-
PDF
- Split View
-
Views
-
Cite
Cite
José Miguel Rivera-Caravaca, Olivier Piot, Inmaculada Roldán-Rabadán, Arnaud Denis, Manuel Anguita, Jacques Mansourati, Alejandro Pérez-Cabeza, Eloi Marijon, Javier García-Seara, Christophe Leclercq, Ignacio García-Bolao, Nicolas Lellouche, Tatjana Potpara, Giuseppe Boriani, Laurent Fauchier, Gregory Y H Lip, Francisco Marín, Characterization of atrial fibrillation in real-world patients: testing the 4S-AF scheme in the Spanish and French cohorts of the EORP-AF Long-Term General Registry, EP Europace, Volume 24, Issue 2, February 2022, Pages 202–210, https://doi.org/10.1093/europace/euab202
Close - Share Icon Share
Abstract
The 4S-AF scheme [Stroke risk, Symptom severity, Severity of atrial fibrillation (AF) burden, Substrate severity] has recently been described as a novel approach to in-depth characterization of AF. We aim to determine if the 4S-AF scheme would be useful for AF characterization and provides prognostic information in real-world AF patients.
The Spanish and French cohorts of the EORP-AF Long-Term General Registry were included. The baseline 4S-AF scheme was calculated and related to the primary management strategy (rhythm or rate control). Follow-up was performed at 1-year with all-cause mortality and the composite of ischaemic stroke/transient ischaemic attack/systemic embolism, major bleeding, and all-cause death, as primary endpoints. A total of 1479 patients [36.9% females, median age 72 interquartile range (IQR 64–80) years] were included. The median 4S-AF scheme score was 5 (IQR 4–7). The 4S-AF scheme, as continuous and as categorical, was associated with the management strategy decided for the patient (both P < 0.001). The predictive performances of the 4S-AF scheme for the actual management strategy were appropriate in its continuous [c-index 0.77, 95% confidence interval (CI) 0.75–0.80] and categorical (c-index 0.75, 95% CI 0.72–0.78) forms. Cox regression analyses showed that ‘red category’ classified patients in the 4S-AF scheme had a higher risk of all-cause death (aHR 1.75, 95% CI 1.02–2.99) and composite outcomes (aHR 1.60, 95% CI 1.05–2.44).
Characterization of AF by using the 4S-AF scheme may aid in identifying AF patients that would be managed by rhythm or rate control and could also help in identifying high-risk AF patients for worse clinical outcomes in a ‘real-world’ setting.
The 4S-AF scheme [Stroke risk, Symptom severity, Severity of atrial fibrillation (AF) burden, Substrate severity] was described to in-depth characterization of AF and included in the recent ESC guidelines.
Approximately, 75% of patients from the Spanish and French cohorts of the EORP-AF Long-Term General Registry were on the management strategy suggested by the 4S-AF scheme, and it was associated with the probability of determining the real strategy decided for the patient.
The 4S-AF scheme presented an appropriate predictive performance for the actual management strategy, as well as high net benefit and clinical usefulness.
Cox regression analyses showed that ‘red category’ 4S-AF scheme patients had a significantly higher risk of all-cause death and composite outcomes.
Application of the 4S-AF scheme in everyday clinical practice would facilitate decision-making, may aid to select the most appropriate management strategy, and could also help in identifying high-risk AF patients for worse clinical outcomes.
Introduction
The management of atrial fibrillation (AF) is complex. Classically, much of the attention has been focused on stroke prevention, but it has been increasingly recognized that AF patients require a more integrated management approach by a coordinated and multidisciplinary team.1 Part of this management could be well-addressed by following the Atrial fibrillation Better Care (ABC) holistic pathway, which encompasses anticoagulation/avoid stroke (A), better symptom management (B), and cardiovascular risk factors and comorbid conditions optimization (C). Adherence to the ABC pathway has shown to improve patient outcomes in different regions of the world.2
The 2020 ESC AF guidelines propose a patient pathway for the management of AF, summed up as ‘CC to ABC’. ABC refers to the ABC pathway which has been detailed above and the CC represents Confirmation of AF and Characterization of AF by using the 4S-AF scheme.3 The 4S-AF scheme was recently described as a novel approach to in-depth characterization of AF.4 This includes four AF-related domains (Stroke risk, Symptom severity, Severity of AF burden, Substrate severity). Implementation of the 4S-AF scheme for AF patients characterization in routine clinical practice could facilitate overall AF management and AF research. However, there is no validation of this scheme to date, so the clinical utility and prognostic value of the 4S-AF scheme remains uncertain.
In this study, we present the first validation of the 4S-AF scheme in a ‘real-world’ setting using the Spanish and French cohorts of the EORP-AF Long-Term General Registry. We aim to determine if this scheme would be useful for the characterization of AF, thus aiding decision-making about the management strategy of AF patients and providing prognostic information.
Methods
A detailed description of the design of the EORP-AF Long-Term General Registry has been published elsewhere.5 In brief, the EORP-AF Long-Term General Registry was a prospective, observational, large-scale multicentre registry sponsored and conducted by the ESC, enrolling AF patients in 250 centres from 27 participating ESC countries.
Patients were enrolled consecutively when presenting with AF as primary or secondary diagnosis both in- and outpatient cardiology services. Main inclusion criteria were: (i) the qualifying AF event had to be recorded by a 12-lead electrocardiogram (ECG), 24 h ECG Holter, or other electrocardiographic documentation within 12 months before enrolment; (ii) age should be ≥18 years; and (iii) written informed consent form provided. Exclusion criteria were the following: (i) no objective proof of AF; (ii) being previously enrolled in the EORP-AF Pilot Registry; or (iii) being or planned to be enrolled in a pharmacological interventional clinical trial.
Inclusion was undertaken from October 2013 to September 2016. Of the 9663 patients enrolled in the EORP-AF Long-Term General Registry who consented for the follow-up phase and had available data about follow-up status, 729 (7.5%) patients were from Spain and 750 (7.8%) were from France. The first Spanish patient was included in 2014 and the first French patient was included in 2015. The study protocol was approved by an institutional review board for every participating institution. The study was performed according to the European Union Note for Guidance on Good Clinical Practice CPMP/ECH/135/95 and the Declaration of Helsinki.
