-
PDF
- Split View
-
Views
-
Cite
Cite
Boris Rudic, Erol Tülümen, Fabian Fastenrath, Susanne Röger, Diana Goranova, Ibrahim Akin, Martin Borggrefe, Jürgen Kuschyk, Incidence, mechanisms, and clinical impact of inappropriate shocks in patients with a subcutaneous defibrillator, EP Europace, Volume 22, Issue 5, May 2020, Pages 761–768, https://doi.org/10.1093/europace/euaa026
Close - Share Icon Share
Abstract
Inappropriate shocks (IAS) remain a challenge for patients and physicians after implantation of the subcutaneous implantable cardioverter-defibrillator (S-ICD). The aims were to assess and characterize different patterns of IAS.
Two hundred and thirty-nine patients were implanted with an S-ICD between 2010 and 2018 for primary and secondary prevention. Follow-up data of at least 6 months were analysed. During a mean follow-up of 34.9 ± 16.0 months, a total of 73 shocks occurred in 38 patients (6%). Forty-three (59%) shocks were considered appropriate due to ventricular tachycardia/ventricular fibrillation, while 30 (41%) were inappropriate and occurred in 19 patients (8%). Myopotentials/noise was the most frequent cause of inappropriate shocks (n = 8), followed by T-wave oversensing (n = 6) and undersensing of the QRS, resulting in adaptation of the automatic gain control and inappropriate shock (n = 5). Seventy-four percent of all IAS occurred on the primary vector, while no IAS occurred on the alternate vector. In seven of eight patients (88%), IAS related to myopotentials have occurred on the primary sensing vector. Multivariate analysis identified taller patients, primary sensing vector and first-generation S-ICD device as predictors for IAS. SMART pass effectively reduced the occurrence of IAS in the second-generation S-ICD system.
Inappropriate therapies are less frequently observed on the alternate vector. The primary vector seems to be unfavourable with regard to oversensing caused by myopotentials. Inappropriate shocks were associated with an increased rate of rehospitalization but not mortality. These observations have implications for the prevention of inappropriate S-ICD shocks.
Inappropriate shocks (IAS) in subcutaneous implantable cardioverter-defibrillator (S-ICD) can be challenging due to limited programming options.
Primary and secondary sensing vectors are programmed in the majority of patients.
Inappropriate shocks may occur in recognizable patterns and seem to be more prevalent in taller patients.
First-generation S-ICD device (1010 SQ-RX) is associated with worse outcome in terms of IAS.
Alternate sensing vector had the least occurrence of IAS and might be considered in patients with inherited arrhythmia syndromes (i.e. Brugada syndrome).
Introduction
Implantable cardioverter-defibrillator (ICD) therapy is proven to reduce mortality.1 However, inappropriate shocks (IAS) are accompanied with multiple adverse effects including impaired quality of life, psychological disturbances, and even increased mortality.2 Inappropriate shocks remain especially a challenge in physically active and younger patients, in whom quality of life and long-term acceptance of the ICD therapy will be highly dependent on prevention of IAS. The subcutaneous implantable cardioverter-defibrillator (S-ICD) was developed to provide an alternative to the transvenous ICD.3 Studies demonstrating the safety and effectiveness of the S-ICD have been published.4,5 The S-ICD is often considered in younger patients with inherited diseases, although it is increasingly implanted in a variety of cardiac conditions for primary and secondary prevention of ventricular tachyarrhythmias.6–8 The S-ICD system uses three sensing configurations to detect electrical activity. These recordings resemble the signal characteristics of the standard surface electrocardiogram (ECG) allowing morphology-based sensing in addition to heart rate analysis. New algorithms have been introduced in order to reduce inappropriate therapy in the S-ICD.9 However, programming of the S-ICD remains restricted and avoidance of IAS using strategic programming limited.10 The purpose of this study was to analyse single episodes of inappropriate S-ICD shocks and their association with comorbidities, patient characteristics, and programming parameters, in order to explore mechanisms of inappropriate shocks in S-ICD patients, along with guidance for IAS prevention.
Methods
Study population
From November 2010 until June 2018, a total of 248 consecutive patients with indication for primary or secondary prevention, not participating in an ongoing prospective clinical trial, were implanted with an S-ICD system (Figure 1). Follow-up of at least 6 months was available in 239 patients, which were included in this study. In the majority of cases, the pulse generator was placed between the anterior portion of the Serratus muscle and the posterior aspect of the Latissimus dorsi muscle. Demographic characteristics and comorbidities of the study population are summarized in Table 1.
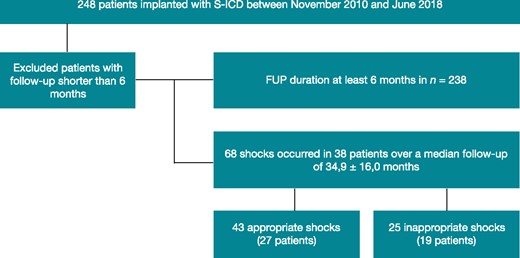
Patient flow chart. FUP, follow-up; S-ICD, subcutaneous implantable cardioverter-defibrillator.
