-
PDF
- Split View
-
Views
-
Cite
Cite
Ahmed M Al-Kaisey, Ramanathan Parameswaran, Stephen A Joseph, Peter M Kistler, Joseph B Morton, Jonathan M Kalman, Extensive right atrial free wall low-voltage zone as the substrate for atrial fibrillation: successful ablation by scar homogenization, EP Europace, Volume 23, Issue 1, January 2021, Pages 59–64, https://doi.org/10.1093/europace/euaa233
Close - Share Icon Share
Abstract
Prior studies have described a variety of mechanisms for atrial fibrillation (AF) originating in the right atrium (RA). In this study, we report a series of patients in whom an extensive right atrial free wall low-voltage zone (LVZ) served as the AF substrate.
Five patients with a clinical syndrome of paroxysmal AF and atrial tachycardia (AT) underwent electrophysiologic evaluation. Five patients (3 M; age 52 ± 7 years) had symptomatic paroxysmal AF for (28 ± 17 months) not responsive to medical therapy. At the initial EP study, AT was inducible in four patients and was spontaneous in one patient. In all patients, tachycardia instability precluded detailed AT mapping. Sinus or pace maps indicated an extensive LVZ in the lateral RA trabeculated free wall which consisted of regions of low amplitude complex signals interspersed between electrically silent areas. Radiofrequency ablation aimed at rendering the LVZ electrical inert was successful in eliminating AF in four of five patients. At a follow-up of 28 ± 15 months, one patient had an isolated recurrence of AF. However, two patients required repeat ablation for recurrent AT.
An extensive LVZ in the trabeculated RA free wall constitutes an unusual substrate for AF. These patients also demonstrate unstable ATs originating from the same zone. Radiofrequency ablation to render the low-voltage zone electrically inert is an effective strategy to manage AF and AT.
Spontaneous extensive low-voltage zones (LVZs) in the right atrium (RA) are an unusual substrate for atrial fibrillation (AF).
We present a cohort of five patients with clinical AF and atrial tachycardia without prior cardiac surgery secondary to LVZs within the RA.
Subsequent radiofrequency ablation aimed at rendering these zones electrically inert was successful in eliminating or markedly reducing AF in this cohort.
The cause of such substrate remains unknown.
Introduction
In many patients with atrial fibrillation (AF), the arrhythmia is maintained by an advanced atrial substrate beyond the pulmonary veins.1 A range of conditions associated with AF such as heart failure, hypertension, obesity, and obstructive sleep apnoea in addition to persistent AF itself can drive the development of this abnormal atrial substrate.2 This substrate is characterized by zones of low-voltage amplitude (LVZ), complex signals, and slowed conduction and may be identified by high-density electro-anatomic mapping or by cardiac Magnetic resonance imaging (MRI).3,4
In recent years, there has been increasing interest in targeting these areas with catheter ablation in order to improve clinical outcomes for patients with persistent AF. Such approaches have involved either scar homogenization or alternatively box isolation of fibrotic areas.5,6 Although early results have been promising, definitive evidence for this approach remains lacking. Notably, these regions have been identified in the left atrium (LA). Prior reports described the presence of spontaneous dense scar (no prior surgery or ablation) in the right atrial free wall as a mechanism underlying the development of atypical right atrial free wall flutter.7–9
In our report, we describe a series of patients in whom clinical symptomatic AF was related to extensive patchy scar in the right atrial free wall and in whom ablation resulted in the elimination of AF.
Methods
Patient population
Five patients referred for ablation of highly symptomatic, medically refractory paroxysmal AF underwent mapping and ablation. In four of the five patients, there was also electrocardiogram (ECG) or rhythm monitoring data consistent with atrial tachycardia (AT). Table 1 presents the patients’ demographics, clinical characteristics, and prior ablation procedures. There were three males, and the mean patient age was 52 ± 7 years. None of the patients had a prior history of cardiac surgery or structural or congenital heart disease. All patients had paroxysmal AF with 12-lead ECG documentation of sustained AF. The mean number of anti-arrhythmic agents failed was 2 ± 0.4. Four patients had already undergone a total of nine prior ablation procedures without sustained improvement in symptoms [four pulmonary vein isolation (PVI) procedures in two patients, three cavotricuspid isthmus ablation, two linear ablations from inferior scar to inferior vena cava (IVC)].