Patients information was collected according to the study procedures previously described.5 This included baseline clinical characteristics and previous clinical history, as well as history of previous interventional procedures, medications, estimation of thrombo-embolic risk according to the CHA2DS2-VASc score, bleeding risk according to HAS-BLED score, and symptomatic status according to the European Heart Rhythm Association (EHRA) score. The primary management strategy of AF (rhythm or rate control) was also recorded at baseline. All data collected were then entered into an electronic case report form.
Follow-up and study outcomes
Follow-up was performed at 1-year after enrolment. During follow-up, all incident major adverse clinical events were recorded. In this study, we primarily focused on all-cause mortality and the composite of ischaemic stroke (IS)/transient ischaemic attack (TIA)/systemic embolism (SE), major bleeding, and all-cause death, but individual outcomes of IS/TIA/SE and major bleeding were also reported. Investigators recorded all available details about the incident major adverse clinical events on the centralized electronic case report form.
The 4S-AF scheme
For the study purpose, we calculated the baseline 4S-AF scheme according to the original publication.4 The scoring system is summarized in Figure 1. In brief, the first step according to 4S-AF scheme is assessing the stroke risk with the CHA2DS2-VASc score and to offer oral anticoagulation (OAC) in non-truly low-risk patients (i.e. ≥1 in males; ≥2 in females). Symptom severity should be assessed by using the EHRA symptom score whereas the severity of AF burden could be evaluated according to AF type (as paroxysmal or first onset, persistent, long-standing persistent, and permanent). In order to be practical and allow using only AF type to evaluate the ‘severity of AF burden’ domain, permanent AF accounted as 3 points instead of 2 as stated in the original publication. Substrate severity focuses mainly in comorbidities and cardiovascular risk factors. Thus, the substrate domain was evaluated according to the presence of high body mass index (≥30 kg/m2), heart failure, diabetes, vascular disease (i.e. coronary artery disease and/or peripheral artery disease), hypercholesterolaemia, history of stroke/TIA/thromboembolism, chronic kidney disease, liver disease, malignancy, hypertension, and chronic obstructive pulmonary disease (COPD)/obstructive sleep apnoea (OSA). In addition, left atrial (LA) enlargement/dysfunction was considered as a LA volume higher than 28 mL/m (mild ≥28; moderate ≥34; severe ≥40).
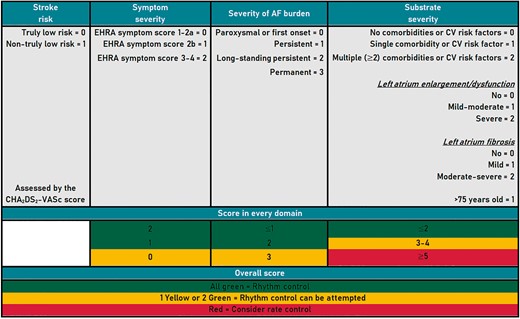
According to the results for the domains ‘symptom severity’, ‘severity of AF burden’, and ‘substrate severity’, three code colours have been defined. Patients with these three domains in ‘green’ should be managed by rhythm control. In patients with one of these domains in ‘yellow’ or two domains in ‘green’ categories, rhythm control can be attempted. On contrary, for patients with ‘red’ colour category, the 4S-AF scheme suggests a rate control strategy.
Statistical analysis
Continuous variables were reported as the median and interquartile range (IQR) or mean ± standard deviation, as appropriate. Categorical variables were reported as absolute frequencies and percentages.
Comparisons between categorical groups were performed using a χ2 test or Fisher’s exact test, accordingly, whereas the Pearson’s χ2 test was used to compare proportions. The Student's t or the Mann–Whitney U tests were used to compare continuous and categorical variables, as appropriate.
Cox proportional hazard regression models were performed to determine the association between the 4S-AF scheme and the risk of study outcomes. The results were expressed as adjusted hazard ratio (aHR) with 95% confidence intervals (CIs). The proportional hazard assumption was checked using the scaled Schoenfeld residuals, and the proportional hazard assumption was supported by a non-significant relationship between residuals and time, and refuted by a significant relationship (data not shown). Plots of Kaplan–Meier curves for study outcomes were performed, and survival distributions were compared using the log-rank test.
The receiver operating characteristic curve was applied to evaluate the predictive ability (expressed as c-index) of the 4S-AF scheme for the primary management strategy. The clinical usefulness and net benefit of using the 4S-AF scheme in its continuous and categorical forms for deciding on rhythm or rate control management strategies were estimated using decision curve analyses (DCAs). In this case, the DCAs show the clinical usefulness of the 4S-AF scheme based on a continuum of potential thresholds for rhythm or rate control (x-axis) and the net benefit of using the scheme to select the management strategy (y-axis). Thus, the x-axis shows threshold values for rhythm or rate control, while the y-axis represents the net benefit for the different threshold values of rhythm or rate control. The farther are the 4S-AF models from the dashed purple line (i.e. assume all patients will be on rate control) and the solid green line (i.e. assume all patients will be on rhythm control), the higher net benefit.
Two-sided P-values <0.05 were accepted as statistically significant. Statistical analyses were performed using SPSS v. 24.0 (SPSS, Inc., Chicago, IL, USA) and STATA v. 15.0 (Stata Corp., College Station, TX, USA) for Windows.