| Number of patients, n (%) | 239 (100) |
| Male, n (%) | 185 (77) |
| Age (years) | 57.3 ± 16.0 |
| Height (cm) | 174 ± 0.1 |
| Weight (kg) | 84.6 ± 21.4 |
| BMI (kg/m2) | 27.9 ± 6.6 |
| Underlying disease, n (%) | |
| Coronary artery disease | 92 (38) |
| Non-ischaemic cardiomyopathy | 66 (28) |
| Brugada syndrome | 24 (10) |
| Hypertrophic cardiomyopathy | 19 (8) |
| Idiopathic ventricular fibrillation | 18 (8) |
| Long-QT syndrome | 8 (3) |
| ARVC | 3 (1) |
| CPVT | 3 (1) |
| Non-compaction cardiomyopathy | 2 (1) |
| Short-QT syndrome | 1 (1) |
| Other cardiomyopathies | 3 (1) |
| Primary prevention indication, n (%) | 171 (72) |
| Mean LVEF (%) | 38 ± 21 |
| History of previous ICD implantation, n (%) | 53 (22) |
| QRS width (ms) | 105 ± 25 |
| Bundle branch block, n (%) | 27 (11) |
| Atrial fibrillation, n (%) | 51 (21) |
| AF paroxysmal, n (%) | 38 (16) |
| AF persistent/permanent, n (%) | 13 (5) |
| 1st gen S-ICD pulse generator (1010 SQ-RX), n (%) | 79 (33) |
| Dual-zone programming, n (%) | 200 (84) |
| SMART Pass enabled (only in A209/A219, n = 160), n (%) | 152 (95) |
| Number of patients, n (%) | 239 (100) |
| Male, n (%) | 185 (77) |
| Age (years) | 57.3 ± 16.0 |
| Height (cm) | 174 ± 0.1 |
| Weight (kg) | 84.6 ± 21.4 |
| BMI (kg/m2) | 27.9 ± 6.6 |
| Underlying disease, n (%) | |
| Coronary artery disease | 92 (38) |
| Non-ischaemic cardiomyopathy | 66 (28) |
| Brugada syndrome | 24 (10) |
| Hypertrophic cardiomyopathy | 19 (8) |
| Idiopathic ventricular fibrillation | 18 (8) |
| Long-QT syndrome | 8 (3) |
| ARVC | 3 (1) |
| CPVT | 3 (1) |
| Non-compaction cardiomyopathy | 2 (1) |
| Short-QT syndrome | 1 (1) |
| Other cardiomyopathies | 3 (1) |
| Primary prevention indication, n (%) | 171 (72) |
| Mean LVEF (%) | 38 ± 21 |
| History of previous ICD implantation, n (%) | 53 (22) |
| QRS width (ms) | 105 ± 25 |
| Bundle branch block, n (%) | 27 (11) |
| Atrial fibrillation, n (%) | 51 (21) |
| AF paroxysmal, n (%) | 38 (16) |
| AF persistent/permanent, n (%) | 13 (5) |
| 1st gen S-ICD pulse generator (1010 SQ-RX), n (%) | 79 (33) |
| Dual-zone programming, n (%) | 200 (84) |
| SMART Pass enabled (only in A209/A219, n = 160), n (%) | 152 (95) |
AF, atrial fibrillation; BMI, body mass index; ICD, implantable cardioverter-defibrillator; S-ICD, subcutaneous implantable cardioverter-defibrillator.
| Number of patients, n (%) | 239 (100) |
| Male, n (%) | 185 (77) |
| Age (years) | 57.3 ± 16.0 |
| Height (cm) | 174 ± 0.1 |
| Weight (kg) | 84.6 ± 21.4 |
| BMI (kg/m2) | 27.9 ± 6.6 |
| Underlying disease, n (%) | |
| Coronary artery disease | 92 (38) |
| Non-ischaemic cardiomyopathy | 66 (28) |
| Brugada syndrome | 24 (10) |
| Hypertrophic cardiomyopathy | 19 (8) |
| Idiopathic ventricular fibrillation | 18 (8) |
| Long-QT syndrome | 8 (3) |
| ARVC | 3 (1) |
| CPVT | 3 (1) |
| Non-compaction cardiomyopathy | 2 (1) |
| Short-QT syndrome | 1 (1) |
| Other cardiomyopathies | 3 (1) |
| Primary prevention indication, n (%) | 171 (72) |
| Mean LVEF (%) | 38 ± 21 |
| History of previous ICD implantation, n (%) | 53 (22) |
| QRS width (ms) | 105 ± 25 |
| Bundle branch block, n (%) | 27 (11) |
| Atrial fibrillation, n (%) | 51 (21) |
| AF paroxysmal, n (%) | 38 (16) |
| AF persistent/permanent, n (%) | 13 (5) |
| 1st gen S-ICD pulse generator (1010 SQ-RX), n (%) | 79 (33) |
| Dual-zone programming, n (%) | 200 (84) |
| SMART Pass enabled (only in A209/A219, n = 160), n (%) | 152 (95) |
| Number of patients, n (%) | 239 (100) |
| Male, n (%) | 185 (77) |
| Age (years) | 57.3 ± 16.0 |
| Height (cm) | 174 ± 0.1 |
| Weight (kg) | 84.6 ± 21.4 |
| BMI (kg/m2) | 27.9 ± 6.6 |
| Underlying disease, n (%) | |
| Coronary artery disease | 92 (38) |
| Non-ischaemic cardiomyopathy | 66 (28) |
| Brugada syndrome | 24 (10) |
| Hypertrophic cardiomyopathy | 19 (8) |
| Idiopathic ventricular fibrillation | 18 (8) |
| Long-QT syndrome | 8 (3) |
| ARVC | 3 (1) |
| CPVT | 3 (1) |
| Non-compaction cardiomyopathy | 2 (1) |
| Short-QT syndrome | 1 (1) |
| Other cardiomyopathies | 3 (1) |
| Primary prevention indication, n (%) | 171 (72) |
| Mean LVEF (%) | 38 ± 21 |
| History of previous ICD implantation, n (%) | 53 (22) |
| QRS width (ms) | 105 ± 25 |
| Bundle branch block, n (%) | 27 (11) |
| Atrial fibrillation, n (%) | 51 (21) |
| AF paroxysmal, n (%) | 38 (16) |
| AF persistent/permanent, n (%) | 13 (5) |
| 1st gen S-ICD pulse generator (1010 SQ-RX), n (%) | 79 (33) |
| Dual-zone programming, n (%) | 200 (84) |
| SMART Pass enabled (only in A209/A219, n = 160), n (%) | 152 (95) |
AF, atrial fibrillation; BMI, body mass index; ICD, implantable cardioverter-defibrillator; S-ICD, subcutaneous implantable cardioverter-defibrillator.
