-
PDF
- Split View
-
Views
-
Cite
Cite
Andrea Di Cori, Angelo Auricchio, François Regoli, Carina Blomström-Lundqvist, Christian Butter, Nikolaos Dagres, Jean-Claude Deharo, Aldo P Maggioni, Andrzej Kutarski, Charles Kennergren, Cécile Laroche, Christopher A Rinaldi, Emilio Vincenzo Dovellini, Pier Giorgio Golzio, Anna Margrethe Thøgersen, Maria Grazia Bongiorni, ESC-EHRA ELECTRa Investigators, Clinical impact of antithrombotic therapy in transvenous lead extraction complications: a sub-analysis from the ESC-EORP EHRA ELECTRa (European Lead Extraction ConTRolled) Registry, EP Europace, Volume 21, Issue 7, July 2019, Pages 1096–1105, https://doi.org/10.1093/europace/euz062
Close - Share Icon Share
A sub-analysis of the ESC-EHRA European Lead Extraction ConTRolled (ELECTRa) Registry to evaluate the clinical impact of antithrombotic (AT) on transvenous lead extraction (TLE) safety and efficacy.
ELECTRa outcomes were compared between patients without AT therapy (No AT Group) and with different pre-operative AT regimens, including antiplatelets (AP), anticoagulants (AC), or both (AP + AC). Out of 3510 pts, 2398 (68%) were under AT pre-operatively. AT patients were older with more comorbidities (P < 0.0001). AT subgroups, defined as AP, AC, or AP + AC, were 1096 (31.2%), 985 (28%), and 317 (9%), respectively. Regarding AP patients, 1413 (40%) were under AP, 1292 (91%) with a single AP, interrupted in 26% about 3.8 ± 3.7 days before TLE. In total, 1302 (37%) patients were under AC, 881 vitamin K antagonist (68%), 221 (17%) direct oral anticoagulants, 155 (12%) low weight molecular heparin, and 45 (3.5%) unfractionated heparin. AC was ‘interrupted without bridging’ in 696 (54%) and ‘interrupted with bridging’ in 504 (39%) about 3.3 ± 2.3 days before TLE, and ‘continued’ in 87 (7%). TLE success rate was high in all subgroups. Only overall in-hospital death (1.4%), but not the procedure-related one, was higher in the AT subgroups (P = 0.0500). Age >65 years and New York Heart Association Class III/IV, but not AT regimens, were independent predictors of death for any cause. Haematomas were more frequent in AT subgroups, especially in AC ‘continued’ (P = 0.025), whereas pulmonary embolism in the No-AT (P < 0.01).
AT minimization is safe in patients undergoing TLE. AT does not seem to predict death but identifies a subset of fragile patients with a worse in-hospital TLE outcome.
Management of antithrombotic (AT) therapy in the case of transvenous lead extraction (TLE) appears to be even more controversial, given the scanty availability of data.
The ESC-EHRA European Lead Extraction ConTRolled (ELECTRa) Registry is a large multicentre study which evaluated the safety and efficacy of TLE in Europe.
The aim of our sub-analysis was to evaluate the impact of AT on in-hospital complications and mortality in patients undergoing TLE in the ESC-EHRA ELECTRa Registry.
Our analysis demonstrates, for the first time, that patients under chronic AT show a high TLE success rate, but with a higher overall mortality and more minor bleeding events compared to no ATs, especially in case of ‘continued’ anticoagulation.
AT minimization is safe in patients undergoing TLE.
AT does not seem to predict death but identifies a subset of fragile patients with a worse in-hospital TLE outcome.
Introduction
The complexity of candidates for transvenous lead extraction (TLE) has shown a parallel increase, both in terms of comorbidities, and of concomitant therapy, including antithrombotic (AT) therapy. Current data indicate that 50% of patients with pacemakers (PMs) and implantable cardiac defibrillators (ICDs) use single or dual antiplatelets,1–3 and the rate of use of anticoagulant therapy ranges from 15% to 35%,1,2 reaching almost 50% in patients with cardiac resynchronization therapies (CRTs).3
The management of candidates for low-risk cardiac implantable electronic device (CIED) procedures, like implantation or replacement, receiving concomitant AT is a debated issue, and only marginally the object of evidence-based recommendations in current guidelines.4,5 Nevertheless, the management of AT in TLE procedures appears to be even more controversial, given the scanty availability of data.6
The ESC-EHRA European Lead Extraction ConTRolled (ELECTRa) Registry is a prospective registry of consecutive TLE procedures conducted by the European Heart Rhythm Association (EHRA) in order to identify the safety and efficacy of the current practice of TLE.7
The present study is a sub-analysis of the ESC-EHRA ELECTRa Registry conducted with the aim of evaluating the clinical impact of AT on TLE safety and efficacy.
Methods
For this study, we used individual patients’ data from the ESC-EHRA ELECTRa Registry.7 The ELECTRa Registry included 73 centres from 19 European countries that enrolled 3555 consecutive patients, of whom 3510 underwent TLE. The executive committee, in collaboration with the EURObservational Research Programme (EORP) provided the study design, protocol, and scientific leadership of the registry under the responsibility of the EHRA Scientific Initiatives Committee (SIC). All EHRA affiliated centres in Europe performing TLE (irrespective of volume) were identified and invited to participate by EHRA and the regional coordinators. Participating centres were required to recruit all consecutive patients with an indication for TLE (excluding those patients primarily requiring surgical extraction) in their institution. No specific protocol or recommendations regarding technique were made for the TLE procedure. Data were prospectively collected using a secure web-based database system. Dedicated data monitors were used to ensure the integrity of the data and to ensure that all consecutive patients were included. The study design comprised a baseline visit at the time of admission for TLE and at the time of hospital discharge and a clinical evaluation at 1-year follow-up. In this investigation, we only included patients with data from both the baseline and the pre-discharge follow-up visit. A detailed description of the study design and of the electronic case report form has been previously described.8
Definitions and endpoints
Study subgroups
For the purpose of this study, we specifically performed a population sub-analysis focused on the AT therapy. According to the AT therapy assumed before TLE, four groups were identified:
No-AT Group: no AP or AC therapy.
AP Group: at least 1 AP drug, from Aspirin, Clopidogrel, Prasugrel, and Ticagrelor.
AC Group: at least 1 AC drug, from warfarin, low weight molecular heparin (LWMH), unfractionated heparin (UFH), and direct oral anticoagulants (DOAC).
AP/AC Group: a combination of at least one AP and one AC.
According to the study design, AT management before a TLE procedure was at the discretion of Operators. In the database, AP or AC interruption, related interruption timing and eventual AC bridging should be clearly indicated. AC patients were further analysed according to the pre-procedural management strategy, as ‘interrupted with bridging’, ‘interrupted without bridging’, and ‘continued’.
Definitions
Definitions published in the guidance documents by HRS6,9 and by EHRA10 were used to define procedural approaches, techniques, and outcomes. Transvenous lead extraction safety and efficacy were calculated by evaluating the rate of procedure-related complications (major and minor) and success/failure (radiological and clinical).