Results
The merged cohort included 1479 patients [36.9% females (546), median age of 72 (IQR 64–80) years], of which 750 patients derived from the French cohort and 729 derived from the Spanish cohort (Table 1). The median score of the 4S-AF scheme was 5 (IQR 4–7), whereas median scores in the Spanish and French cohorts were 6 (IQR 5–8) and 5 (IQR 3–6), respectively.
| . | Overall N = 1479 . | Spanish cohort N = 729 . | French cohort N = 750 . | P-value . |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 933 (63.1) | 416 (57.1) | 517 (68.9) | <0.001 |
| Age (years), median (IQR) | 72 (64–80) | 75 (67–81) | 69 (61–78) | <0.001 |
| AF-related conditions | ||||
| Type of AF | ||||
| First-onset/paroxysmal | 596 (40.3) | 293 (40.2) | 303 (40.4) | 0.874 |
| Persistent | 310 (20.9) | 86 (11.8) | 224 (29.9) | 0.002 |
| Long-standing persistent | 54 (3.6) | 11 (1.5) | 43 (5.7) | 0.835 |
| Permanent | 500 (33.8) | 338 (46.4) | 162 (21.6) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 790 (53.4) | 444 (60.9) | 346 (46.1) | <0.001 |
| Diabetes mellitus | 357 (24.1) | 219 (30.0) | 138 (18.4) | 0.020 |
| Heart failure | 527 (35.6) | 270 (37.0) | 257 (34.3) | 0.578 |
| History of stroke/TIA/thromboembolism | 200 (13.5) | 101 (13.9) | 99 (13.2) | 0.951 |
| Peripheral vascular disease | 104 (7.0) | 44 (6.0) | 60 (8.0) | 0.995 |
| Renal impairment | 155 (10.5) | 88 (12.1) | 67 (8.9) | 0.707 |
| Coronary artery disease | 344 (23.3) | 144 (19.8) | 200 (26.7) | 0.176 |
| Hypercholesterolaemia | 643 (43.5) | 358 (49.1) | 285 (38.0) | 0.006 |
| Current smoking habit | 143 (9.7) | 51 (7.0) | 92 (12.3) | 0.479 |
| History of previous bleeding | 101 (6.8) | 59 (8.1) | 42 (5.6) | 0.929 |
| Concomitant malignant disease | 147 (9.9) | 74 (10.2) | 73 (9.7) | 0.862 |
| Hyperthyroidism | 54 (3.7) | 14 (1.9) | 40 (5.3) | 0.824 |
| Hypothyroidism | 148 (10.0) | 80 (11.0) | 68 (9.1) | 0.914 |
| COPD/OSA | 135 (9.1) | 72 (9.9) | 63 (8.4) | 0.998 |
| Valvular disease | 432 (29.2) | 234 (32.1) | 198 (26.4) | 0.237 |
| Concomitant treatment, n (%) | ||||
| Oral anticoagulation | 1321 (89.3) | 634 (87.0) | 687 (91.6) | 0.346 |
| Any VKA | 673 (45.5) | 389 (53.4) | 284 (37.9) | <0.001 |
| Any DOAC | 648 (43.8) | 245 (33.6) | 403 (53.7) | <0.001 |
| Anti-arrhythmics | 522 (35.3) | 118 (16.2) | 404 (53.9) | <0.001 |
| Digoxin | 111 (7.5) | 72 (9.9) | 39 (5.2) | 0.619 |
| Calcium antagonist | 171 (11.6) | 76 (10.4) | 95 (12.7) | 0.821 |
| Beta-blockers | 905 (61.2) | 427 (58.6) | 478 (63.7) | 0.133 |
| Statins | 609 (41.2) | 330 (45.3) | 279 (37.2) | 0.053 |
| Diuretics | 717 (48.5) | 384 (52.7) | 333 (44.4) | 0.032 |
| Angiotensin-converting enzyme inhibitors | 484 (32.7) | 219 (30.0) | 265 (35.4) | 0.246 |
| Angiotensin-renin blockers | 316 (21.4) | 212 (29.1) | 104 (13.9) | 0.005 |
| Oral anti-diabetics | 273 (18.5) | 175 (24.0) | 98 (13.1) | 0.046 |
| CHA2DS2-VASc score, median (IQR) | 3 (2–4) | 3 (2–5) | 3 (1–4) | <0.001 |
| HAS-BLED score, median (IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) | <0.001 |
| . | Overall N = 1479 . | Spanish cohort N = 729 . | French cohort N = 750 . | P-value . |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 933 (63.1) | 416 (57.1) | 517 (68.9) | <0.001 |
| Age (years), median (IQR) | 72 (64–80) | 75 (67–81) | 69 (61–78) | <0.001 |
| AF-related conditions | ||||
| Type of AF | ||||
| First-onset/paroxysmal | 596 (40.3) | 293 (40.2) | 303 (40.4) | 0.874 |
| Persistent | 310 (20.9) | 86 (11.8) | 224 (29.9) | 0.002 |
| Long-standing persistent | 54 (3.6) | 11 (1.5) | 43 (5.7) | 0.835 |
| Permanent | 500 (33.8) | 338 (46.4) | 162 (21.6) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 790 (53.4) | 444 (60.9) | 346 (46.1) | <0.001 |
| Diabetes mellitus | 357 (24.1) | 219 (30.0) | 138 (18.4) | 0.020 |
| Heart failure | 527 (35.6) | 270 (37.0) | 257 (34.3) | 0.578 |
| History of stroke/TIA/thromboembolism | 200 (13.5) | 101 (13.9) | 99 (13.2) | 0.951 |
| Peripheral vascular disease | 104 (7.0) | 44 (6.0) | 60 (8.0) | 0.995 |
| Renal impairment | 155 (10.5) | 88 (12.1) | 67 (8.9) | 0.707 |
| Coronary artery disease | 344 (23.3) | 144 (19.8) | 200 (26.7) | 0.176 |
| Hypercholesterolaemia | 643 (43.5) | 358 (49.1) | 285 (38.0) | 0.006 |
| Current smoking habit | 143 (9.7) | 51 (7.0) | 92 (12.3) | 0.479 |
| History of previous bleeding | 101 (6.8) | 59 (8.1) | 42 (5.6) | 0.929 |
| Concomitant malignant disease | 147 (9.9) | 74 (10.2) | 73 (9.7) | 0.862 |
| Hyperthyroidism | 54 (3.7) | 14 (1.9) | 40 (5.3) | 0.824 |
| Hypothyroidism | 148 (10.0) | 80 (11.0) | 68 (9.1) | 0.914 |
| COPD/OSA | 135 (9.1) | 72 (9.9) | 63 (8.4) | 0.998 |