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | |
|---|---|---|---|---|---|---|
| Age (years) | 48 | 58 | 49 | 63 | 43 | |
| Sex (M/F) | M | M | M | F | F | |
| Clinical presentation | Paroxysmal AF (Y/N) | Y | Y | Y | Y | Y |
| Co-existing AT or flutter (Y/N) | Y | Y | Y | Y | N | |
| Duration of symptoms (months) | 12 | 18 | 42 | 54 | 12 | |
| Failed anti-arrhythmic drugs (n) | 2 | 2 | 2 | 2 | 3 | |
| Comorbidities | Hypertension (Y/N) | Y | Y | N | Y | N |
| Other | N | OSA | N | N | N | |
| Transthoracic echocardiography | LV systolic function | Normal | Normal | Normal | Normal | Normal |
| LA size | Normal | Mild | Moderate | Mild | Mild | |
| RA size | Normal | Mild | Moderate | Normal | Normal | |
| Prior ablation | PVI (Y/N) | N | N | Y (×2) | N | Y (×2) |
| CTI (Y/N) | N | N | Y | Y | Y | |
| Linear ablation scar to IVC (Y/N) | Y | N | N | Y | N | |
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | |
|---|---|---|---|---|---|---|
| Age (years) | 48 | 58 | 49 | 63 | 43 | |
| Sex (M/F) | M | M | M | F | F | |
| Clinical presentation | Paroxysmal AF (Y/N) | Y | Y | Y | Y | Y |
| Co-existing AT or flutter (Y/N) | Y | Y | Y | Y | N | |
| Duration of symptoms (months) | 12 | 18 | 42 | 54 | 12 | |
| Failed anti-arrhythmic drugs (n) | 2 | 2 | 2 | 2 | 3 | |
| Comorbidities | Hypertension (Y/N) | Y | Y | N | Y | N |
| Other | N | OSA | N | N | N | |
| Transthoracic echocardiography | LV systolic function | Normal | Normal | Normal | Normal | Normal |
| LA size | Normal | Mild | Moderate | Mild | Mild | |
| RA size | Normal | Mild | Moderate | Normal | Normal | |
| Prior ablation | PVI (Y/N) | N | N | Y (×2) | N | Y (×2) |
| CTI (Y/N) | N | N | Y | Y | Y | |
| Linear ablation scar to IVC (Y/N) | Y | N | N | Y | N | |
AF, atrial fibrillation; AT, atrial tachycardia; CTI, cavotricuspid isthmus ablation; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; OSA, obstructive sleep apnoea; PVI, pulmonary vein isolation; RA, right atrium.
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | |
|---|---|---|---|---|---|---|
| Age (years) | 48 | 58 | 49 | 63 | 43 | |
| Sex (M/F) | M | M | M | F | F | |
| Clinical presentation | Paroxysmal AF (Y/N) | Y | Y | Y | Y | Y |
| Co-existing AT or flutter (Y/N) | Y | Y | Y | Y | N | |
| Duration of symptoms (months) | 12 | 18 | 42 | 54 | 12 | |
| Failed anti-arrhythmic drugs (n) | 2 | 2 | 2 | 2 | 3 | |
| Comorbidities | Hypertension (Y/N) | Y | Y | N | Y | N |
| Other | N | OSA | N | N | N | |
| Transthoracic echocardiography | LV systolic function | Normal | Normal | Normal | Normal | Normal |
| LA size | Normal | Mild | Moderate | Mild | Mild | |
| RA size | Normal | Mild | Moderate | Normal | Normal | |
| Prior ablation | PVI (Y/N) | N | N | Y (×2) | N | Y (×2) |
| CTI (Y/N) | N | N | Y | Y | Y | |
| Linear ablation scar to IVC (Y/N) | Y | N | N | Y | N | |
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | |
|---|---|---|---|---|---|---|
| Age (years) | 48 | 58 | 49 | 63 | 43 | |
| Sex (M/F) | M | M | M | F | F | |
| Clinical presentation | Paroxysmal AF (Y/N) | Y | Y | Y | Y | Y |
| Co-existing AT or flutter (Y/N) | Y | Y | Y | Y | N | |
| Duration of symptoms (months) | 12 | 18 | 42 | 54 | 12 | |
| Failed anti-arrhythmic drugs (n) | 2 | 2 | 2 | 2 | 3 | |
| Comorbidities | Hypertension (Y/N) | Y | Y | N | Y | N |
| Other | N | OSA | N | N | N | |
| Transthoracic echocardiography | LV systolic function | Normal | Normal | Normal | Normal | Normal |
| LA size | Normal | Mild | Moderate | Mild | Mild | |
| RA size | Normal | Mild | Moderate | Normal | Normal | |
| Prior ablation | PVI (Y/N) | N | N | Y (×2) | N | Y (×2) |
| CTI (Y/N) | N | N | Y | Y | Y | |
| Linear ablation scar to IVC (Y/N) | Y | N | N | Y | N | |
AF, atrial fibrillation; AT, atrial tachycardia; CTI, cavotricuspid isthmus ablation; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; OSA, obstructive sleep apnoea; PVI, pulmonary vein isolation; RA, right atrium.