Implantation procedure and device programming
All patients eligible for S-ICD implantation were screened using the recommended tool provided by the manufacturer. Screening was performed during supine and standing/sitting position. Patients were required to have at least one screening vector passing the screening test.
All implantations were performed using local anaesthesia and conscious sedation with intermittent boluses of midazolam (5–15 µg/kg), fentanyl (0.5–1.0 µg/kg), and propofol (1–2 mg/kg). The operation technique was described earlier.11 In all patients, the S-ICD pulse generator was implanted between the serratus anterior and latissimus dorsi muscle. Further, in all 239 patients, a successful intraoperative defibrillation testing was performed. Sensing vectors were determined automatically at the end of the implantation procedure. Forty-nine implantations were performed in three-incision technique, while in the remainder of implantations the two-incision technique was applied.12 Exercise screening of appropriate QRS/T wave detection was performed in 207 patients (87%) on post-operative day 1. In the remainder of patients (n = 32), exercise screening was not performed due to comorbidities or inability. Sensing vectors were manually modified in 7 patients (3%), following results of the exercise screening, in order to obtain a better QRS-T-wave ratio and by judgement of the operator. Thirty-nine patients (16%) were programmed to a single shock zone ≥250 b.p.m. Two hundred patients (84%) had a dual therapy zone with the lower therapy cut-off starting from 190 b.p.m. to 230 b.p.m. (see Table 1).
Follow-up of patients and data collection
Patients were routinely followed every 3 months at the outpatient clinic. On each visit, device interrogations were performed and patients were asked for adverse events or shocks. Additional follow-up visits took place in the event of shock delivery or individual device-related complications. Forty of 239 patients were followed using the Latitude telemonitoring system. All events were evaluated by two investigators blinded to patient’s history. In case of multiple IAS in the same patient, the programmed sensing vector at the time of the first IAS was used for statistical analysis. The study was approved by the local institutional review board (IRB).
Statistical analysis
Categorical data are displayed as frequencies and percentages. Continuous data are described as mean ± SD. To analyse inappropriate shocks and programmed sensing vectors, the Fisher’s exact test was used. In seven patients with appropriate shocks, IAS occurred independently over time. These patients were counted to the IAS group. Multivariate analysis was performed with logistic regression analysis using block entry of variables with significant result in the univariate analysis. P-values ≤0.05 were considered significant. The SPSS software package (Chicago, IL, USA; version 20) was used for analysis.
Results
Baseline characteristics
A total of 239 patients (185 males; mean age: 57 ± 16 years) underwent successful screening and implantation of an S-ICD. Follow-up data of at least 6 months were evaluated. Ischaemic, non-ischaemic, and hypertrophic cardiomyopathy accounted for the majority of indications (n = 177; 74%), while other cardiomyopathies and channelopathies constituted the remainder (n = 62; 26%). Primary preventive ICD indication was present in 171 (72%) patients. Twenty-two percent had history of previous transvenous ICD implantation. Patient characteristics are summarized in Table 1. Permanent sensing vector was programmed to primary in 118 patients (49%), to secondary in 89 patients (37%), and to alternate in 32 patients (14%) (Table 2).