| Valvular disease | 432 (29.2) | 234 (32.1) | 198 (26.4) | 0.237 |
| Concomitant treatment, n (%) | ||||
| Oral anticoagulation | 1321 (89.3) | 634 (87.0) | 687 (91.6) | 0.346 |
| Any VKA | 673 (45.5) | 389 (53.4) | 284 (37.9) | <0.001 |
| Any DOAC | 648 (43.8) | 245 (33.6) | 403 (53.7) | <0.001 |
| Anti-arrhythmics | 522 (35.3) | 118 (16.2) | 404 (53.9) | <0.001 |
| Digoxin | 111 (7.5) | 72 (9.9) | 39 (5.2) | 0.619 |
| Calcium antagonist | 171 (11.6) | 76 (10.4) | 95 (12.7) | 0.821 |
| Beta-blockers | 905 (61.2) | 427 (58.6) | 478 (63.7) | 0.133 |
| Statins | 609 (41.2) | 330 (45.3) | 279 (37.2) | 0.053 |
| Diuretics | 717 (48.5) | 384 (52.7) | 333 (44.4) | 0.032 |
| Angiotensin-converting enzyme inhibitors | 484 (32.7) | 219 (30.0) | 265 (35.4) | 0.246 |
| Angiotensin-renin blockers | 316 (21.4) | 212 (29.1) | 104 (13.9) | 0.005 |
| Oral anti-diabetics | 273 (18.5) | 175 (24.0) | 98 (13.1) | 0.046 |
| CHA2DS2-VASc score, median (IQR) | 3 (2–4) | 3 (2–5) | 3 (1–4) | <0.001 |
| HAS-BLED score, median (IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) | <0.001 |
COPD/OSA, chronic obstructive pulmonary disease/obstructive sleep apnoea; DOAC, direct-acting oral anticoagulant; IQR, interquartile range; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
| . | Overall N = 1479 . | Spanish cohort N = 729 . | French cohort N = 750 . | P-value . |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 933 (63.1) | 416 (57.1) | 517 (68.9) | <0.001 |
| Age (years), median (IQR) | 72 (64–80) | 75 (67–81) | 69 (61–78) | <0.001 |
| AF-related conditions | ||||
| Type of AF | ||||
| First-onset/paroxysmal | 596 (40.3) | 293 (40.2) | 303 (40.4) | 0.874 |
| Persistent | 310 (20.9) | 86 (11.8) | 224 (29.9) | 0.002 |
| Long-standing persistent | 54 (3.6) | 11 (1.5) | 43 (5.7) | 0.835 |
| Permanent | 500 (33.8) | 338 (46.4) | 162 (21.6) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 790 (53.4) | 444 (60.9) | 346 (46.1) | <0.001 |
| Diabetes mellitus | 357 (24.1) | 219 (30.0) | 138 (18.4) | 0.020 |
| Heart failure | 527 (35.6) | 270 (37.0) | 257 (34.3) | 0.578 |
| History of stroke/TIA/thromboembolism | 200 (13.5) | 101 (13.9) | 99 (13.2) | 0.951 |
| Peripheral vascular disease | 104 (7.0) | 44 (6.0) | 60 (8.0) | 0.995 |
| Renal impairment | 155 (10.5) | 88 (12.1) | 67 (8.9) | 0.707 |
| Coronary artery disease | 344 (23.3) | 144 (19.8) | 200 (26.7) | 0.176 |
| Hypercholesterolaemia | 643 (43.5) | 358 (49.1) | 285 (38.0) | 0.006 |
| Current smoking habit | 143 (9.7) | 51 (7.0) | 92 (12.3) | 0.479 |
| History of previous bleeding | 101 (6.8) | 59 (8.1) | 42 (5.6) | 0.929 |
| Concomitant malignant disease | 147 (9.9) | 74 (10.2) | 73 (9.7) | 0.862 |
| Hyperthyroidism | 54 (3.7) | 14 (1.9) | 40 (5.3) | 0.824 |
| Hypothyroidism | 148 (10.0) | 80 (11.0) | 68 (9.1) | 0.914 |
| COPD/OSA | 135 (9.1) | 72 (9.9) | 63 (8.4) | 0.998 |
| Valvular disease | 432 (29.2) | 234 (32.1) | 198 (26.4) | 0.237 |
| Concomitant treatment, n (%) | ||||
| Oral anticoagulation | 1321 (89.3) | 634 (87.0) | 687 (91.6) | 0.346 |
| Any VKA | 673 (45.5) | 389 (53.4) | 284 (37.9) | <0.001 |
| Any DOAC | 648 (43.8) | 245 (33.6) | 403 (53.7) | <0.001 |
| Anti-arrhythmics | 522 (35.3) | 118 (16.2) | 404 (53.9) | <0.001 |
| Digoxin | 111 (7.5) | 72 (9.9) | 39 (5.2) | 0.619 |
| Calcium antagonist | 171 (11.6) | 76 (10.4) | 95 (12.7) | 0.821 |
| Beta-blockers | 905 (61.2) | 427 (58.6) | 478 (63.7) | 0.133 |
| Statins | 609 (41.2) | 330 (45.3) | 279 (37.2) | 0.053 |
| Diuretics | 717 (48.5) | 384 (52.7) | 333 (44.4) | 0.032 |
| Angiotensin-converting enzyme inhibitors | 484 (32.7) | 219 (30.0) | 265 (35.4) | 0.246 |
| Angiotensin-renin blockers | 316 (21.4) | 212 (29.1) | 104 (13.9) | 0.005 |
| Oral anti-diabetics | 273 (18.5) | 175 (24.0) | 98 (13.1) | 0.046 |
| CHA2DS2-VASc score, median (IQR) | 3 (2–4) | 3 (2–5) | 3 (1–4) | <0.001 |
| HAS-BLED score, median (IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) | <0.001 |
| . | Overall N = 1479 . | Spanish cohort N = 729 . | French cohort N = 750 . | P-value . |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 933 (63.1) | 416 (57.1) | 517 (68.9) | <0.001 |
| Age (years), median (IQR) | 72 (64–80) | 75 (67–81) | 69 (61–78) | <0.001 |
| AF-related conditions | ||||
| Type of AF | ||||
| First-onset/paroxysmal | 596 (40.3) | 293 (40.2) | 303 (40.4) | 0.874 |
| Persistent | 310 (20.9) | 86 (11.8) | 224 (29.9) | 0.002 |
| Long-standing persistent | 54 (3.6) | 11 (1.5) | 43 (5.7) | 0.835 |
| Permanent | 500 (33.8) | 338 (46.4) | 162 (21.6) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 790 (53.4) | 444 (60.9) | 346 (46.1) | <0.001 |