Electrophysiological and mapping procedure
All patients provided written informed consent. The procedure was performed under general anaesthesia and all anti-arrhythmic medications were discontinued for at least five half-lives.
The EPMed (EPMed Systems, West Berlin, NJ, USA) digital electrophysiology system was used for recording of intracardiac electrograms and the digital 12-lead ECG. Intracardiac electrograms were filtered between 30 and 500 Hz and the surface ECG was filtered between 0.05 and 40 Hz. Multipolar catheters were positioned via the right femoral vein: (i) a quadripolar electrode catheter was inserted to the His position; (ii) a decapolar electrode catheter into the coronary sinus with the proximal pole at the CS ostium; (iii) a high-density mapping catheter was used to create right atrial maps (pentaray in three patients and Orion in two patients); (iv) a 4 mm ablation catheter was deployed via a long sheath. A baseline electrophysiology study was performed using standard right ventricular and atrial programmed stimulation and incremental atrial pacing with isoproterenol infusion. Right atrial substrate maps were created in sinus rhythm or pacing to define the abnormal atrial substrate. In three patients, the CARTO mapping system (Biosense Webster, Diamond Barr, CA, USA) was used and in two patients the Rhythmia mapping system (Boston Scientific, Marlborough, MA, USA) was used. Strict criteria were employed to ensure good tissue contact with the mapping catheter with point collection performed by experienced operators after careful assessment of geometry and fluoroscopic motion. During mapping, an even point cloud was created of the right atrium (RA) but with careful attention given to the region of abnormal signals. Electrically silent areas were defined by the absence of recordable activity or a bipolar voltage amplitude < 0.05 mV. Low-voltage zones were defined by the presence of a bipolar voltage < 0.5 mV and were confirmed by the presence of markedly abnormal low amplitude, multi-component electrograms consistently recorded over 10 consecutive beats. The anatomic location of complex multi-component signals was annotated within the LVZ. The region around the low-voltage and scarred areas was mapped carefully to delineate the border between the two areas.
Ablation
Prior to performing ablation, high output pacing (20 mA) was performed using the ablation catheter in the postero-lateral RA to delineate the course of the phrenic nerve which was annotated in all patients. No ablation was performed over the phrenic nerve which coursed from superior vena cava to IVC immediately posterior to the scar zone in all five patients.
After delineation of the LVZ, radiofrequency ablation (RFA) was performed to all tagged fractionated potentials in order to render the scar electrically inert. Ablation was performed at a power of 25–30 W using an irrigated-tip ablation catheter (17 mL/min). Radiofrequency delivery was continued until the local signal was eliminated. Confirmation that the scar was electrically inert was obtained by demonstrating the absence of capture (output of 20 mA at a pulse width of 2 ms) with pacing throughout the LVZ.
Outcome
Acute procedural success was defined by the absence of fractionated signals in the mapped LVZ area, absence of pace capture throughout the LVZ, and non-inducibility of sustained AT or AF (using a standardized induction protocol with programmed atrial stimulation, burst atrial pacing, and addition of isoproterenol). Long-term follow-up included a 3-month blanking period and consisted of a 6-month assessment of clinical symptoms, ECG, and Holter monitoring.