Major complications were defined as those related to the procedure that were life-threatening or resulted in death, or any unexpected event that caused persistent or significant disability, or any event that required significant surgical intervention to prevent any major outcomes6,9
Minor complications were defined as any undesired event related to the procedure that required medical intervention or minor procedural intervention to remedy, and did not limit persistently or significantly the patient’s function; nor did it threaten life or cause death.
Intra-procedural complications were defined as any event related to the performance of the procedure that occurred or became evident from the time the patient entered the operating room or catheterization laboratory until the time the patient left the operating room.
Post-procedural complications were defined as any other such event occurring after the procedure until patient discharge. All-cause in-hospital major complications including deaths were all major complications including deaths, irrespective of their classified relation to the procedure.7
Endpoints
The primary endpoint was major complications and deaths observed during the hospitalization, in the non-AT and AT subgroups. Predictors of major complications were also evaluated. Secondary endpoints included procedural success rates of TLE among non-AT and AT groups, as well as baseline patient and lead characteristics, indications for TLE, techniques, and tools used.
Statistical analysis
Univariate analysis was applied to both continuous and categorical variables. Results were summarized by AT therapy (No-AT vs. AP vs. AC vs. AP/AC). Continuous variables were reported as mean ± standard deviation (SD) or as median and interquartile range (IQR). Among-group comparisons were made using a non-parametric test (Kruskal–Wallis test). Categorical variables were reported as percentages (without missing values if applicable). Among-group comparisons were made using a χ2 test or the Fisher’s exact test (if any expected cell count was <5). A stepwise multiple Cox regression was used to determine the predictors of intra- and post-procedural major related complications (Model A) and all-cause mortality (Model B) including in the models all the candidate variables (variables with P < 0.05 in univariate, except those with a high number of missing data, and variables considered of relevant clinical interest). No interaction was tested. A two-sided P-value of 0.05 was considered as statistically significant. All the analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
From 1 November 2012 to 31 May 2014, 116 European centres across all regions of the European continent were invited to participate, of which 73 from 19 countries participated in the study. A total of 3555 consecutive patients were enrolled and 3510 (98.7%) underwent TLE.
Patient material
Among 3510 patients, 1112 (32%) were No-AT and 2398 (68%) were AT patients. Particularly, the number of AT patients, defined as AP, AC, or AP + AC, was 1096 (31.2%), 985 (28%), and 317 (9%), respectively. The overall cohort was 72.2% male, with a mean age of 64.8 ± 15.6 years. Comorbidities present were as follows: hypertension 54%, coronary artery disease 40%, primary electrical disease 27%, dilated cardiomyopathy 26%, diabetes 22%, chronic kidney disease 18%, and COPD 9%. Baseline characteristics were significantly different among groups (Table 1).
Comparison of baseline characteristics between patients not treated with AT, treated with AP, with AC and both AP + AC
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Characteristics, n/N (%) | ||||||
| Age (years) | 64.9 ± 15.6 | 58.7 ± 19.5 | 68.3 ± 11.4 | 68.9 ± 12.3 | 68.9 ± 11.0 | <0.0001 |
| Male gender | 2539/3510 (72) | 704/1112 (63) | 883/1096 (81) | 684/985 (69) | 268/317 (85) | <0.0001 |
| BMI (kg/m2), mean ± SD | 26.6 ± 4.8) | 25.6 ± 4.5 | 27.4 ± 4.8 | 27.0 ± 4.8 | 26.7 ± 4.8 | <0.0001 |
| Comorbidities, n/N (%) | ||||||
| Hypertension | 1888/3478 (54) | 387/1107 (35) | 711/1081 (66) | 584/976 (60) | 206/314 (66) | <0.0001 |
| Coronary artery disease | 1375/3482 (40) | 152/1104 (14) | 658/1088 (61) | 320/974 (33) | 245/316 (78) | <0.0001 |
| Primary electrical disease | 950/3483 (27) | 411/1102 (37) | 240/1088 (22) | 243/978 (25) | 56/315 (18) | <0.0001 |
| Dilated cardiomyopathy | 917/3492 (26) | 197/1109 (18) | 320/1090 (29) | 297/977 (30) | 103/316 (33) | <0.0001 |
| Hypertrophic cardiomyopathy | 158/3502 (5) | 70/1109 (6) | 27/1094 (3) | 50/983 (5) | 11/316 (4) | 0.0001 |
| Valvular heart disease | 514/3500 (15) | 75/1109 (6.8) | 98/1093 (9) | 266/981 (27) | 75/317 (24) | <0.0001 |
| Chronic heart failure | 1557/3488 (45) | 243/1103 (22) | 584/1090 (54) | 528/979 (54) | 202/316 (64) | <0.0001 |
| NYHA, Class III/IV | 486/3472 (14) | 72/1101 (6) | 147/1086 (13) | 188/975 (19) | 79/310 (25) | <0.0001 |
| LVEF (%), mean ± SD | 45.5 ± 14.7 | 52.5 ± 12.9 | 42.9 ± 14.2 | 43.4 ± 14.2 | 38.3 ± 14.1 | <0.0001 |
| Diabetes mellitus | 781/3487 (22) | 156/1108 (14) | 285/1084 (26) | 233/980 (24) | 107/315 (34) | <0.0001 |
| Chronic kidney disease | 613/3493 (18) | 89/1108 (8) | 219/1086 (20) | 221/983 (22) | 84/316 (27) | <0.0001 |
| COPD | 297/3483 (9) | 54/1108 (5) | 104/1080 (10) | 100/980 (10) | 39/315 (12) | <0.0001 |
| Medical therapy, n/N (%) | ||||||
| Antipatelets | ||||||
| ASA | 1288/1413 (91) | NA | 1004/1096 (92) | NA | 284/317 (90) | 0.2656 |
| Clopidogrel | 204/1413 (14) | NA | 153/1096 (14) | NA | 51/317 (16) | 0.3423 |