| Diabetes mellitus | 357 (24.1) | 219 (30.0) | 138 (18.4) | 0.020 |
| Heart failure | 527 (35.6) | 270 (37.0) | 257 (34.3) | 0.578 |
| History of stroke/TIA/thromboembolism | 200 (13.5) | 101 (13.9) | 99 (13.2) | 0.951 |
| Peripheral vascular disease | 104 (7.0) | 44 (6.0) | 60 (8.0) | 0.995 |
| Renal impairment | 155 (10.5) | 88 (12.1) | 67 (8.9) | 0.707 |
| Coronary artery disease | 344 (23.3) | 144 (19.8) | 200 (26.7) | 0.176 |
| Hypercholesterolaemia | 643 (43.5) | 358 (49.1) | 285 (38.0) | 0.006 |
| Current smoking habit | 143 (9.7) | 51 (7.0) | 92 (12.3) | 0.479 |
| History of previous bleeding | 101 (6.8) | 59 (8.1) | 42 (5.6) | 0.929 |
| Concomitant malignant disease | 147 (9.9) | 74 (10.2) | 73 (9.7) | 0.862 |
| Hyperthyroidism | 54 (3.7) | 14 (1.9) | 40 (5.3) | 0.824 |
| Hypothyroidism | 148 (10.0) | 80 (11.0) | 68 (9.1) | 0.914 |
| COPD/OSA | 135 (9.1) | 72 (9.9) | 63 (8.4) | 0.998 |
| Valvular disease | 432 (29.2) | 234 (32.1) | 198 (26.4) | 0.237 |
| Concomitant treatment, n (%) | ||||
| Oral anticoagulation | 1321 (89.3) | 634 (87.0) | 687 (91.6) | 0.346 |
| Any VKA | 673 (45.5) | 389 (53.4) | 284 (37.9) | <0.001 |
| Any DOAC | 648 (43.8) | 245 (33.6) | 403 (53.7) | <0.001 |
| Anti-arrhythmics | 522 (35.3) | 118 (16.2) | 404 (53.9) | <0.001 |
| Digoxin | 111 (7.5) | 72 (9.9) | 39 (5.2) | 0.619 |
| Calcium antagonist | 171 (11.6) | 76 (10.4) | 95 (12.7) | 0.821 |
| Beta-blockers | 905 (61.2) | 427 (58.6) | 478 (63.7) | 0.133 |
| Statins | 609 (41.2) | 330 (45.3) | 279 (37.2) | 0.053 |
| Diuretics | 717 (48.5) | 384 (52.7) | 333 (44.4) | 0.032 |
| Angiotensin-converting enzyme inhibitors | 484 (32.7) | 219 (30.0) | 265 (35.4) | 0.246 |
| Angiotensin-renin blockers | 316 (21.4) | 212 (29.1) | 104 (13.9) | 0.005 |
| Oral anti-diabetics | 273 (18.5) | 175 (24.0) | 98 (13.1) | 0.046 |
| CHA2DS2-VASc score, median (IQR) | 3 (2–4) | 3 (2–5) | 3 (1–4) | <0.001 |
| HAS-BLED score, median (IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) | <0.001 |
COPD/OSA, chronic obstructive pulmonary disease/obstructive sleep apnoea; DOAC, direct-acting oral anticoagulant; IQR, interquartile range; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
According to the 4S-AF scheme, 379 (25.6%) patients in the merged cohort should be managed by a rhythm control strategy, in 394 (26.7%) a rhythm control strategy could be attempted, and in 706 (47.7%) a rate control should be considered. There were significant differences between the Spanish and French cohorts regarding the management proposed by the 4S-AF scheme (P < 0.001) (Supplementary material online, Table S1).
Application of the 4S-AF scheme would suggest rhythm control in 621/1258 (49.4%) patients (rhythm control should be chosen or could be attempted), rate control in 637/1258 (50.6%) patients; and the actual figures of patients managed by rhythm control and rate control were similar: 619/1258 (49.2%) and 639/1258 (50.8%), respectively (Supplementary material online, Table S2). Despite most patients were on the management strategy suggested by the 4S-AF scheme, 75.5% (481/637) of patients suggested to be managed by rate control were actually on rate control, and 74.6% (463/621) of patients suggested to be managed by rhythm control were on rhythm control (P < 0.001). Thus, ∼25% of patients were on a management strategy different from suggested by the 4S-AF scheme (Supplementary material online, Figure S1).
The 4S-AF scheme as a continuous variable was associated with the probability of determining the real strategy decided for the patient (OR 1.55, 95% CI 1.47–1.65; P < 0.001) (Hosmer–Lemeshow P-value = 0.139). This was also confirmed by the 4S-AF scheme as a dichotomic variable based on the score of each item (i.e. rhythm control should be chosen/attempted vs. rate control), with an OR of 9.04 (95% CI 7.00–11.66; P < 0.001).
The predictive performances of the 4S-AF scheme for the actual management strategy decided for the patient were good, with a c-index of 0.77 (95% CI 0.75–0.80, P < 0.001) in its continuous form, and a c-index of 0.75 (95% CI 0.72–0.78, P < 0.001) in its categorical form (Figure 2). DCAs graphically demonstrate that using the 4S-AF scheme for deciding on the primary management strategy may result into an important improvement of net benefit, and thus, of clinical usefulness, during a large threshold of probabilities (Figure 3).
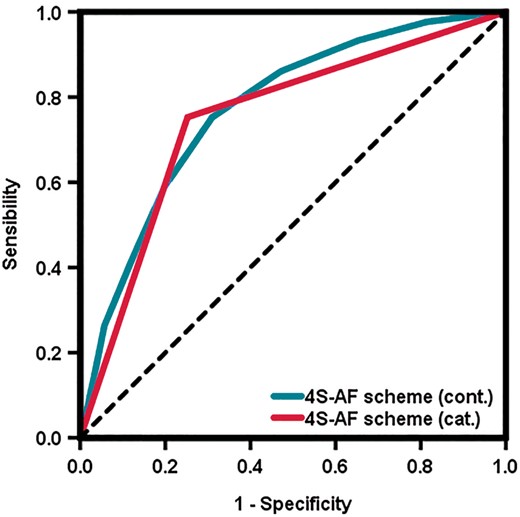
Receiver operating characteristic curves for the 4S-AF scheme for the prediction of rhythm or rate control management strategies in the merged cohort.
