOLUTION underscores the frailty of patients undergoing LAAO. Patients were enrolled for EWOLUTION from 2013 to 2015, and during the early days of LAAO, this treatment option was often reserved as a last resort. This may have led to the selection of patients with more comorbidities and therefore poorer prognosis. The all-cause mortality rate in EWOLUTION is similar to that in the AMULET observational study, which enrolled patients around the same period.14 Moreover, 2-year mortality rate in EWOLUTION also appears very comparable with a cohort of AF patients with similar CHA2DS2-VASc score not undergoing LAAO, both on and off OAT.15 The more recent PINNACLE FLX and FLXibility registries investigating Watchman FLX describe a 1-year mortality rate of respectively 6.6% and 10.8%16,17 and 1-year results from the National Cardiovascular Data Registry (NCDR) show a mortality rate of 8.5%.18 These data may indicate the improved prognosis of contemporary LAAO candidates in comparison with the 10.3% 1-year mortality rate observed in EWOLUTION. Importantly, devices have improved over the last years, as well as imaging techniques and overall procedural workflow, making procedures faster and the burden on the patient much smaller. Furthermore, newer devices such as the Watchman FLX Pro and the Laminar device may be less thrombogenic than their predecessors and thereby limit the need for post-procedural DAPT or OAC, thus reducing post-procedural bleeding risk. This could improve survival rate, as deceased patients had substantially more major bleeding events in our data. Recent guidelines and consensus papers show more liberal recommendations for LAAO,19 with the most recent American College of Cardiology AF guideline describing LAAO as a reasonable treatment option for patients with a long-term contraindication for OAT.20 This provides a rationale for LAAO in a broader selection of patients, including also those with less comorbidities and therefore better prognosis.
Nevertheless, patient selection and risk stratification remain of vital importance for all medical therapies. Implantable cardioverter-defibrillator treatment for sudden cardiac death prevention is only considered for patients with an expected survival of at least 1 year, although this may be deemed arbitrary. In the EWOLUTION cohort, predictors of death included valvular disease, congestive heart failure, and abnormal renal or liver function. The definitions of these variables are also arbitrary. Abnormal renal function was scored at a serum creatinine ≥ 200 µmol/L and also in patients on chronic dialysis or after renal transplantation. This definition includes a wide range of patients and potentially introduces a difference in mortality risk within this range. Patients with advanced kidney disease on dialysis typically have poor 5-year survival and studies investigating LAAO in these patients show up to half of patients dying within this time frame,21 although mortality may be higher in patients on dialysis not receiving LAAO.22 We observed a 30% 2-year mortality rate in patients with renal disease, which seems in line with other literature.23
The presence of various comorbidities is accompanied by substantial mortality rates in our data. This may suggest that we should withhold LAAO from these patients. On the other hand, a recent propensity score-matched comparison in patients with a CHA2DS2-VASc score ≥ 5 (and thus multiple comorbidities) showed a benefit of LAAO over DOACs with regard to a composite endpoint of CV death, thromboembolic events, and clinically relevant bleeding.24 Although patients with multiple comorbidities stand to benefit the most from stroke prevention, they may not live long enough to justify the costly intervention. Randomized data focusing on subgroups would be the best method to gain insights in the efficacy and cost-effectiveness of LAAO within specific subpopulations. However, given the fact that indirect evidence on the protective effect of LAAO within patients contraindicated to OAT already exists, a randomized trial comparing LAAO to standard of care without OAT has proven hard to perform due to physician and patient preference for the LAAO arm.
In a study by Mesnier et al.,25 predictors of 1-year mortality after LAAO included older age, diabetes, heart failure, lower body mass index (BMI), and lower estimated glomerular filtration rate. Our data largely support these findings, although diabetes was only associated with 2-year mortality in the univariate analysis within our cohort. In addition to the Mesnier model, we found valvular disease, vascular disease, and liver disease as possible predictors of early death. It would have been insightful to validate the model by Mesnier et al.25 within our cohort, but this was not feasible due to the absence of BMI data in EWOLUTION.