| Prasugrel | 12/1413 (0.85) | NA | 10/1096 (0.91) | NA | 2/317 (0.63) | 0.6305 |
| Ticagrelor | 7/1413 (0.50) | NA | 6/1096 (0.55) | NA | 1/317 (0.32) | 0.6043 |
| Dual antiplatelets | 121/1413 (8.5) | NA | 97/1096 (8.8) | NA | 24/317 (7.56) | 0.5137 |
| Antiplatelets interruption | 371/1413 (26) | NA | 290/1096 (27) | NA | 81/317 (26) | 0.7463 |
| Interruption of AP (days) | 3.8 ± 3.7 | NA | 3.9 ± 3.7 | NA | 3.7 ± 3.7 | 0.6383 |
| Anticoagulants | ||||||
| Treatments | 0.0028 | |||||
| VKA | 881/1302 (68) | NA | NA | 690/985 (70) | 191/317 (60) | |
| LMWH | 155/1302 (12) | NA | NA | 103/985 (11) | 52/317 (16) | |
| UFH | 45/1302 (4) | NA | NA | 29/985 (3) | 16/317 (5) | |
| DOAC | 221/1302 (17) | NA | NA | 163/985 (17) | 58/317 (18) | |
| Interruption | 0.8493 | |||||
| Interrupted AC without bridging | 696/1287 (54) | NA | NA | 523/985 (53.1) | 173/317 (54.6) | |
| Interrupted AC with bridging | 504/1287 (39) | NA | NA | 385/985 (39) | 119/317 (37.5) | |
| Continued AC | 87/1287 (7) | NA | NA | 67/985 (6.8) | 20/317 (6.3) | |
| Interruption of AC (days) | 3.3 ± 2.3 | NA | NA | 3.3 ± 2.2 | 3.28 ± 2.6 | 0.4890 |
| Other therapy | ||||||
| Antibiotics | 1587/3510 (45) | 459/1112 (41) | 474/1096 (43) | 493/985 (50) | 161/317 (51) | <0.0001 |
| Digoxin | 277/2842 (10) | 35/617 (6) | 47/1000 (5) | 156/920 (17) | 39/305 (13) | <0.0001 |
| Diuretics | 1648/2842 (58) | 246/617 (40) | 574/1000 (57) | 615/920 (67) | 213/305 (70) | <0.0001 |
| Ace/ATII-inhibitors | 1981/2842 (70) | 364/617 (59) | 781/1000 (78) | 628/920 (68) | 208/305 (68) | <0.0001 |
| Calcium antagonists | 360/2842 (13) | 68/617 (11) | 147/1000 (15) | 108/920 (12) | 37/305 (12) | 0.1095 |
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Characteristics, n/N (%) | ||||||
| Age (years) | 64.9 ± 15.6 | 58.7 ± 19.5 | 68.3 ± 11.4 | 68.9 ± 12.3 | 68.9 ± 11.0 | <0.0001 |
| Male gender | 2539/3510 (72) | 704/1112 (63) | 883/1096 (81) | 684/985 (69) | 268/317 (85) | <0.0001 |
| BMI (kg/m2), mean ± SD | 26.6 ± 4.8) | 25.6 ± 4.5 | 27.4 ± 4.8 | 27.0 ± 4.8 | 26.7 ± 4.8 | <0.0001 |
| Comorbidities, n/N (%) | ||||||
| Hypertension | 1888/3478 (54) | 387/1107 (35) | 711/1081 (66) | 584/976 (60) | 206/314 (66) | <0.0001 |
| Coronary artery disease | 1375/3482 (40) | 152/1104 (14) | 658/1088 (61) | 320/974 (33) | 245/316 (78) | <0.0001 |
| Primary electrical disease | 950/3483 (27) | 411/1102 (37) | 240/1088 (22) | 243/978 (25) | 56/315 (18) | <0.0001 |
| Dilated cardiomyopathy | 917/3492 (26) | 197/1109 (18) | 320/1090 (29) | 297/977 (30) | 103/316 (33) | <0.0001 |
| Hypertrophic cardiomyopathy | 158/3502 (5) | 70/1109 (6) | 27/1094 (3) | 50/983 (5) | 11/316 (4) | 0.0001 |
| Valvular heart disease | 514/3500 (15) | 75/1109 (6.8) | 98/1093 (9) | 266/981 (27) | 75/317 (24) | <0.0001 |
| Chronic heart failure | 1557/3488 (45) | 243/1103 (22) | 584/1090 (54) | 528/979 (54) | 202/316 (64) | <0.0001 |
| NYHA, Class III/IV | 486/3472 (14) | 72/1101 (6) | 147/1086 (13) | 188/975 (19) | 79/310 (25) | <0.0001 |
| LVEF (%), mean ± SD | 45.5 ± 14.7 | 52.5 ± 12.9 | 42.9 ± 14.2 | 43.4 ± 14.2 | 38.3 ± 14.1 | <0.0001 |
| Diabetes mellitus | 781/3487 (22) | 156/1108 (14) | 285/1084 (26) | 233/980 (24) | 107/315 (34) | <0.0001 |
| Chronic kidney disease | 613/3493 (18) | 89/1108 (8) | 219/1086 (20) | 221/983 (22) | 84/316 (27) | <0.0001 |
| COPD | 297/3483 (9) | 54/1108 (5) | 104/1080 (10) | 100/980 (10) | 39/315 (12) | <0.0001 |
| Medical therapy, n/N (%) | ||||||
| Antipatelets | ||||||
| ASA | 1288/1413 (91) | NA | 1004/1096 (92) | NA | 284/317 (90) | 0.2656 |
| Clopidogrel | 204/1413 (14) | NA | 153/1096 (14) | NA | 51/317 (16) | 0.3423 |
| Prasugrel | 12/1413 (0.85) | NA | 10/1096 (0.91) | NA | 2/317 (0.63) | 0.6305 |
| Ticagrelor | 7/1413 (0.50) | NA | 6/1096 (0.55) | NA | 1/317 (0.32) | 0.6043 |
| Dual antiplatelets | 121/1413 (8.5) | NA | 97/1096 (8.8) | NA | 24/317 (7.56) | 0.5137 |
| Antiplatelets interruption | 371/1413 (26) | NA | 290/1096 (27) | NA | 81/317 (26) | 0.7463 |
| Interruption of AP (days) | 3.8 ± 3.7 | NA | 3.9 ± 3.7 | NA | 3.7 ± 3.7 | 0.6383 |
| Anticoagulants | ||||||
| Treatments | 0.0028 | |||||
| VKA | 881/1302 (68) | NA | NA | 690/985 (70) | 191/317 (60) | |
| LMWH | 155/1302 (12) | NA | NA | 103/985 (11) | 52/317 (16) | |
| UFH | 45/1302 (4) | NA | NA | 29/985 (3) | 16/317 (5) | |
| DOAC | 221/1302 (17) | NA | NA | 163/985 (17) | 58/317 (18) | |
| Interruption | 0.8493 | |||||
| Interrupted AC without bridging | 696/1287 (54) | NA | NA | 523/985 (53.1) | 173/317 (54.6) | |
| Interrupted AC with bridging | 504/1287 (39) | NA | NA | 385/985 (39) | 119/317 (37.5) | |
| Continued AC | 87/1287 (7) | NA | NA | 67/985 (6.8) | 20/317 (6.3) | |
| Interruption of AC (days) | 3.3 ± 2.3 | NA | NA | 3.3 ± 2.2 | 3.28 ± 2.6 | 0.4890 |
| Other therapy | ||||||
| Antibiotics | 1587/3510 (45) | 459/1112 (41) | 474/1096 (43) | 493/985 (50) | 161/317 (51) | <0.0001 |
| Digoxin | 277/2842 (10) | 35/617 (6) | 47/1000 (5) | 156/920 (17) | 39/305 (13) | <0.0001 |
| Diuretics | 1648/2842 (58) | 246/617 (40) | 574/1000 (57) | 615/920 (67) | 213/305 (70) | <0.0001 |
| Ace/ATII-inhibitors | 1981/2842 (70) | 364/617 (59) | 781/1000 (78) | 628/920 (68) | 208/305 (68) | <0.0001 |
| Calcium antagonists | 360/2842 (13) | 68/617 (11) | 147/1000 (15) | 108/920 (12) | 37/305 (12) | 0.1095 |
Patients characteristics were calculated on the population of 3555 consecutive patients enrolled. Leads characteristics were calculated on the population of 3510 consecutive patients enrolled who underwent the intervention (TLE). In the calculations all values unknown were excluded.