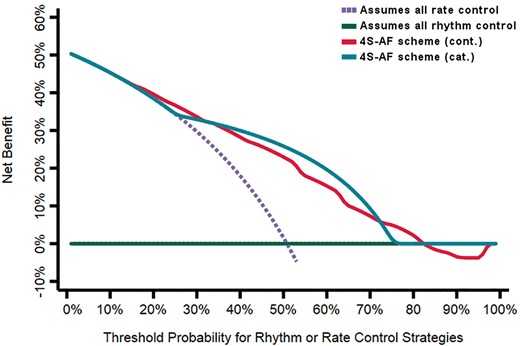
Decision curve analysis for the 4S-AF scheme for rhythm or rate control management strategies in the merged cohort.
The 4S-AF scheme as a predictor of adverse clinical outcomes
During the follow-up, there were 87 (5.88 per 100 patient-years) all-cause deaths and 136 (9.20 per 100 patient-years) composite outcome events. Other outcomes are shown in Supplementary material online, Table S3.
As expected, the incidence of all-cause mortality (7.20 per 100 patient-years vs. 4.40 per 100 patient-years, P = 0.038) and composite outcome events (11.50 per 100 patient-years vs. 6.90 per 100 patient-years, P = 0.005) was higher in patients on rate control. In those patients managed with concordance to the 4S-AF scheme (i.e. the scheme suggested rate control and the patient was on rate control or the scheme suggested rhythm control and the patient was on rhythm control), the incidences of all-cause mortality (5.69 per 100 patient-years vs. 6.17 per 100 patient-years, P = 0.764) and composite outcome events (9.02 per 100 patient-years vs. 9.74 per 100 patient-years, P = 0.719) were numerically lower, although the results were not statistically different.
Multivariable Cox proportional regression models adjusted by CHA2DS2-VASc and HAS-BLED scores were performed in the Spanish and French cohorts, as well as in the merged cohort, to investigate if the 4S-AF scheme may identify patients at higher risk of mortality and composite outcomes (i.e. IS/TIA/SE, major bleeding, or all-cause death). In the Spanish cohort, ‘red’ category patients showed a higher risk of all-cause death (aHR 1.57, 95% CI 0.61–4.04; P = 0.346) and composite outcomes (aHR 1.21, 95% CI 0.62–2.35; P = 0.585), but not significantly. Similar findings were observed in the French cohort (for all-cause death: aHR 2.03, 95% CI 0.92–4.48; P = 0.079 and for composite outcomes: aHR 1.61, 95% CI 0.84–3.09; P = 0.151). However, in the merged cohort, patients classified as ‘red’ category in the 4S-AF scheme demonstrated a significantly higher risk of all-cause death (aHR 1.75, 95% CI 1.02–2.99; P = 0.041) and composite outcomes (aHR 1.60, 95% CI 1.05–2.44; P = 0.028) (Table 2). Thus, patients for who the 4S-AF scheme suggests that may be managed by rhythm control (recommended or considered) had a significantly lower risk of these events.
Univariate and multivariate Cox proportional regression analyses in the overall (merged) cohort
| . | Univariate analysis HR (95% CI) . | P-value . | Multivariate analysis HR (95% CI) . | P-value . |
|---|---|---|---|---|
| All-cause death | ||||
| CHA2DS2-VASc | 1.39 (1.24–1.55) | <0.001 | 1.26 (1.09–1.45) | 0.002 |
| HAS-BLED | 1.40 (1.17–1.67) | <0.001 | 1.09 (0.88–1.35) | 0.433 |
| 4S-AF scheme (red category) | 2.91 (1.83–4.63) | <0.001 | 1.75 (1.02–2.99) | 0.041 |
| Composite outcomesa | ||||
| CHA2DS2-VASc | 1.33 (1.21–1.45) | <0.001 | 1.17 (1.05–1.32) | 0.006 |
| HAS-BLED | 1.49 (1.30–1.72) | <0.001 | 1.24 (1.05–1.47) | 0.010 |
| 4S-AF scheme (red category) | 2.58 (1.80–3.71) | <0.001 | 1.60 (1.05–2.44) | 0.028 |
| . | Univariate analysis HR (95% CI) . | P-value . | Multivariate analysis HR (95% CI) . | P-value . |
|---|---|---|---|---|
| All-cause death | ||||
| CHA2DS2-VASc | 1.39 (1.24–1.55) | <0.001 | 1.26 (1.09–1.45) | 0.002 |
| HAS-BLED | 1.40 (1.17–1.67) | <0.001 | 1.09 (0.88–1.35) | 0.433 |
| 4S-AF scheme (red category) | 2.91 (1.83–4.63) | <0.001 | 1.75 (1.02–2.99) | 0.041 |
| Composite outcomesa | ||||
| CHA2DS2-VASc | 1.33 (1.21–1.45) | <0.001 | 1.17 (1.05–1.32) | 0.006 |
| HAS-BLED | 1.49 (1.30–1.72) | <0.001 | 1.24 (1.05–1.47) | 0.010 |
| 4S-AF scheme (red category) | 2.58 (1.80–3.71) | <0.001 | 1.60 (1.05–2.44) | 0.028 |
Any of the following: IS/TIA/SE, major bleeding, and all-cause death.
CI, confidence interval; HR, hazard ratio.