| Underlying disease . | Sensing vector set to primary . | Sensing vector set to secondary . | Sensing vector set to alternate . |
|---|---|---|---|
| Coronary artery disease, n (%) | 52 (57) | 29 (31) | 11 (12) |
| Non-ischaemic cardiomyopathy, n (%) | 31 (47) | 28 (43) | 7 (10) |
| Brugada syndrome, n (%) | 10 (42) | 8 (33) | 6 (25) |
| Hypertrophic cardiomyopathy, n (%) | 8 (42) | 9 (47) | 2 (11) |
| Idiopathic ventricular fibrillation, n (%) | 5 (28) | 7 (39) | 6 (33) |
| Long-QT syndrome, n (%) | 6 (75) | 2 (25) | 0 |
| ARVC, n (%) | 3 (100) | 0 | 0 |
| CPVT, n (%) | 1 (25) | 2 (75) | 0 |
| Non-compaction cardiomyopathy, n (%) | 1 (50) | 1 (50) | 0 |
| Short-QT syndrome, n (%) | 0 | 1 (100) | 0 |
| Other cardiomyopathies, n (%) | 1 (25) | 2 (75) | 0 |
| Total, n (%) | 118 (49) | 89 (37) | 32 (14) |
| Underlying disease . | Sensing vector set to primary . | Sensing vector set to secondary . | Sensing vector set to alternate . |
|---|---|---|---|
| Coronary artery disease, n (%) | 52 (57) | 29 (31) | 11 (12) |
| Non-ischaemic cardiomyopathy, n (%) | 31 (47) | 28 (43) | 7 (10) |
| Brugada syndrome, n (%) | 10 (42) | 8 (33) | 6 (25) |
| Hypertrophic cardiomyopathy, n (%) | 8 (42) | 9 (47) | 2 (11) |
| Idiopathic ventricular fibrillation, n (%) | 5 (28) | 7 (39) | 6 (33) |
| Long-QT syndrome, n (%) | 6 (75) | 2 (25) | 0 |
| ARVC, n (%) | 3 (100) | 0 | 0 |
| CPVT, n (%) | 1 (25) | 2 (75) | 0 |
| Non-compaction cardiomyopathy, n (%) | 1 (50) | 1 (50) | 0 |
| Short-QT syndrome, n (%) | 0 | 1 (100) | 0 |
| Other cardiomyopathies, n (%) | 1 (25) | 2 (75) | 0 |
| Total, n (%) | 118 (49) | 89 (37) | 32 (14) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; LVEF, left ventricular ejection fraction; IQR, interquartile range; EGM, electrogram.
| Underlying disease . | Sensing vector set to primary . | Sensing vector set to secondary . | Sensing vector set to alternate . |
|---|---|---|---|
| Coronary artery disease, n (%) | 52 (57) | 29 (31) | 11 (12) |
| Non-ischaemic cardiomyopathy, n (%) | 31 (47) | 28 (43) | 7 (10) |
| Brugada syndrome, n (%) | 10 (42) | 8 (33) | 6 (25) |
| Hypertrophic cardiomyopathy, n (%) | 8 (42) | 9 (47) | 2 (11) |
| Idiopathic ventricular fibrillation, n (%) | 5 (28) | 7 (39) | 6 (33) |
| Long-QT syndrome, n (%) | 6 (75) | 2 (25) | 0 |
| ARVC, n (%) | 3 (100) | 0 | 0 |
| CPVT, n (%) | 1 (25) | 2 (75) | 0 |
| Non-compaction cardiomyopathy, n (%) | 1 (50) | 1 (50) | 0 |
| Short-QT syndrome, n (%) | 0 | 1 (100) | 0 |
| Other cardiomyopathies, n (%) | 1 (25) | 2 (75) | 0 |
| Total, n (%) | 118 (49) | 89 (37) | 32 (14) |
| Underlying disease . | Sensing vector set to primary . | Sensing vector set to secondary . | Sensing vector set to alternate . |
|---|---|---|---|
| Coronary artery disease, n (%) | 52 (57) | 29 (31) | 11 (12) |
| Non-ischaemic cardiomyopathy, n (%) | 31 (47) | 28 (43) | 7 (10) |
| Brugada syndrome, n (%) | 10 (42) | 8 (33) | 6 (25) |
| Hypertrophic cardiomyopathy, n (%) | 8 (42) | 9 (47) | 2 (11) |
| Idiopathic ventricular fibrillation, n (%) | 5 (28) | 7 (39) | 6 (33) |
| Long-QT syndrome, n (%) | 6 (75) | 2 (25) | 0 |
| ARVC, n (%) | 3 (100) | 0 | 0 |
| CPVT, n (%) | 1 (25) | 2 (75) | 0 |
| Non-compaction cardiomyopathy, n (%) | 1 (50) | 1 (50) | 0 |
| Short-QT syndrome, n (%) | 0 | 1 (100) | 0 |
| Other cardiomyopathies, n (%) | 1 (25) | 2 (75) | 0 |
| Total, n (%) | 118 (49) | 89 (37) | 32 (14) |
ARVC, arrhythmogenic right ventricular cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; LVEF, left ventricular ejection fraction; IQR, interquartile range; EGM, electrogram.
Incidence of defibrillator shocks
During a median follow-up of 34.9 ± 16.0 months, 38 patients (16%) experienced a total of 73 shocks (incidence 4.9 per 100 patient-years). Median time from implantation to any shock was 154.5 days (interquartile range 327.8 days). Twenty-seven patients (11%) had 43 appropriate shocks, while 19 patients (8%) experienced a total of 30 inappropriate shocks. The median time from implantation to inappropriate shock was 67.0 days (IQR 386.0 days). Thirteen patients experienced one and six patients had two or more inappropriate therapies during follow-up. Patients with IAS were significantly taller than patients without IAS (179 ± 0.1 cm vs. 174 ± 0.1 cm; P = 0.006). Inappropriate shocks were more prevalent in patients with the first-generation S-ICD, as compared to the second generation (11% vs. 6%). Further, in the second S-ICD generation, activation of SMART pass filter led to a reduced incidence of IAS in all enabled devices. Table 3 shows relevant characteristics of patients with IAS.