AC, anticoagulants; AP, antiplatelets; ASA, acetyl salicylic acid; AT, antithrombotic; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; LVEF, left ventricular ejection fraction; LWMH, low molecular weight heparin; NYHA, New York Heart Association; UFH, unfractionated heparin; VKA, vitamin K antagonists.
Comparison of baseline characteristics between patients not treated with AT, treated with AP, with AC and both AP + AC
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Characteristics, n/N (%) | ||||||
| Age (years) | 64.9 ± 15.6 | 58.7 ± 19.5 | 68.3 ± 11.4 | 68.9 ± 12.3 | 68.9 ± 11.0 | <0.0001 |
| Male gender | 2539/3510 (72) | 704/1112 (63) | 883/1096 (81) | 684/985 (69) | 268/317 (85) | <0.0001 |
| BMI (kg/m2), mean ± SD | 26.6 ± 4.8) | 25.6 ± 4.5 | 27.4 ± 4.8 | 27.0 ± 4.8 | 26.7 ± 4.8 | <0.0001 |
| Comorbidities, n/N (%) | ||||||
| Hypertension | 1888/3478 (54) | 387/1107 (35) | 711/1081 (66) | 584/976 (60) | 206/314 (66) | <0.0001 |
| Coronary artery disease | 1375/3482 (40) | 152/1104 (14) | 658/1088 (61) | 320/974 (33) | 245/316 (78) | <0.0001 |
| Primary electrical disease | 950/3483 (27) | 411/1102 (37) | 240/1088 (22) | 243/978 (25) | 56/315 (18) | <0.0001 |
| Dilated cardiomyopathy | 917/3492 (26) | 197/1109 (18) | 320/1090 (29) | 297/977 (30) | 103/316 (33) | <0.0001 |
| Hypertrophic cardiomyopathy | 158/3502 (5) | 70/1109 (6) | 27/1094 (3) | 50/983 (5) | 11/316 (4) | 0.0001 |
| Valvular heart disease | 514/3500 (15) | 75/1109 (6.8) | 98/1093 (9) | 266/981 (27) | 75/317 (24) | <0.0001 |
| Chronic heart failure | 1557/3488 (45) | 243/1103 (22) | 584/1090 (54) | 528/979 (54) | 202/316 (64) | <0.0001 |
| NYHA, Class III/IV | 486/3472 (14) | 72/1101 (6) | 147/1086 (13) | 188/975 (19) | 79/310 (25) | <0.0001 |
| LVEF (%), mean ± SD | 45.5 ± 14.7 | 52.5 ± 12.9 | 42.9 ± 14.2 | 43.4 ± 14.2 | 38.3 ± 14.1 | <0.0001 |
| Diabetes mellitus | 781/3487 (22) | 156/1108 (14) | 285/1084 (26) | 233/980 (24) | 107/315 (34) | <0.0001 |
| Chronic kidney disease | 613/3493 (18) | 89/1108 (8) | 219/1086 (20) | 221/983 (22) | 84/316 (27) | <0.0001 |
| COPD | 297/3483 (9) | 54/1108 (5) | 104/1080 (10) | 100/980 (10) | 39/315 (12) | <0.0001 |
| Medical therapy, n/N (%) | ||||||
| Antipatelets | ||||||
| ASA | 1288/1413 (91) | NA | 1004/1096 (92) | NA | 284/317 (90) | 0.2656 |
| Clopidogrel | 204/1413 (14) | NA | 153/1096 (14) | NA | 51/317 (16) | 0.3423 |
| Prasugrel | 12/1413 (0.85) | NA | 10/1096 (0.91) | NA | 2/317 (0.63) | 0.6305 |
| Ticagrelor | 7/1413 (0.50) | NA | 6/1096 (0.55) | NA | 1/317 (0.32) | 0.6043 |
| Dual antiplatelets | 121/1413 (8.5) | NA | 97/1096 (8.8) | NA | 24/317 (7.56) | 0.5137 |
| Antiplatelets interruption | 371/1413 (26) | NA | 290/1096 (27) | NA | 81/317 (26) | 0.7463 |
| Interruption of AP (days) | 3.8 ± 3.7 | NA | 3.9 ± 3.7 | NA | 3.7 ± 3.7 | 0.6383 |
| Anticoagulants | ||||||
| Treatments | 0.0028 | |||||
| VKA | 881/1302 (68) | NA | NA | 690/985 (70) | 191/317 (60) | |
| LMWH | 155/1302 (12) | NA | NA | 103/985 (11) | 52/317 (16) | |
| UFH | 45/1302 (4) | NA | NA | 29/985 (3) | 16/317 (5) | |
| DOAC | 221/1302 (17) | NA | NA | 163/985 (17) | 58/317 (18) | |
| Interruption | 0.8493 | |||||
| Interrupted AC without bridging | 696/1287 (54) | NA | NA | 523/985 (53.1) | 173/317 (54.6) | |
| Interrupted AC with bridging | 504/1287 (39) | NA | NA | 385/985 (39) | 119/317 (37.5) | |
| Continued AC | 87/1287 (7) | NA | NA | 67/985 (6.8) | 20/317 (6.3) | |
| Interruption of AC (days) | 3.3 ± 2.3 | NA | NA | 3.3 ± 2.2 | 3.28 ± 2.6 | 0.4890 |
| Other therapy | ||||||
| Antibiotics | 1587/3510 (45) | 459/1112 (41) | 474/1096 (43) | 493/985 (50) | 161/317 (51) | <0.0001 |
| Digoxin | 277/2842 (10) | 35/617 (6) | 47/1000 (5) | 156/920 (17) | 39/305 (13) | <0.0001 |
| Diuretics | 1648/2842 (58) | 246/617 (40) | 574/1000 (57) | 615/920 (67) | 213/305 (70) | <0.0001 |
| Ace/ATII-inhibitors | 1981/2842 (70) | 364/617 (59) | 781/1000 (78) | 628/920 (68) | 208/305 (68) | <0.0001 |
| Calcium antagonists | 360/2842 (13) | 68/617 (11) | 147/1000 (15) | 108/920 (12) | 37/305 (12) | 0.1095 |
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Characteristics, n/N (%) | ||||||
| Age (years) | 64.9 ± 15.6 | 58.7 ± 19.5 | 68.3 ± 11.4 | 68.9 ± 12.3 | 68.9 ± 11.0 | <0.0001 |
| Male gender | 2539/3510 (72) | 704/1112 (63) | 883/1096 (81) | 684/985 (69) | 268/317 (85) | <0.0001 |
| BMI (kg/m2), mean ± SD | 26.6 ± 4.8) | 25.6 ± 4.5 | 27.4 ± 4.8 | 27.0 ± 4.8 | 26.7 ± 4.8 | <0.0001 |
| Comorbidities, n/N (%) | ||||||
| Hypertension | 1888/3478 (54) | 387/1107 (35) | 711/1081 (66) | 584/976 (60) | 206/314 (66) | <0.0001 |
| Coronary artery disease | 1375/3482 (40) | 152/1104 (14) | 658/1088 (61) | 320/974 (33) | 245/316 (78) | <0.0001 |