Importantly, the presence of these risk factors is a surrogate marker for frailty. A recent publication of NCDR LAAO data showed a five-fold increased risk of in-hospital mortality for frail patients, and 2.1% of frail patients dying within 45 days after discharge.26 Also, Wang et al.27 showed an increasing risk for mortality after LAAO within higher risk groups for frailty, even when adjusted for age, sex, and comorbidities. Incidence of 1-year mortality was 11.2% among the most frail patients, which was significantly higher than patients with intermediate (6.8%) and low (2.8%) frailty risk. However, the determination of frailty scores requires prospective assessment, and these were not collected within EWOLUTION.
Other unknown factors, such as the presence of malignancy or chronic obstructive pulmonary disease, likely influence mortality rate after LAAO, especially since there was a high rate of non-CV death in our analysis. Additionally, biometric mortality predictors such as lower systolic blood pressure, higher serum uric acid concentration, lower haemoglobin level, higher cardiac troponin T level, and higher N-terminal pro-brain natriuretic peptide level, might be extrapolated from the heart failure population to the LAAO population.28 Moreover, physicians should be critical before indicating patients with a shorter life span for a costly invasive procedure to achieve long-term stroke prevention.
Limitations
Although most definitions for clinical endpoints and patient characteristics were pre-defined, our study is limited by some covariates being scored per physician’s discretion, such as OAT eligibility and presence of valvular disease. Lack of a standardized definition for risk factors introduces inter-observer variability and hampers the uniformity of an eventual risk model. Furthermore, the unavailability of potential predictors of early mortality, such as frailty score and BMI, forms a hiatus in the multivariate analysis. As a consequence, no internal or external validation of the risk model was carried out. Nevertheless, the observed risk factors of 2-year mortality after LAAO do provide insight in what patients are most susceptible to early death. The EWOLUTION registry included a real-world population that did not have to meet strict clinical trial criteria, thus positively contributing to the generalizability of our findings to the actual LAAO population.
Conclusion
One in six patients died within 2 years after LAAO within EWOLUTION, with the majority of patients dying from a non-CV cause. Independent predictors of death included older age, valvular disease, heart failure, vascular disease, and abnormal renal or kidney function. When combined in a risk model, mortality risk was 46% in patients demonstrating more than four risk factors. These findings underscore the importance of careful patient selection for LAAO, as non-procedure-related factors may introduce procedure futility due to early all-cause death.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
The authors would like to acknowledge all EWOLUTION investigators provided in Supplementary material online, Table S3.
Funding
The original EWOLUTION registry was funded by the Boston Scientific Corporation. No additional funding was provided for the current study.
Conflict of interest
H.I. is a proctor for Boston Scientific and received personal fees from Boston Scientific, outside the submitted work. B.S. reports personal fees from Boston Scientific and St Jude Medical, outside the submitted work. T.B. reports personal fees from Boston Scientific, outside the submitted work. P.M. is a proctor for Boston Scientific and a consultant for St Jude Medical. M.G. is a Boston Scientific advisory board member, receives honoraria for lectures from Boston Scientific and Abbott, and is a proctor for Boston Scientific and Abbott. E.V. and K.S. are employees and shareholders at Boston Scientific. T.D.P. reports fees to the cardiology department research fund from Boston Scientific, outside the submitted work. M.W.B. reports personal fees from Boston Scientific, St Jude Medical, Biosense Webster, and Johnson & Johnson, outside the submitted work. H.S. reports personal fees from Abbott, Aptus, Atrium, Biosense Webster, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, CardioMEMS, Cardiox, Celonova, CGuard, Coherex, Comed B.V., Contego, Covidien, CSI, CVRx, ev3, FlowCardia, Gardia, Gore, GTIMD Medical, Guided Delivery Systems, Hemoteq, InSeal Medical, InspireMD, Kona Medical, Lumen Biomedical, LifeTech, Lutonix, Maya Medical, Medtronic, Occlutech, pfm Medical, Recor, Trireme, Trivascular, Valtech, Vascular Dynamics, Venus Medical, Veryan, and Vessix, outside the submitted work, and he reports stock options in Cardiokinetix, Access Closure, Coherex, and SMT, outside the submitted work. L.V.A.B. reports fees to the Cardiology Department from Boston Scientific, and Medtronic, outside the submitted work. The other authors report no conflicts.
Data availability
All relevant data of the EWOLUTION study cohort are held by Boston Scientific. Access may be obtained with limitations upon request.