Differences between patients with appropriate vs. inappropriate vs. no shocks
| . | Patients with inappropriate shocks (n = 19)a . | Patients with appropriate shocks (n = 20)a . | Patients without shocks (n = 200) . | P-value . |
|---|---|---|---|---|
| Age (years) | 58 ± 16 | 64 ± 13 | 58 ± 16 | NS |
| Male (%) | 17 (89) | 16 (80) | 153 (76) | NS |
| Height (cm) | 179 ± 0.1 | 175 ± 0.1 | 174 ± 0.1 | 0.006b |
| Weight (kg) | 82.5 ± 23.9 | 77.6 ± 20.0 | 84.1 ± 21.2 | NS |
| BMI | 26.1 ± 6.8 | 26.0 ± 5.8 | 26.8 ± 6.7 | NS |
| Two-incision technique | 14 (74) | 15 (75) | 161 (81) | NS |
| Three-incision technique | 5 (26) | 5 (25) | 39 (19) | NS |
| Generation of S-ICD pulse generator | ||||
| First gen (1010) | 9/79 (11) | 14/79 (18) | 56/79 (70) | 0.001 |
| Second gen (A209) | 10/160 (6) | 6/160 (4) | 144/160 (90) | 0.001 |
| SMART Pass on | 5/10 (50) | 3/6 (50) | 117/144 (81) | 0.03c |
| SMART Pass off | 5/10 (50) | 3/6 (50) | 27/144 (19) | |
| Programmed vector at the time of shock | ||||
| Primary (%) | 15 (79) | 10 (50) | 94 (47) | 0.03b |
| Secondary (%) | 4 (21) | 7 (35) | 77 (38) | NS |
| Alternate (%) | 0 (0) | 3 (20) | 29 (14) | NA |
| . | Patients with inappropriate shocks (n = 19)a . | Patients with appropriate shocks (n = 20)a . | Patients without shocks (n = 200) . | P-value . |
|---|---|---|---|---|
| Age (years) | 58 ± 16 | 64 ± 13 | 58 ± 16 | NS |
| Male (%) | 17 (89) | 16 (80) | 153 (76) | NS |
| Height (cm) | 179 ± 0.1 | 175 ± 0.1 | 174 ± 0.1 | 0.006b |
| Weight (kg) | 82.5 ± 23.9 | 77.6 ± 20.0 | 84.1 ± 21.2 | NS |
| BMI | 26.1 ± 6.8 | 26.0 ± 5.8 | 26.8 ± 6.7 | NS |
| Two-incision technique | 14 (74) | 15 (75) | 161 (81) | NS |
| Three-incision technique | 5 (26) | 5 (25) | 39 (19) | NS |
| Generation of S-ICD pulse generator | ||||
| First gen (1010) | 9/79 (11) | 14/79 (18) | 56/79 (70) | 0.001 |
| Second gen (A209) | 10/160 (6) | 6/160 (4) | 144/160 (90) | 0.001 |
| SMART Pass on | 5/10 (50) | 3/6 (50) | 117/144 (81) | 0.03c |
| SMART Pass off | 5/10 (50) | 3/6 (50) | 27/144 (19) | |
| Programmed vector at the time of shock | ||||
| Primary (%) | 15 (79) | 10 (50) | 94 (47) | 0.03b |
| Secondary (%) | 4 (21) | 7 (35) | 77 (38) | NS |
| Alternate (%) | 0 (0) | 3 (20) | 29 (14) | NA |
BMI, body mass index; IAS, inappropriate shocks; S-ICD, subcutaneous implantable cardioverter-defibrillator.
Seven patients had AS and IAS. For the purpose of the Fisher’s exact test, patients with AS and IAS were included in the IAS group and excluded from AS group.
Comparison patients with IAS vs. patients without shocks.
Comparison among SMART pass-enabled devices (A209) patients with IAS vs. patients without shocks.
Differences between patients with appropriate vs. inappropriate vs. no shocks
| . | Patients with inappropriate shocks (n = 19)a . | Patients with appropriate shocks (n = 20)a . | Patients without shocks (n = 200) . | P-value . |
|---|---|---|---|---|
| Age (years) | 58 ± 16 | 64 ± 13 | 58 ± 16 | NS |
| Male (%) | 17 (89) | 16 (80) | 153 (76) | NS |
| Height (cm) | 179 ± 0.1 | 175 ± 0.1 | 174 ± 0.1 | 0.006b |
| Weight (kg) | 82.5 ± 23.9 | 77.6 ± 20.0 | 84.1 ± 21.2 | NS |
| BMI | 26.1 ± 6.8 | 26.0 ± 5.8 | 26.8 ± 6.7 | NS |
| Two-incision technique | 14 (74) | 15 (75) | 161 (81) | NS |
| Three-incision technique | 5 (26) | 5 (25) | 39 (19) | NS |
| Generation of S-ICD pulse generator | ||||
| First gen (1010) | 9/79 (11) | 14/79 (18) | 56/79 (70) | 0.001 |
| Second gen (A209) | 10/160 (6) | 6/160 (4) | 144/160 (90) | 0.001 |
| SMART Pass on | 5/10 (50) | 3/6 (50) | 117/144 (81) | 0.03c |
| SMART Pass off | 5/10 (50) | 3/6 (50) | 27/144 (19) | |
| Programmed vector at the time of shock | ||||
| Primary (%) | 15 (79) | 10 (50) | 94 (47) | 0.03b |
| Secondary (%) | 4 (21) | 7 (35) | 77 (38) | NS |
| Alternate (%) | 0 (0) | 3 (20) | 29 (14) | NA |
| . | Patients with inappropriate shocks (n = 19)a . | Patients with appropriate shocks (n = 20)a . | Patients without shocks (n = 200) . | P-value . |
|---|---|---|---|---|
| Age (years) | 58 ± 16 | 64 ± 13 | 58 ± 16 | NS |
| Male (%) | 17 (89) | 16 (80) | 153 (76) | NS |
| Height (cm) | 179 ± 0.1 | 175 ± 0.1 | 174 ± 0.1 | 0.006b |
| Weight (kg) | 82.5 ± 23.9 | 77.6 ± 20.0 | 84.1 ± 21.2 | NS |
| BMI | 26.1 ± 6.8 | 26.0 ± 5.8 | 26.8 ± 6.7 | NS |
| Two-incision technique | 14 (74) | 15 (75) | 161 (81) | NS |
| Three-incision technique | 5 (26) | 5 (25) | 39 (19) | NS |
| Generation of S-ICD pulse generator | ||||
| First gen (1010) | 9/79 (11) | 14/79 (18) | 56/79 (70) | 0.001 |
| Second gen (A209) | 10/160 (6) | 6/160 (4) | 144/160 (90) | 0.001 |
| SMART Pass on | 5/10 (50) | 3/6 (50) | 117/144 (81) | 0.03c |
| SMART Pass off | 5/10 (50) | 3/6 (50) | 27/144 (19) | |
| Programmed vector at the time of shock | ||||
| Primary (%) | 15 (79) | 10 (50) | 94 (47) | 0.03b |
| Secondary (%) | 4 (21) | 7 (35) | 77 (38) | NS |
| Alternate (%) | 0 (0) | 3 (20) | 29 (14) | NA |
BMI, body mass index; IAS, inappropriate shocks; S-ICD, subcutaneous implantable cardioverter-defibrillator.