| Primary electrical disease | 950/3483 (27) | 411/1102 (37) | 240/1088 (22) | 243/978 (25) | 56/315 (18) | <0.0001 |
| Dilated cardiomyopathy | 917/3492 (26) | 197/1109 (18) | 320/1090 (29) | 297/977 (30) | 103/316 (33) | <0.0001 |
| Hypertrophic cardiomyopathy | 158/3502 (5) | 70/1109 (6) | 27/1094 (3) | 50/983 (5) | 11/316 (4) | 0.0001 |
| Valvular heart disease | 514/3500 (15) | 75/1109 (6.8) | 98/1093 (9) | 266/981 (27) | 75/317 (24) | <0.0001 |
| Chronic heart failure | 1557/3488 (45) | 243/1103 (22) | 584/1090 (54) | 528/979 (54) | 202/316 (64) | <0.0001 |
| NYHA, Class III/IV | 486/3472 (14) | 72/1101 (6) | 147/1086 (13) | 188/975 (19) | 79/310 (25) | <0.0001 |
| LVEF (%), mean ± SD | 45.5 ± 14.7 | 52.5 ± 12.9 | 42.9 ± 14.2 | 43.4 ± 14.2 | 38.3 ± 14.1 | <0.0001 |
| Diabetes mellitus | 781/3487 (22) | 156/1108 (14) | 285/1084 (26) | 233/980 (24) | 107/315 (34) | <0.0001 |
| Chronic kidney disease | 613/3493 (18) | 89/1108 (8) | 219/1086 (20) | 221/983 (22) | 84/316 (27) | <0.0001 |
| COPD | 297/3483 (9) | 54/1108 (5) | 104/1080 (10) | 100/980 (10) | 39/315 (12) | <0.0001 |
| Medical therapy, n/N (%) | ||||||
| Antipatelets | ||||||
| ASA | 1288/1413 (91) | NA | 1004/1096 (92) | NA | 284/317 (90) | 0.2656 |
| Clopidogrel | 204/1413 (14) | NA | 153/1096 (14) | NA | 51/317 (16) | 0.3423 |
| Prasugrel | 12/1413 (0.85) | NA | 10/1096 (0.91) | NA | 2/317 (0.63) | 0.6305 |
| Ticagrelor | 7/1413 (0.50) | NA | 6/1096 (0.55) | NA | 1/317 (0.32) | 0.6043 |
| Dual antiplatelets | 121/1413 (8.5) | NA | 97/1096 (8.8) | NA | 24/317 (7.56) | 0.5137 |
| Antiplatelets interruption | 371/1413 (26) | NA | 290/1096 (27) | NA | 81/317 (26) | 0.7463 |
| Interruption of AP (days) | 3.8 ± 3.7 | NA | 3.9 ± 3.7 | NA | 3.7 ± 3.7 | 0.6383 |
| Anticoagulants | ||||||
| Treatments | 0.0028 | |||||
| VKA | 881/1302 (68) | NA | NA | 690/985 (70) | 191/317 (60) | |
| LMWH | 155/1302 (12) | NA | NA | 103/985 (11) | 52/317 (16) | |
| UFH | 45/1302 (4) | NA | NA | 29/985 (3) | 16/317 (5) | |
| DOAC | 221/1302 (17) | NA | NA | 163/985 (17) | 58/317 (18) | |
| Interruption | 0.8493 | |||||
| Interrupted AC without bridging | 696/1287 (54) | NA | NA | 523/985 (53.1) | 173/317 (54.6) | |
| Interrupted AC with bridging | 504/1287 (39) | NA | NA | 385/985 (39) | 119/317 (37.5) | |
| Continued AC | 87/1287 (7) | NA | NA | 67/985 (6.8) | 20/317 (6.3) | |
| Interruption of AC (days) | 3.3 ± 2.3 | NA | NA | 3.3 ± 2.2 | 3.28 ± 2.6 | 0.4890 |
| Other therapy | ||||||
| Antibiotics | 1587/3510 (45) | 459/1112 (41) | 474/1096 (43) | 493/985 (50) | 161/317 (51) | <0.0001 |
| Digoxin | 277/2842 (10) | 35/617 (6) | 47/1000 (5) | 156/920 (17) | 39/305 (13) | <0.0001 |
| Diuretics | 1648/2842 (58) | 246/617 (40) | 574/1000 (57) | 615/920 (67) | 213/305 (70) | <0.0001 |
| Ace/ATII-inhibitors | 1981/2842 (70) | 364/617 (59) | 781/1000 (78) | 628/920 (68) | 208/305 (68) | <0.0001 |
| Calcium antagonists | 360/2842 (13) | 68/617 (11) | 147/1000 (15) | 108/920 (12) | 37/305 (12) | 0.1095 |
Patients characteristics were calculated on the population of 3555 consecutive patients enrolled. Leads characteristics were calculated on the population of 3510 consecutive patients enrolled who underwent the intervention (TLE). In the calculations all values unknown were excluded.
AC, anticoagulants; AP, antiplatelets; ASA, acetyl salicylic acid; AT, antithrombotic; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; LVEF, left ventricular ejection fraction; LWMH, low molecular weight heparin; NYHA, New York Heart Association; UFH, unfractionated heparin; VKA, vitamin K antagonists.
In particularly, AT patient subgroups (AP, AC, and AP + AC) were significantly older, with a higher prevalence of cardiac disease, heart failure, and comorbidities, as shown in Table 1.
Regarding AP, 1413 (40%) patients were under AP, 1292 (91%) with a single AP and 121 (9%) with a dual AP therapy. Among AP patients, 1288 (91%) were under acetyl salicylic acid, 204 (14%) under Clopidogrel, 12 (0.8%) under Prasugrel, and 7 (0.5%) under Ticagrelor. Antiplatelet was ‘continued’ in 1042 (74%) and ‘interrupted’ in 371 (26%) pts about 3.8 ± 3.7 days before the procedure (Figure 1).
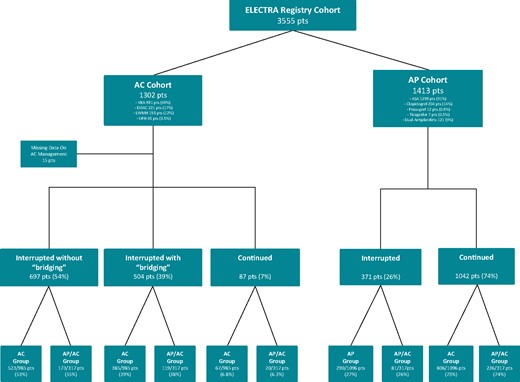
ELECTRa registry patient flow diagram presenting the proportion of patients of the total enrolled patient cohort of the registry under chronic anticoagulation (AC) and antiplatelet (AP) therapy. Details are provided on the AC and AP agent chronically prescribed as well as the pre-operative strategies. ASA, acetyl salicylic acid; DOAC, direct oral anticoagulants; LMWH, low-molecular weight heparin; VKA, vitamin K antagonist.