Results
Table 2 presents the procedural characteristics and clinical follow-up details. All patients had paroxysmal AF and presented at the time of ablation in sinus rhythm (SR). At baseline electrophysiology study, programmed stimulation induced a right AT in four patients and in one patient this was induced during catheter manipulation. However, limited attempts to map the tachycardia were prevented by the instability of the arrhythmia in all patients. The arrhythmia either degenerated and terminated, changed from one unstable AT to another, or rapidly degenerated into AF. Therefore, a detailed voltage map was created during SR or stable atrial pacing. In all patients, there was a large area LVZ confined within the trabeculated lateral RA extending anteriorly to near the tricuspid annulus and posteriorly to the region of the crista terminalis (Figure 1). The crista terminalis showed relatively large amplitude double component signals as previously described and did not show the very low amplitude fractionated potentials that were observed in the RA free wall. The inferior extent of the LVZ approached the IVC-RA junction and the superior extent reached the superior aspect of the atrium extending variably towards the right atrial appendage tip but did not significantly involve the superior septum. In all patients, the LVZ consisted of regions of dense scar with intervening regions of low amplitude complex signals (Figure 1). Right atrium maps had a mean of 6413 (range 2122–13 701) points. Two patients had undergone left atrial mapping which did not demonstrate a similar substrate and left atrial voltages appeared normal.
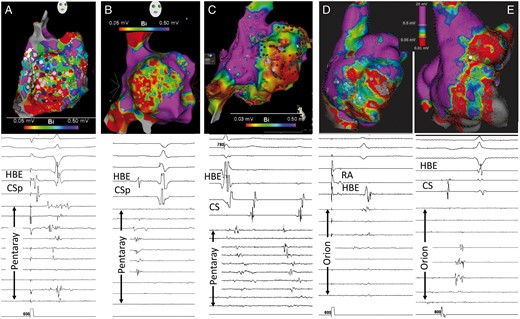
Electroanatomic mapping of the right atrium. The upper panels present the right lateral voltage map for each of the five patients. Voltage range is from 0.03/0.05 mV to 0.5 mV. Note that all five maps demonstrate an extensive LVZ occupying virtually the entire RA free wall from the crista terminalis posteriorly to the tricuspid annulus anteriorly. Superiorly the LVZ reaches the superior RA and inferiorly merges with the inferior vena cava. The purple and pink dots in the left most panel indicate sites of complex electrograms and the red dots in the middle panel indicate ablation points. The lower panels show representative electrogram examples from within the LVZ using either the pentaray (A–C panels) or the orion (D and E panels) mapping catheters. Recordings are made during sinus rhythm or atrial pacing with the exception of the middle panel (C), which demonstrates electrograms during an unstable atrial arrhythmia. Note the low amplitude, multi-component complex electrograms. There are also some channels with no recordable near field electrograms despite adequate tissue contact indicating regions of scarring. LVZ, low-voltage zone; HBE, His bundle electrogram; CS, coronary sinus; RA, right atrium.
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Presenting rhythm | SR | SR | SR | SR | SR |
| Baseline P wave duration (ms) | 77 | 87 | 101 | 83 | 100 |
| Baseline PR interval (ms) | 150 | 143 | 130 | 140 | 170 |
| Baseline HV interval (ms) | 35 | 55 | 45 | 50 | 43 |
| Inducible AT at baseline (Y/N) | Y | N | Y | Y | Y |
| Stable/mappable AT (Y/N) | N | N | N | N | N |
| Multiple unstable ATs (Y/N) | Y | Y | Y | Y | Y |
| AF triggered by AT (Y/N) | Y | N | Y | Y | Y |
| 3D mapping system | CARTO | CARTO | Rhythmia | CARTO | Rhythmia |
| No. of points/map (n) | 2173 | 3029 | 13 701 | 4000 | 9301 |
| RF duration (min) | 23 | 47 | 34 | 29 | 42 |
| Fluoroscopy time (min) | 9 | 14 | 9 | 9 | 20 |
| Procedure time (min) | 152 | 166 | 156 | 137 | 253 |
| Follow-up from the first scar homogenization procedure (months) | 52 | 23 | 37 | 6 | 22 |