Univariate and multivariate Cox proportional regression analyses in the overall (merged) cohort
| . | Univariate analysis HR (95% CI) . | P-value . | Multivariate analysis HR (95% CI) . | P-value . |
|---|---|---|---|---|
| All-cause death | ||||
| CHA2DS2-VASc | 1.39 (1.24–1.55) | <0.001 | 1.26 (1.09–1.45) | 0.002 |
| HAS-BLED | 1.40 (1.17–1.67) | <0.001 | 1.09 (0.88–1.35) | 0.433 |
| 4S-AF scheme (red category) | 2.91 (1.83–4.63) | <0.001 | 1.75 (1.02–2.99) | 0.041 |
| Composite outcomesa | ||||
| CHA2DS2-VASc | 1.33 (1.21–1.45) | <0.001 | 1.17 (1.05–1.32) | 0.006 |
| HAS-BLED | 1.49 (1.30–1.72) | <0.001 | 1.24 (1.05–1.47) | 0.010 |
| 4S-AF scheme (red category) | 2.58 (1.80–3.71) | <0.001 | 1.60 (1.05–2.44) | 0.028 |
| . | Univariate analysis HR (95% CI) . | P-value . | Multivariate analysis HR (95% CI) . | P-value . |
|---|---|---|---|---|
| All-cause death | ||||
| CHA2DS2-VASc | 1.39 (1.24–1.55) | <0.001 | 1.26 (1.09–1.45) | 0.002 |
| HAS-BLED | 1.40 (1.17–1.67) | <0.001 | 1.09 (0.88–1.35) | 0.433 |
| 4S-AF scheme (red category) | 2.91 (1.83–4.63) | <0.001 | 1.75 (1.02–2.99) | 0.041 |
| Composite outcomesa | ||||
| CHA2DS2-VASc | 1.33 (1.21–1.45) | <0.001 | 1.17 (1.05–1.32) | 0.006 |
| HAS-BLED | 1.49 (1.30–1.72) | <0.001 | 1.24 (1.05–1.47) | 0.010 |
| 4S-AF scheme (red category) | 2.58 (1.80–3.71) | <0.001 | 1.60 (1.05–2.44) | 0.028 |
Any of the following: IS/TIA/SE, major bleeding, and all-cause death.
CI, confidence interval; HR, hazard ratio.
Kaplan–Meier curves confirmed that in the merged cohort, ‘red category patients’ according to the 4S-AF scheme had lower event-free survival (log-rank P-value for both events <0.001) (Figure 4). These results were also confirmed in the Spanish cohort (log-rank P-value: for all-cause death = 0.021; for composite outcomes = 0.026) and in the French cohort (log-rank P-value for both events <0.001).
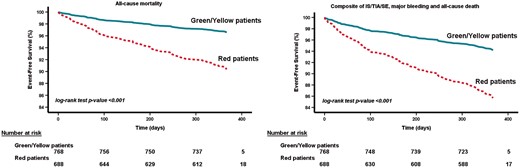
Survival curves for all-cause mortality and composite outcomes according to the 4S-AF categories in the merged cohort. IS, ischaemic stroke; SE, systemic embolism; TIA, transient ischaemic attack.
Discussion
In this first study testing the 4S-AF scheme, we demonstrate how this approach could be useful in everyday clinical practice. Hence, the novelty of our results is two-fold: (i) the 4S-AF scheme may help in identifying patients that would be managed by rhythm or rate control and (ii) the 4S-AF scheme could also help in identifying high-risk patients who have worse clinical outcomes.
There is consensus about the complexity of the management of AF, and how it should be more integrated and holistic.6,7 Indeed, the recent 2020 ESC AF guidelines support this approach.3 Despite all the conditions and variables that need to be considered, there are two central pillars in the AF management, i.e., the need of OAC for stroke prevention and the appropriate choice of rate and/or rhythm control to improve symptoms and prevent AF complications. The 4S-AF is a simple and practical scheme which may provide the information needed to characterize, evaluate, and guide treatment decision-making. This novel approach supposes a paradigm shift, from the basic classification to a more structured characterization of AF including the assessment of stroke risk, symptoms severity, AF burden, and substrate severity.4 An important advantage of the 4S-AF scheme is that currently widely used and well-known assessment tools/classifications could be applied for the assessment and characterization of specific domains (e.g. CHA2DS2-VASc score, EHRA symptom score, comorbidities and cardiovascular risk factors review, etc.).
The 4S-AF scheme includes a first and necessary simple step: assessing the risk of stroke using the CHA2DS2-VASc score, whereby the default should be to offer OAC unless they are low-risk patients.3,8 This is important as guideline-adherent OAC therapy significantly reduces the risk of stroke and improves survival.9,10 The choice of a rate or rhythm control strategy depends on the assessment of the other three 4S-AF domains (Symptom severity, Severity of AF burden, and Substrate severity), and there are several factors influencing this decision. Importantly, a substantial proportion of AF patients attend the emergency department every year, not always in relation to ischaemic or bleeding events.11 Hence, the proper characterization of AF may help in deciding the correct management, leading to a reduction of AF symptoms and recurrence. For example, the EAST-AFNET 4 trial demonstrated that early rhythm-control therapy was associated with a lower risk of adverse cardiovascular outcomes compared to usual care among patients with early AF and cardiovascular conditions.12 Finally, the 4S-AF scheme also allows the review, identification, and management of the most important risk factors, which complements stroke prevention and reduces AF burden and symptom severity.13,14
Herein, we clearly show that the 4S-AF scheme may assist in the complicated decision of rhythm or rate control. Indeed, our DCA supports the use of the 4S-AF scheme for this task, showing that the net benefit would (in theory) be high during a threshold of probabilities comprised between 50–80%, approximately. To date, there are no other tools to compare against the 4S-AF scheme, but the present results from the DCA gave the idea that the application of this novel scheme in everyday clinical practice would facilitate decision-making, and may help to select the most appropriate management strategy for the patient. Nevertheless, it is important to note that a higher or lower score in the 4S-AF scheme does not necessarily suggest one management strategy over the other. Taking as a practical example two patients, the first one is a 76-year-old female patient with long-standing persistent AF, moderate symptoms (EHRA = 3), and hypertension. Her AF characterization based on the 4S-AF scheme is St = 0, Sy = 2, Sb = 2, Su = 2, with an overall score of 6. The second patient is a 78-year-old male patient with persistent AF and no symptoms (EHRA = 1). As comorbid conditions, he also has diabetes, and heart failure, with demonstrated mild LA dysfunction and fibrosis in the transthoracic echocardiogram. His 4S-AF characterization is St = 0, Sy = 0, Sb = 1, Su = 5, which again denotes an overall 4S-AF score of 6. Therefore, both patients have the same score, but the first one is a ‘yellow’ patient in who rhythm control should be attempted whereas the second one is a ‘red’ patient in who rate control would be probably the best option. For this reason, the overall score in the 4S-AF scheme should not be considered alone but always be accompanied by the critical interpretation of that score and by information on the patient age, comorbidities, and personal preferences.