Regarding AC, 1302 (37%) patients were under AC. Among AC patients, 881 were under vitamin K antagonist (68%), 221 (17%) under DOAC, 155 (12%) under LWMH, and 45 (3.5%) under UFH. AC pre-procedural management strategy included ‘interruption without bridging’ in 696 (54%), ‘interruption with bridging’ in 504 (39%) and a ‘continued’ strategy in 87 (7%) (Figure 1). AC was interrupted about 3.3 ± 2.3 days before TLE. The last dose of LWMH was usually administered the night before.
Patient demographics are shown in Table 1.
Cardiac implantable electronic device data and procedural data
Regarding devices, 52.9% of patients had a PM and 47.1% an ICD. A CRT device was found in 20.6% of patients. AT subgroups (AP, AC, and AP + AC) showed a high prevalence of CRT-D devices (P < 0.0001). Regarding procedural indications, in the overall population infection was the most frequent indication (53%). Infection and thrombosis were statistically more frequent in the AT subgroups (P = 0.0005 and P = 0.0096, respectively), whereas chronic pain and recalled leads were more frequent in the No-AT group (P = 0.0354 and P = 0.0002, respectively). Cardiac implantable electronic device characteristics and TLE indications are reported in Table 2.
Comparison of CIED, lead characteristics, and procedural indications between patients not treated with AT, treated with AP, with AC, and both AP + AC
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Device type, n/N (%) | ||||||
| CRT-pacemaker | 127/3510 (3.6) | 37/1112 (3.3) | 32/1096 (2.9) | 47/985 (4.8) | 11/317 (3.5) | 0.1339 |
| CRT-defibrillator | 607/3510 (17) | 104/1112 (9.3) | 233/1096 (21) | 189/985 (19) | 81/317 (26) | <0.0001 |
| Lead type extracted, n/N (%)a | ||||||
| PM | 4584/6493 (71) | 1547/1987 (78) | 1339/2056 (65) | 1322/1843 (72) | 376/607 (62) | <0.0001 |
| ICD | 1576/6493 (24) | 388/1987 (20) | 613/2056 (30) | 392/1843 (21) | 183/607 (30) | |
| LV | 333/6493 (5) | 52/1987 (3) | 104/2056 (5) | 129/1843 (7) | 48/607 (8) | |
| Lead extracted tip location, n/N (%)a | ||||||
| Right atrium | 2219/6493 (34) | 708/1987 (36) | 707/2056 (34) | 598/1843 (32) | 206/607 (34) | <0.0001 |
| Right ventricle | 3587/6493 (55) | 1125/1987 (57) | 1130/2056 (55) | 1016/1843 (55) | 316/607 (52) | |
| Coronary sinus | 547/6493 (8) | 89/1987 (5) | 185/2056 (9) | 197/1843 (11) | 76/607 (13) | |
| Other | 140/6493 (2) | 65/1987 (3) | 34/2056 (2) | 32/1843 (2) | 9/607 (2) | |
| Lead dwelling time (years) | 6.4 ± 5.4 | 7.2 ± 5.8 | 5.8 ± 5.1 | 6.6 ± 5.5 | 5.3 ± 4.4 | |
| Indication for TLE, n/N (%) | ||||||
| Infections | 1865/3499 (53) | 550/1110 (50) | 570/1092 (52) | 553/981 (56) | 192/316 (61) | 0.0005 |
| Chronic pain | 180/3510 (5.1) | 74/1112 (6.7) | 53/1096 (4.9) | 41/985 (4.2) | 12/317 (3.8) | 0.0354 |
| Thrombosis or venous stenosis | 160/3510 (4.6) | 34/1112 (3.1) | 49/1096 (4.5) | 57/985 (5.8) | 20/317 (6.3) | 0.0096 |
| Functional leads | 2023/3510 (58) | 620/1112 (56) | 634/1096 (58) | 593/985 (60) | 176/317 (56) | 0.1815 |
| Non-functional leads | 1331/3510 (38) | 448/1112 (40) | 443/1096 (40) | 341/985 (35) | 99/317 (31) | 0.0010 |
| Recalled leads (updated) | 440/3510 (13) | 139/1112 (13) | 173/1096 (16) | 94/985 (10) | 34/317 (11) | 0.0002 |
| Upgrading indication | 248/3510 (7) | 43/1112 (4) | 78/1096 (7) | 98/985 (10) | 29/317 (9) | <0.0001 |
| MRI indication | 26/3510 (0.7) | 15/1112 (1.3) | 6/1096 (0.5) | 4/985 (0.4) | 1/317 (0.3) | 0.0378 |
| Other | 54/3510 (1.5) | 22/1112 (2.0) | 10/1096 (0.9) | 16/985 (1.6) | 6/317 (1.9) | 0.2063 |
| Previous attempt of lead extraction | 171/3510 (5) | 67/1112 (6) | 48/1096 (4) | 42/985 (4) | 14/317 (4) | 0.1957 |
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Device type, n/N (%) | ||||||
| CRT-pacemaker | 127/3510 (3.6) | 37/1112 (3.3) | 32/1096 (2.9) | 47/985 (4.8) | 11/317 (3.5) | 0.1339 |
| CRT-defibrillator | 607/3510 (17) | 104/1112 (9.3) | 233/1096 (21) | 189/985 (19) | 81/317 (26) | <0.0001 |
| Lead type extracted, n/N (%)a | ||||||
| PM | 4584/6493 (71) | 1547/1987 (78) | 1339/2056 (65) | 1322/1843 (72) | 376/607 (62) | <0.0001 |
| ICD | 1576/6493 (24) | 388/1987 (20) | 613/2056 (30) | 392/1843 (21) | 183/607 (30) | |
| LV | 333/6493 (5) | 52/1987 (3) | 104/2056 (5) | 129/1843 (7) | 48/607 (8) | |
| Lead extracted tip location, n/N (%)a | ||||||
| Right atrium | 2219/6493 (34) | 708/1987 (36) | 707/2056 (34) | 598/1843 (32) | 206/607 (34) | <0.0001 |
| Right ventricle | 3587/6493 (55) | 1125/1987 (57) | 1130/2056 (55) | 1016/1843 (55) | 316/607 (52) | |
| Coronary sinus | 547/6493 (8) | 89/1987 (5) | 185/2056 (9) | 197/1843 (11) | 76/607 (13) | |
| Other | 140/6493 (2) | 65/1987 (3) | 34/2056 (2) | 32/1843 (2) | 9/607 (2) | |
| Lead dwelling time (years) | 6.4 ± 5.4 | 7.2 ± 5.8 | 5.8 ± 5.1 | 6.6 ± 5.5 | 5.3 ± 4.4 | |
| Indication for TLE, n/N (%) | ||||||
| Infections | 1865/3499 (53) | 550/1110 (50) | 570/1092 (52) | 553/981 (56) | 192/316 (61) | 0.0005 |
| Chronic pain | 180/3510 (5.1) | 74/1112 (6.7) | 53/1096 (4.9) | 41/985 (4.2) | 12/317 (3.8) | 0.0354 |
| Thrombosis or venous stenosis | 160/3510 (4.6) | 34/1112 (3.1) | 49/1096 (4.5) | 57/985 (5.8) | 20/317 (6.3) | 0.0096 |
| Functional leads | 2023/3510 (58) | 620/1112 (56) | 634/1096 (58) | 593/985 (60) | 176/317 (56) | 0.1815 |
| Non-functional leads | 1331/3510 (38) | 448/1112 (40) | 443/1096 (40) | 341/985 (35) | 99/317 (31) | 0.0010 |
| Recalled leads (updated) | 440/3510 (13) | 139/1112 (13) | 173/1096 (16) | 94/985 (10) | 34/317 (11) | 0.0002 |
| Upgrading indication | 248/3510 (7) | 43/1112 (4) | 78/1096 (7) | 98/985 (10) | 29/317 (9) | <0.0001 |
| MRI indication | 26/3510 (0.7) | 15/1112 (1.3) | 6/1096 (0.5) | 4/985 (0.4) | 1/317 (0.3) | 0.0378 |
| Other | 54/3510 (1.5) | 22/1112 (2.0) | 10/1096 (0.9) | 16/985 (1.6) | 6/317 (1.9) | 0.2063 |
| Previous attempt of lead extraction | 171/3510 (5) | 67/1112 (6) | 48/1096 (4) | 42/985 (4) | 14/317 (4) | 0.1957 |
AC, anticoagulants; AP, antiplatelets; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; MRI, magnetic resonance imaging; PM, pacemaker; TLE, transvenous lead extraction.