| AF recurrence (Y/N) | N | N | N | Y | N |
| AT recurrence (Y/N) | N | Y | Y | N | Y |
| Repeat scar homogenization procedure (Y/N) | N | Y | N | N | Y (×2) |
| Arrhythmia free after last ablation procedure (Y/N) | Y | Y | N | N | Y |
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Presenting rhythm | SR | SR | SR | SR | SR |
| Baseline P wave duration (ms) | 77 | 87 | 101 | 83 | 100 |
| Baseline PR interval (ms) | 150 | 143 | 130 | 140 | 170 |
| Baseline HV interval (ms) | 35 | 55 | 45 | 50 | 43 |
| Inducible AT at baseline (Y/N) | Y | N | Y | Y | Y |
| Stable/mappable AT (Y/N) | N | N | N | N | N |
| Multiple unstable ATs (Y/N) | Y | Y | Y | Y | Y |
| AF triggered by AT (Y/N) | Y | N | Y | Y | Y |
| 3D mapping system | CARTO | CARTO | Rhythmia | CARTO | Rhythmia |
| No. of points/map (n) | 2173 | 3029 | 13 701 | 4000 | 9301 |
| RF duration (min) | 23 | 47 | 34 | 29 | 42 |
| Fluoroscopy time (min) | 9 | 14 | 9 | 9 | 20 |
| Procedure time (min) | 152 | 166 | 156 | 137 | 253 |
| Follow-up from the first scar homogenization procedure (months) | 52 | 23 | 37 | 6 | 22 |
| AF recurrence (Y/N) | N | N | N | Y | N |
| AT recurrence (Y/N) | N | Y | Y | N | Y |
| Repeat scar homogenization procedure (Y/N) | N | Y | N | N | Y (×2) |
| Arrhythmia free after last ablation procedure (Y/N) | Y | Y | N | N | Y |
AF, atrial fibrillation; AT, atrial tachycardia; RF, radiofrequency; SR, sinus rhythm.
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Presenting rhythm | SR | SR | SR | SR | SR |
| Baseline P wave duration (ms) | 77 | 87 | 101 | 83 | 100 |
| Baseline PR interval (ms) | 150 | 143 | 130 | 140 | 170 |
| Baseline HV interval (ms) | 35 | 55 | 45 | 50 | 43 |
| Inducible AT at baseline (Y/N) | Y | N | Y | Y | Y |
| Stable/mappable AT (Y/N) | N | N | N | N | N |
| Multiple unstable ATs (Y/N) | Y | Y | Y | Y | Y |
| AF triggered by AT (Y/N) | Y | N | Y | Y | Y |
| 3D mapping system | CARTO | CARTO | Rhythmia | CARTO | Rhythmia |
| No. of points/map (n) | 2173 | 3029 | 13 701 | 4000 | 9301 |
| RF duration (min) | 23 | 47 | 34 | 29 | 42 |
| Fluoroscopy time (min) | 9 | 14 | 9 | 9 | 20 |
| Procedure time (min) | 152 | 166 | 156 | 137 | 253 |
| Follow-up from the first scar homogenization procedure (months) | 52 | 23 | 37 | 6 | 22 |
| AF recurrence (Y/N) | N | N | N | Y | N |
| AT recurrence (Y/N) | N | Y | Y | N | Y |
| Repeat scar homogenization procedure (Y/N) | N | Y | N | N | Y (×2) |
| Arrhythmia free after last ablation procedure (Y/N) | Y | Y | N | N | Y |
| Patient no. . | 1 . | 2 . | 3 . | 4 . | 5 . |
|---|---|---|---|---|---|
| Presenting rhythm | SR | SR | SR | SR | SR |
| Baseline P wave duration (ms) | 77 | 87 | 101 | 83 | 100 |
| Baseline PR interval (ms) | 150 | 143 | 130 | 140 | 170 |
| Baseline HV interval (ms) | 35 | 55 | 45 | 50 | 43 |
| Inducible AT at baseline (Y/N) | Y | N | Y | Y | Y |
| Stable/mappable AT (Y/N) | N | N | N | N | N |
| Multiple unstable ATs (Y/N) | Y | Y | Y | Y | Y |
| AF triggered by AT (Y/N) | Y | N | Y | Y | Y |
| 3D mapping system | CARTO | CARTO | Rhythmia | CARTO | Rhythmia |
| No. of points/map (n) | 2173 | 3029 | 13 701 | 4000 | 9301 |
| RF duration (min) | 23 | 47 | 34 | 29 | 42 |
| Fluoroscopy time (min) | 9 | 14 | 9 | 9 | 20 |
| Procedure time (min) | 152 | 166 | 156 | 137 | 253 |
| Follow-up from the first scar homogenization procedure (months) | 52 | 23 | 37 | 6 | 22 |
| AF recurrence (Y/N) | N | N | N | Y | N |
| AT recurrence (Y/N) | N | Y | Y | N | Y |
| Repeat scar homogenization procedure (Y/N) | N | Y | N | N | Y (×2) |
| Arrhythmia free after last ablation procedure (Y/N) | Y | Y | N | N | Y |
AF, atrial fibrillation; AT, atrial tachycardia; RF, radiofrequency; SR, sinus rhythm.