Seven patients had AS and IAS. For the purpose of the Fisher’s exact test, patients with AS and IAS were included in the IAS group and excluded from AS group.
Comparison patients with IAS vs. patients without shocks.
Comparison among SMART pass-enabled devices (A209) patients with IAS vs. patients without shocks.
Results obtained during home monitoring (Latitude™)
In total, 40/239 patients were enrolled in LATITUDE home monitoring. Eight of 19 patients who previously experienced IAS were enrolled in home monitoring for closer follow-up, 4 patients with IAS refused home monitoring and in the remainder of 7 patients home monitoring was not available or offered to the patient. One patient experienced IAS despite being followed over LATITUDE, and 2 patients had short episodes of inappropriate tachycardia detection which led to prompt response and reprogramming of the sensing vector. In these two patients, re-occurrence IAS could be prevented. In the group of patients who received LATITUDE for other reasons than IAS, no patient had documented episodes of sustained or non-sustained inappropriate episodes/sensing.
Causes of appropriate and inappropriate therapies
Twenty-eight out of 43 appropriate therapies were caused by ventricular tachycardia/ventricular fibrillation (VT/VF) faster than 250 beats per minute, while in the remaining 15 episodes a monomorphic VT ≤ 250 b.p.m. was present. Inappropriate shocks were classified into three groups, based on their electrocardiographic pattern: over-sensing, caused by high-frequency signals (Figure 2), was the most frequent cause of IAS and occurred in eight patients. T-wave oversensing (TWOS) was observed in six patients and was exclusively caused by slow monomorphic VTs with widening of the QRS complex and change in the R/T ratio (Figure 3). Finally, under-sensing of the QRS leading to an extreme gain of the sensitivity and IAS (Figure 4) occurred in another five patients. This form of IAS occurred in all of cases within the first 24 h post-operatively. In patients who underwent post-operative exercise screening, 15/193 patients (7%) experienced IAS in comparison to 4/72 patients (13%) who did not undergo exercise screening. The difference was not statistically significant.
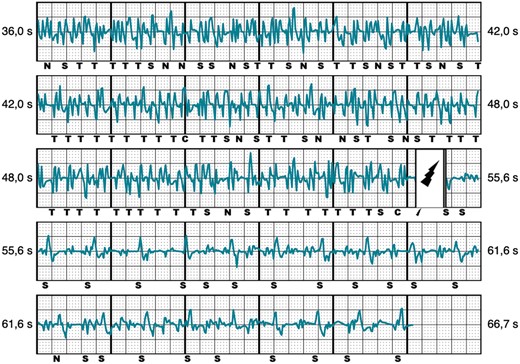
Inappropriate shock due to myopotentials/noise. Representative example of IAS caused by myopotentials/noise.  , shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; N, noise; T, tachy detection.
, shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; N, noise; T, tachy detection.
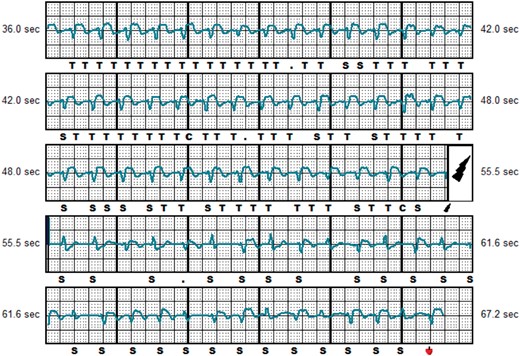
Inappropriate shock caused by TWOS. Representative example of IAS caused by TWOS during slow ventricular tachycardia of 155 b.p.m.  , shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; S, QRS sensing; T, tachy detection; TWOS, T-wave oversensing.
, shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; S, QRS sensing; T, tachy detection; TWOS, T-wave oversensing.