Data are expressed per leads.
Comparison of CIED, lead characteristics, and procedural indications between patients not treated with AT, treated with AP, with AC, and both AP + AC
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Device type, n/N (%) | ||||||
| CRT-pacemaker | 127/3510 (3.6) | 37/1112 (3.3) | 32/1096 (2.9) | 47/985 (4.8) | 11/317 (3.5) | 0.1339 |
| CRT-defibrillator | 607/3510 (17) | 104/1112 (9.3) | 233/1096 (21) | 189/985 (19) | 81/317 (26) | <0.0001 |
| Lead type extracted, n/N (%)a | ||||||
| PM | 4584/6493 (71) | 1547/1987 (78) | 1339/2056 (65) | 1322/1843 (72) | 376/607 (62) | <0.0001 |
| ICD | 1576/6493 (24) | 388/1987 (20) | 613/2056 (30) | 392/1843 (21) | 183/607 (30) | |
| LV | 333/6493 (5) | 52/1987 (3) | 104/2056 (5) | 129/1843 (7) | 48/607 (8) | |
| Lead extracted tip location, n/N (%)a | ||||||
| Right atrium | 2219/6493 (34) | 708/1987 (36) | 707/2056 (34) | 598/1843 (32) | 206/607 (34) | <0.0001 |
| Right ventricle | 3587/6493 (55) | 1125/1987 (57) | 1130/2056 (55) | 1016/1843 (55) | 316/607 (52) | |
| Coronary sinus | 547/6493 (8) | 89/1987 (5) | 185/2056 (9) | 197/1843 (11) | 76/607 (13) | |
| Other | 140/6493 (2) | 65/1987 (3) | 34/2056 (2) | 32/1843 (2) | 9/607 (2) | |
| Lead dwelling time (years) | 6.4 ± 5.4 | 7.2 ± 5.8 | 5.8 ± 5.1 | 6.6 ± 5.5 | 5.3 ± 4.4 | |
| Indication for TLE, n/N (%) | ||||||
| Infections | 1865/3499 (53) | 550/1110 (50) | 570/1092 (52) | 553/981 (56) | 192/316 (61) | 0.0005 |
| Chronic pain | 180/3510 (5.1) | 74/1112 (6.7) | 53/1096 (4.9) | 41/985 (4.2) | 12/317 (3.8) | 0.0354 |
| Thrombosis or venous stenosis | 160/3510 (4.6) | 34/1112 (3.1) | 49/1096 (4.5) | 57/985 (5.8) | 20/317 (6.3) | 0.0096 |
| Functional leads | 2023/3510 (58) | 620/1112 (56) | 634/1096 (58) | 593/985 (60) | 176/317 (56) | 0.1815 |
| Non-functional leads | 1331/3510 (38) | 448/1112 (40) | 443/1096 (40) | 341/985 (35) | 99/317 (31) | 0.0010 |
| Recalled leads (updated) | 440/3510 (13) | 139/1112 (13) | 173/1096 (16) | 94/985 (10) | 34/317 (11) | 0.0002 |
| Upgrading indication | 248/3510 (7) | 43/1112 (4) | 78/1096 (7) | 98/985 (10) | 29/317 (9) | <0.0001 |
| MRI indication | 26/3510 (0.7) | 15/1112 (1.3) | 6/1096 (0.5) | 4/985 (0.4) | 1/317 (0.3) | 0.0378 |
| Other | 54/3510 (1.5) | 22/1112 (2.0) | 10/1096 (0.9) | 16/985 (1.6) | 6/317 (1.9) | 0.2063 |
| Previous attempt of lead extraction | 171/3510 (5) | 67/1112 (6) | 48/1096 (4) | 42/985 (4) | 14/317 (4) | 0.1957 |
| Variable . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| Device type, n/N (%) | ||||||
| CRT-pacemaker | 127/3510 (3.6) | 37/1112 (3.3) | 32/1096 (2.9) | 47/985 (4.8) | 11/317 (3.5) | 0.1339 |
| CRT-defibrillator | 607/3510 (17) | 104/1112 (9.3) | 233/1096 (21) | 189/985 (19) | 81/317 (26) | <0.0001 |
| Lead type extracted, n/N (%)a | ||||||
| PM | 4584/6493 (71) | 1547/1987 (78) | 1339/2056 (65) | 1322/1843 (72) | 376/607 (62) | <0.0001 |
| ICD | 1576/6493 (24) | 388/1987 (20) | 613/2056 (30) | 392/1843 (21) | 183/607 (30) | |
| LV | 333/6493 (5) | 52/1987 (3) | 104/2056 (5) | 129/1843 (7) | 48/607 (8) | |
| Lead extracted tip location, n/N (%)a | ||||||
| Right atrium | 2219/6493 (34) | 708/1987 (36) | 707/2056 (34) | 598/1843 (32) | 206/607 (34) | <0.0001 |
| Right ventricle | 3587/6493 (55) | 1125/1987 (57) | 1130/2056 (55) | 1016/1843 (55) | 316/607 (52) | |
| Coronary sinus | 547/6493 (8) | 89/1987 (5) | 185/2056 (9) | 197/1843 (11) | 76/607 (13) | |
| Other | 140/6493 (2) | 65/1987 (3) | 34/2056 (2) | 32/1843 (2) | 9/607 (2) | |
| Lead dwelling time (years) | 6.4 ± 5.4 | 7.2 ± 5.8 | 5.8 ± 5.1 | 6.6 ± 5.5 | 5.3 ± 4.4 | |
| Indication for TLE, n/N (%) | ||||||
| Infections | 1865/3499 (53) | 550/1110 (50) | 570/1092 (52) | 553/981 (56) | 192/316 (61) | 0.0005 |
| Chronic pain | 180/3510 (5.1) | 74/1112 (6.7) | 53/1096 (4.9) | 41/985 (4.2) | 12/317 (3.8) | 0.0354 |
| Thrombosis or venous stenosis | 160/3510 (4.6) | 34/1112 (3.1) | 49/1096 (4.5) | 57/985 (5.8) | 20/317 (6.3) | 0.0096 |
| Functional leads | 2023/3510 (58) | 620/1112 (56) | 634/1096 (58) | 593/985 (60) | 176/317 (56) | 0.1815 |
| Non-functional leads | 1331/3510 (38) | 448/1112 (40) | 443/1096 (40) | 341/985 (35) | 99/317 (31) | 0.0010 |
| Recalled leads (updated) | 440/3510 (13) | 139/1112 (13) | 173/1096 (16) | 94/985 (10) | 34/317 (11) | 0.0002 |
| Upgrading indication | 248/3510 (7) | 43/1112 (4) | 78/1096 (7) | 98/985 (10) | 29/317 (9) | <0.0001 |
| MRI indication | 26/3510 (0.7) | 15/1112 (1.3) | 6/1096 (0.5) | 4/985 (0.4) | 1/317 (0.3) | 0.0378 |
| Other | 54/3510 (1.5) | 22/1112 (2.0) | 10/1096 (0.9) | 16/985 (1.6) | 6/317 (1.9) | 0.2063 |
| Previous attempt of lead extraction | 171/3510 (5) | 67/1112 (6) | 48/1096 (4) | 42/985 (4) | 14/317 (4) | 0.1957 |
AC, anticoagulants; AP, antiplatelets; CRT, cardiac resynchronization therapy; ICD, implantable cardiac defibrillator; MRI, magnetic resonance imaging; PM, pacemaker; TLE, transvenous lead extraction.