Radiofrequency ablation to homogenize the scar required a mean RFA duration of 35 ± 8 min (Figure 2). The mean fluoroscopy time was 12 ± 4 min. Although these patients demonstrated an extensive region of right atrial free wall scar, no significant prolongation of the PR or HV intervals were observed in any patient. In addition, P wave duration was within normal limits and P wave morphology demonstrated only minor inferior lead notching in two patients. To date, no patient has demonstrated features of clinically significant sinus node dysfunction.

Ablation targeting low-voltage zone in the lateral wall of the right atrium. This slide indicates the right lateral voltage map of the right atrium for four patients indicating the region covered by ablation in order to render the LVZ completely electrically inert (no recordable electrograms and an inability to capture during high output pacing). LVZ, low-voltage zone.
During a mean follow-up of 28 ± 15 months following the initial scar homogenization procedure, only one patient (Patient 4) had an AF recurrence. This was an episode of AF outside the blanking period. Due to marked improvement in symptoms, this patient has declined a further ablation. Moreover, three patients had recurrent symptoms documented to be due to right AT:
One patient (Patient 2) had further ablation of residual RA LVZ channels and has been arrhythmia free over the ensuing 14 months.
One patient (Patient 3) has had two episodes of documented AT over a 3-year period and in view of marked clinical improvement and minimal symptoms have declined a further ablation.
One patient (Patient 5) required two further procedures both for documented AT recurrence. Both procedures targeted residual channels in an extensive RA LVZ. The patient is currently free of AT recurrence 3 months following the last procedure.
There have been no procedural complications.
Discussion
We describe a clinical syndrome of paroxysmal atrial arrhythmias including both sustained AF and unstable ATs which is due to an extensive region of abnormal substrate with multiple channels in the lateral trabeculated region of the RA. Homogenization of this abnormal substrate perhaps analogous to the approach to ischaemic ventricular tachycardia (VT) resulted in either elimination or marked reduction of AF and AT. In this series, the clue to detecting RA low-voltage areas came from the clinical scenario that included both unstable AT and AF. In addition, during the initial EP study looking for an underlying supraventricular tachycardia (SVT) mechanism, unstable AT was observed. This occasioned a search for an underlying mechanism. It is therefore in this clinical contact that we would recommend an evaluation of RA substrate looking for this unusual finding.
Mechanism of right atrial remodelling
Kornej et al.10 have demonstrated the relationship between traditional risk factors and the development of left atrial substrate. Both the DR-FLASH score (based on Diabetes mellitus, Renal dysfunction, persistent AF type, LA enlargement, Age >65 years, female Sex, and Hypertension) and the APPLE score (Age > 65 years, Persistent AF, imPaired eGFR, LA enlargement, impaired ejection fraction (EF)) were strongly and independently predictive of development of LVZs supporting findings from earlier mapping studies.2,11,12
The aetiology of the right atrial substrate in the current study however remains unclear. The only prevalent comorbidity was hypertension (three out of five patients) and only one patient had more than mild atrial enlargement. Only two of the patients in the current study were keen athletes although not at an elite level and neither demonstrated any other typical features of the athlete’s heart. In addition, it is unlikely that this substrate was indicative of a wider biatrial process as: (i) in two patients, the LA was mapped and a similar substrate was not apparent; (ii) in these two patients PVI did not improve the arrhythmia and; (iii) in all five patients with ongoing symptomatic AF, RA LVZ homogenization eliminated or markedly reduced AF. Prior series describing atypical RA free wall flutter involving a region of RA free wall scar have similarly not found an obvious association or cause for this abnormality.7–9 Lastly, none of our five patients demonstrated any evolving cardiac or extracardiac manifestations of scar promoting cardiac conditions (such as sarcoidosis and amyloidosis) at baseline and during follow-up.