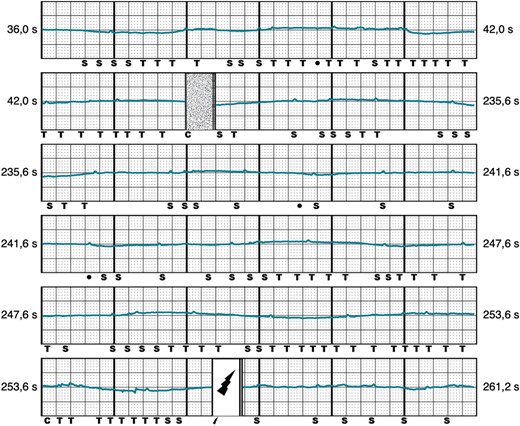
Inappropriate shock caused by under-sensing. Representative example of IAS shock caused by under-sensing, 8 h after successful implantation of the S-ICD.  , shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; S, QRS sensing; S-ICD, subcutaneous implantable cardioverter-defibrillator; T, tachy detection.
, shock delivered; C, capacitor charging start/end; IAS, inappropriate shocks; S, QRS sensing; S-ICD, subcutaneous implantable cardioverter-defibrillator; T, tachy detection.
Correlation between sensing vectors and defibrillator shocks
A total of 73 shocks was available for evaluation. Table 3 provides a grouped overview based on the underlying cause of ICD shock. In terms of appropriate shock, there were no differences among the distribution of sensing vectors as compared to patients without any shock (P = 0.9). In the group of patients with IAS, we found a significantly higher proportion of patients with the primary sensing vector as compared to patients without shocks (P = 0.03). Further, IAS caused by myopotentials were almost exclusively registered on the primary sensing vector (seven of eight cases). Inappropriate shocks caused by TWOS occurred in 6 patients and 17 episodes with equal distribution among the primary and secondary vector. No inappropriate therapies were evidenced with the alternate vector.
Patients with IAS caused by myopotentials were on average taller (mean 183 ± 0.1 cm) and younger (mean 46.3 ± 13.5 years) than patients without IAS (P = 0.034 and P = 0.001, respectively), in contrast to patients with IAS related to TWOS and QRS under-sensing. No other clinical parameters, e.g., atrial fibrillation, QRS duration, presence of bundle branch block, or underlying disease were associated with IAS. Further, we found no association between the implanting technique (two-incision/three-incision) and occurrence of IAS.
In the multivariate analysis of predictors for IAS, we found that a body height ≥182 cm, a programmed primary sensing vector and presence of the first-generation S-ICD device (1010) was independently and significantly associated with IAS (Table 4).
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | CI . | P-value . | OR . | CI . | P-value . |
| Primary sensing vector | 3.1 | 1.1–9.0 | 0.03 | 5.3 | 1.6–17.4 | 0.005 |
| Height ≥ 182 cm | 3.5 | 1.3–9.7 | 0.02 | 6.8 | 2.1–22.0 | 0.001 |
| First gen device (1010) | 2.9 | 1.6–5.2 | 0.001 | 3.0 | 1.1–8.6 | 0.04 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | CI . | P-value . | OR . | CI . | P-value . |
| Primary sensing vector | 3.1 | 1.1–9.0 | 0.03 | 5.3 | 1.6–17.4 | 0.005 |
| Height ≥ 182 cm | 3.5 | 1.3–9.7 | 0.02 | 6.8 | 2.1–22.0 | 0.001 |
| First gen device (1010) | 2.9 | 1.6–5.2 | 0.001 | 3.0 | 1.1–8.6 | 0.04 |
CI, confidence interval; OR, odds ratio.
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | CI . | P-value . | OR . | CI . | P-value . |
| Primary sensing vector | 3.1 | 1.1–9.0 | 0.03 | 5.3 | 1.6–17.4 | 0.005 |
| Height ≥ 182 cm | 3.5 | 1.3–9.7 | 0.02 | 6.8 | 2.1–22.0 | 0.001 |
| First gen device (1010) | 2.9 | 1.6–5.2 | 0.001 | 3.0 | 1.1–8.6 | 0.04 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| . | OR . | CI . | P-value . | OR . | CI . | P-value . |
| Primary sensing vector | 3.1 | 1.1–9.0 | 0.03 | 5.3 | 1.6–17.4 | 0.005 |
| Height ≥ 182 cm | 3.5 | 1.3–9.7 | 0.02 | 6.8 | 2.1–22.0 | 0.001 |
| First gen device (1010) | 2.9 | 1.6–5.2 | 0.001 | 3.0 | 1.1–8.6 | 0.04 |
CI, confidence interval; OR, odds ratio.
Inappropriate shocks led to a higher rate of rehospitalization as compared to patients without any shocks. Other major adverse events (death, occurrence of VT/VF, and myocardial infarction) were not increased in patients with IAS as compared to patients with AS or patients without shocks.
Patients with IAS were reprogrammed to the next best sensing vector (R/T ratio, SMART pass availability, dynamic and isometric provocation testing, etc.). In the majority of cases, this approach mitigated the occurrence of further shocks. In three patients (16%), a second reprogramming was necessary due to further IAS recurrence. After the reprogramming, these patients remained free of symptoms for a median follow-up of 14 months.