In the original 4S-AF scheme publication, the authors stated that this scheme could also provide prognostic information, but the clinical usefulness and prognostic value require further validations.4 We have now addressed this and found differences between ‘green/yellow category’ patients and ‘red category’ patients in terms of prognosis, i.e. ‘red category’ patients for who the 4S-AF scheme recommends considering rate control had a significantly higher risk of all-cause mortality and composite outcomes. This reflects the fact the patients on rate control are usually those more complex to manage.15 These novel findings are of great interest since beyond helping with the best strategy for the control of AF symptoms, the characterization of AF with the 4S-AF would identify those patients who require more careful review and follow-up; particularly because to date neither rhythm nor rate control strategies have demonstrated to be superior over the other.
For example, in the ORBIT-AF Registry, rhythm control was not superior to rate control strategy in reducing stroke, heart failure, or mortality, but was associated with more cardiovascular hospitalizations.16 Investigators from the AF-CHF Trial demonstrated that in AF patients with congestive heart failure, a routine strategy of rhythm control did not reduce the rate of cardiovascular death, as compared with a rate-control strategy.17 This is in accordance with the CASTLE-AF trial which showed that in AF patients with heart failure, a rhythm control strategy with antiarrhythmic drugs did not significantly reduce the primary composite endpoint of all-cause mortality and heart failure hospitalization when compared with a rate control strategy.18 In elderly patients, the ‘Get With The Guidelines-Heart Failure’ Registry found that there was higher all-cause mortality at 1-year in AF patients with heart failure and preserved ejection fraction under rate control compared with the patients under rhythm control.19 However, this is still controversial since in the REPOSI Study there were no differences between rate and rhythm control strategies for cardiovascular and all-cause death among elderly AF patients.20
Limitations
There are some limitations in relation to this manuscript. The main limitation of the study is related to its observational nature and the short follow-up. Second, adverse events were not centrally adjudicated, although this limitation is shared by almost all real-life observational registries. Another limitation is related to the study setting since most AF patients in the EORP-AF Long-Term General Registry were included in cardiology clinics. Since AF patients are also commonly managed by different health professionals (i.e. general practitioners, internal medicine specialists, geriatricians), our data need to be cautiously interpreted when extended to the entire AF population. However, the Spanish cohort was composed also by AF patients derived from internal medicine physicians, primary care physicians, clinical cardiologists, and electrophysiologists, to give a more global vision of AF management in Spain.
Also, as this is the first test of the 4S-AF scheme in a real-world setting, there are no previous data which could be used as an example. We conceived this study as a retrospective validation of the 4S-AF scheme using only simple and practical currently used assessment tools (CHA2DS2-VASc score, EHRA symptom score, temporal pattern of AF, and clinical assessment of comorbidities/cardiovascular risk factors). Thus, we introduced a modification of the ‘Severity of AF burden’ domain, taking into account only the AF type and scoring permanent AF as 3 points instead of the 2 points, in order to be able to reach the maximum of 3 points in such domain (in a range from 0 for paroxysmal/first onset AF to 3 for permanent AF). Despite this slight modification of the original 4S-AF scheme, the authors already declared in the original publication that they did not propose this system as a definitive treatment-decision tool and modifications would be necessary. Regarding the ‘Substrate’ domain, we did not have data on transoesophageal echocardiography, cardiac computed tomography, or nuclear magnetic resonance imaging, which may provide additional indices of atrial dysfunction and structural alterations including fibrosis. For this reason, LA fibrosis was not completely evaluated in our study and included in the ‘Substrate’ domain.
Finally, it should be noted that prospective validation of the 4S-AF scheme through a randomized clinical trial is required before final conclusions about the usefulness of this approach can be drawn.
Conclusions
In this ‘real-world’ study including Spanish and French AF patients from the EORP-AF Long-Term General Registry, we demonstrated for the first time that the 4S-AF scheme may be useful in everyday clinical practice. Characterization of AF by using the 4S-AF approach may help in identifying patients that would be managed by rhythm or rate control and could also help in identifying high-risk patients for worse clinical outcomes.
Supplementary material
Supplementary material is available at Europace online.
Funding
Since the start of EORP, the following companies have supported the programme: Abbott Vascular Int. (2011–14), Amgen Cardiovascular (2009–18), AstraZeneca (2014–17), Bayer AG (2009–18), Boehringer Ingelheim (2009–19), Boston Scientific (2009–12), The Bristol Myers Squibb and Pfizer Alliance (2011–16), The Alliance Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2011–17), Edwards (2016–19), Gedeon Richter Plc. (2014–17), Menarini Int. Op. (2009–12), MSD-Merck & Co. (2011–14), Novartis Pharma AG (2014–17), ResMed (2014–16), Sanofi (2009–11), SERVIER (2009–18). The Atrial Fibrillation NETwork (AFNET), conducting the registry in Germany, received support from The Bristol Myers Squibb/Pfizer Alliance (2014–18) and the German Centre for Cardiovascular Research (DZHK). Funding from Daiichi-Sankyo and Boehringer-Ingelheim has been received for conducting the registry in Spain. Funding from BMS-Pfizer Alliance was received to support the programme in France.
Conflict of interest: J.M.R-C has received a grant from Sociedad Española de Trombosis y Hemostasia (grant for short international training stays 2020) and the First Contact Initiative Grant 2020 from the European Society of Cardiology Council on Basic Cardiovascular Science. O.P. has served as consultant for Bayer and BMS-Pfizer Alliance, outside the submitted work. J.G.S has received a grant from Daiichi-Sankyo, outside the submitted work. G.Y.H.L. has served as consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. F.M. reports personal fees from Boehringer Ingelheim, Bayer and Pfizer-BMS, outside the submitted work.
Data availability
The data underlying this article was provided by the European Society of Cardiology under permission. Data can be shared on request to the Executive Committee and Steering Committee of the EORP-AF Long-Term General Registry.
References
Author notes
Gregory Y.H. Lip and Francisco Marín authors are joint senior authors.