Role of the right atrium in the mechanism of atrial fibrillation
The potential role of the RA in the mechanism of different forms of AF has long been recognized. A study by Hocini et al. described that in 20% of patients with persistent AF, left atrial ablation unmasked a right to left cycle length gradient indicating the RA as the arrhythmia driver. Right atrium ablation which included substrate modification terminated the arrhythmia by 55%.13 Other studies have also described the ablation of AF in which there was a critical mechanism in the RA. These have involved the targeting of non-pulmonary vein triggers arising from sites such as the crista terminalis, the coronary sinus ostium, and other right atrial sites.14,15 Other studies have described the targeting of complex fractionated electrograms in the RA or the identification of right atrial rotors using phase mapping approaches.16–18
In a series of elegant studies over a decade ago, the group of Chen and coworkers described both atypical flutter and AF originating in the RA in different patient populations.19,20 In these studies, using Ensite mapping, the authors identified channels in the region of the crista terminalis and an LVZ located predominantly along the lower crista terminalis. The LVZ extended on to the RA free wall in 27% of patients in this series. The authors observed that the mean number of channels requiring ablation was 2.2 with successful long-term outcomes in 66% of patients.
In contrast, in the current study, we describe patients with an extensive LVZ occupying most of the RA trabeculated free wall and with extensive channels indicated by multiple regions of complex low amplitude fractionated signals in between electrically inert areas. Ablation required a mean RF duration of 35 min in order to completely homogenize the scar zone.
Substrate ablation in the left atrium
Substrate-based approaches to ablation in the LA have gained traction since the original description by Kottkamp of box isolation.5 In a series of 41 patients with paroxysmal and persistent AF, box isolation of fibrotic left atrial regions identified by electroanatomic voltage mapping resulted in maintenance of sinus rhythm of up to 83% of patients during 12 months of follow-up. A randomized study of PVI plus linear ablation vs. PVI plus voltage guided substrate ablation in paroxysmal and persistent AF demonstrated improved 12 months of outcomes in the substrate ablation group.21 Low-voltage zones in this study were all in the LA (roof, posterior wall, and septum). Similarly, the stable SR study randomized patients with persistent AF to either PVI plus left atrial substrate ablation or stepwise ablation and found similar efficacy but with significantly less ablation required in the substrate group.6 In contrast, Marrouche et al.3 have extensively described the successful use of cardiac magnetic resonance (CMR) to identify regions of left atrial fibrosis as target zones for ablation. A prospective multicentre randomized study is currently ongoing to examine the efficacy of atrial fibrosis (based on MRI LGE) guided ablation intervention in treatment patients with persistent AF (DECAAF-II, NCT02529319).
However, to date, the identification of fibrotic substrate in the RA either with electroanatomic mapping or CMR has not been routine. In the current study, we identified five cases with RA free wall substrate as the AF driver region from an overall AF ablation series of over 3500 patients indicating that this is indeed an unusual mechanism for AF.
Limitations
We do not have detailed electroanatomic mapping of the LA in all patients in this series. However, all five patients have had elimination or marked reduction of AF with an approach targeting the right atrial substrate indicating that this is likely the dominant AF mechanism.
Conclusion
We describe a small series of patients in whom an extensive right atrial free wall LVZ represented the substrate for a clinical phenotype that includes both AF and unstable atypical ATs. This was demonstrated when substrate mapping was performed following the finding of either spontaneous or inducible right atrial AT. These patients have evidence of multiple channels through this region in between areas of dense scar. Ablative homogenization of this substrate resulted in elimination of AF. The aetiology of this substrate remains unclear.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: R.P. is supported by the National Health and Medical Research Council (NHMRC) research scholarship. P.M.K. has received funding from Abbott for consultancy and speaking engagements. J.M.K. is supported by practitioner fellowships from the NHMRC, has reported receiving research support from Biosense Webster, Boston Scientific, Abbott, and Medtronic and has served on the advisory board of Boston Scientific and Biosense Webster. Other authors declare no conflicts of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.