Discussion
In the present study, IAS occurred in 7.9%, with an annual rate of 2.6 IAS/year in patients with an S-ICD. This is in line with the published long-term data of the largest S-ICD registry, in which 13.5% IAS were noted within 5 years.13
The findings of our study indicate that IAS in patients with S-ICD occur in three main patterns: over-sensing of cardiac and extracardiac signals (TWOS/myopotentials/noise) and under specific circumstances under-sensing caused by change in automatic gain control. While it remains unclear which muscle groups are exactly involved in IAS caused by myopotentials, in our study, these patients were on average younger and taller. We found that the majority of these patients has primary vector programmed as the permanent sensing direction. This finding might indicate involvement of the diaphragm or back muscles, rather than the pectoral muscle in development of IAS caused by myopotentials. In case of taller patients, improper placement of the proximal ring electrode towards the diaphragm may lead to inadvertent IAS. This underlines the importance of pre-operative fluoroscopy with dummy electrode and S-ICD pulse generator in order to determine the optimal position of proximal and distal sensing ring and their relationship to the can.
A subgroup analysis of data from the EFFORTLESS registry on IAS in patients with S-ICD was previously published.14 The authors described the alternate vector as most vulnerable to occurrences of IAS. Interestingly, in our cohort of patients no shocks have occurred on the alternate vector. There might be several explanations for this finding: first, the frequency of the alternate vector in our study population was lower than for primary and secondary vector. This might result in a statistically relevant under-representation in our cohort. Secondly, in contrast to the data published in the EFFORTLESS registry, all patients were implanted by the same operator, utilizing one single operation technique with deep insertion of the defibrillator lead directly on the sternum and intermuscular placement of the pulse generator between Musculus serratus anterior and Musculus latissimus dorsi. This fact could have a relevant impact on the occurrences of IAS on the alternate vector. Finally, different cardiovascular diseases and comorbidities could have influenced the outcome. In our study, 13% of all patients had alternate vector programmed after implantation. However, in patients without relevant structural heart disease (Brugada syndrome and idiopathic VF) the proportion of patients with programmed alternate vector was up to 33%. This implies that patients, who are commonly at younger age and without significant heart disease and pathologic ECG, could benefit from the alternate vector in terms of IAS.
In the study by Olde Nordkamp et al.,14 atrial fibrillation was significantly associated with IAS. In the present study, although the prevalence of atrial fibrillation was higher than in the EFFORTLESS registry, atrial fibrillation was not associated with IAS. One reason might be the introduction of algorithms that reduce IAS, e.g., SMART pass filter with the second-generation pulse generator (Emblem A209) and the fact that different therapy cut-offs for the conditional zone were programmed. In our study population, the rate of IAS was lower in patients with the Emblem A209 system, as compared to the 1010 SQ-RX device (6% vs. 11%), which is mainly due to the availability and activation of the SMART pass filter. Complication rate and inappropriate S-ICD therapies correlate with the experience of the operator. Knops et al.15 reported a non-significant trend towards fewer IAS with the increasing experience of the implanter. We could also observe that the incidence of IAS was reduced with increased experience of the implanter. The proportion of patients with IAS decreased over time, starting with 16,8% in the first year, 11% in the second, and 8% in the third. This underlines the importance of a meticulous implantation technique which appears to influence the occurrence of IAS. Early shocks that resulted from undersensing of the EGM caused by air pockets surrounding the sensing ring and/or the pulse generator can effectively be overcome by manual compression of the incision sites or irrigation of saline. After establishing these manoeuvres, we did not experience further IAS shocks caused by air entrapment. Speculatively, in this case, the total incidence of IAS could have been reduced by 2.1–5.8%.
Knops et al.15 further reported that IAS were lowered by increased use of dual-zone programming. In our cohort, the rate of dual-zone programming was slightly lower than that reported in the EFFORTLESS registry (71%). This is mainly driven by a high proportion of patients with cardiac channelopathies and subsequently a different programming of therapy cut-offs. We did not observe a correlation between single- and dual-zone programming. This may be explained by high rate programming (≥230 b.p.m.) in patients with a single zone setting.
Finally, based on our results, post-operative exercise screening was unable to mitigate the occurrence rate of IAS. However, not all patients have undergone exercise screening and secondly in as many as 20% of patients with IAS, exercise screening was established after the first IAS. Therefore and especially in the first-generation of S-ICD, without SMART pass filter, exercise screening may show a benefit.
Study limitations
This study has several limitations. It was not performed as a randomized trial comparing different treatment strategies and relies on different physician-related programming which could have influenced the outcome. Programming settings could also change during follow-up which may have affected the likelihood for IAS. No serial chest X-ray examinations were performed. Although we did not observe different outcome in patients undergoing two- vs. three-incision implantation, slight migration of the pulse generator or the shock coil cannot be fully ruled out. All patients were implanted by the same cardiologist. Different implantation technique could have changed the outcome. Finally, the follow-up time of 2.9 years may not be sufficiently long to represent possible IAS on the alternate vector, as IAS could also occur over later period of time, although the median time to first IAS in our patients was 197 days.
Conclusion
Taller patients and patients programmed on the primary sensing vector were at higher risk of experiencing IAS. Alternate vector showed promising results with regard to prevention of IAS in S-ICD recipients; however, this finding must be validated in a larger cohort of patients with multiple implanters. Careful analysis and interpretation of different mechanisms underlying IAS may help to determine the most appropriate sensing vector and further reduce IAS.
Conflict of interest: B.R. has received travel grants from Boston Scientific. J.K. is a consultant for and has received grant support from Boston Scientific. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.