Data are expressed per leads.
Regarding TLE technique and approaches, locking stylets were frequently used (67%). Manual traction was effective in removing 27.3% of total leads and was more effective in the AT subgroups (25% vs. 28% vs. 28% vs. 32%, for No-AT, AP, AC, and AP/AC, respectively, P = 0.0077). Dilatation was required in 63% of leads and included mechanical not powered (36%), mechanical rotational (8%), and laser sheath (19%). Mechanical not powered and mechanical rotational sheaths were more frequently used in the No-AT patients (P < 0.0001), while no differences were observed in the use of laser sheaths (P = 0.3547) among groups. The majority of patients in all groups required dilatation through the subclavian venous entry site, while alternative approaches like femoral (4.7%) or jugular (5.4%) were rarely used, without differences among groups (P = 0.2483).
Regarding leads extracted, 75.7% were PM leads and 24.3% ICD leads. Leads extracted were ventricular (55%), atrial (34%), and LV (8.4%). Among groups, the majority of leads extracted in the No-AT group were pacing leads, while ICD and LV ventricular leads were mainly removed in the AT subgroups (P < 0.0001). The average implantation time was 6.4 ± 5.4 years and was statistically significantly different between groups, with a longer implantation time in the No-AT group (P < 0.0001).
Procedural data are shown in Table 3.
TLE outcomes: technique, success and complications between patients not treated with AT, treated with AP, with AC, and both AP + AC
| Variables . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| TLE technique, n/N (%)a | ||||||
| Locking stylets | 4360/6493 (67.15) | 1403/1987 (70.61) | 1335/2056 (64.93) | 1245/1843 (67.55) | 377/607 (62.11) | <0.0001 |
| Lead removed with traction alone | 1741/6376 (27) | 479/1925 (25) | 574/2036 (28) | 498/1812 (28) | 190/603 (32) | 0.0077 |
| Sheaths used, n/N (%)a | ||||||
| Mechanical not powered sheaths | 2359/6492 (36) | 721/1986 (36) | 796/2056 (39) | 669/1843 (36) | 173/607 (29) | <0.0001 |
| Mechanical rotational sheaths | 500/6492 (8) | 254/1986 (13) | 111/2056 (5) | 106/1843 (6) | 29/607 (5) | <0.0001 |
| Laser sheaths | 1250/6492 (19) | 372/1986 (19) | 380/2056 (19) | 370/1843 (20) | 128/607 (21) | 0.3547 |
| Alternative TLE approach, n/N (%)a | ||||||
| Femoral | 308/6492 (4.74) | 89/1986 (4.48) | 97/2056 (4.72) | 83/1843 (4.50) | 39/607 (6.43) | 0.2483 |
| Jugular | 352/6492 (5.4) | 110/1986 (5.5) | 97/2056 (5.3) | 94/1843 (5.1) | 39/607 (6.43) | 0.2483 |
| Procedural success, n/N (%)a | ||||||
| Complete radiological success | 6212/6493 (96) | 1863/1987 (94) | 1991/2056 (97) | 1764/1843 (96) | 594/607 (98) | <0.0001 |
| Partial radiological success | 184/6493 (2.83) | 81/1987 (4.08) | 39/2056 (1.90) | 52/1843 (2.82) | 12/607 (1.98) | <0.0001 |
| Failure | 97/6493 (1.49) | 43/1987 (2.16) | 26/2056 (1.26) | 27/1843 (1.47) | 1/607 (0.16) | <0.0001 |
| Acute and post-procedural complications | ||||||
| Major, n/N (%) | 95/3510 (2.7) | 30/1112 (2.7) | 28/1096 (2.6) | 26/985 (2.6) | 11/317 (3.5) | 0.8459 |
| Intra-procedural | 37/3510 (1.1) | 17/1112 (1.5) | 10/1096 (0.9) | 8/985 (0.8) | 2/317 (0.6) | 0.2945 |
| Post-procedural | 21/3510 (0.6) | 6/1112 (0.5) | 7/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.9915 |
| Procedure-related deaths | 17/3510 (0.5) | 3/1112 (0.3) | 6/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.6566 |
| In-hospital-death | 50/3510 (1.4) | 8/1112 (0.7) | 16/1096 (1.5) | 18/985 (1.8) | 8/317 (2.5) | 0.0500 |
| Cardiovascular | 28/50 (56) | 2/8 (25) | 11/16 (69) | 10/18 (56) | 5/8 (63) | 0.2294 |
| Not cardiovascular | 22/50 (44) | 6/8 (75) | 5/16 (31) | 8/18 (44) | 3/8 (37) | |
| Minor, n/N (%) | 174/3510 (5) | 50/1112 (4.5) | 53/1096 (4.8) | 53/985 (5.4) | 18/317 (5.7) | 0.7386 |
| Intra-procedural | 34/3510 (1) | 13/1112 (1.2) | 11/1096 (1.0) | 7/985 (0.7) | 3/317 (0.9) | 0.7614 |
| Post-procedural | 131/3510 (3.7) | 32/1112 (2.9) | 40/1096 (3.7) | 44/985 (4.5) | 15/317 (4.7) | 0.1999 |
| Haematoma at surgical siteb | 40/3510 (1.1) | 5/1112 (0.5) | 11/1096 (1.0) | 18/985 (1.8) | 6/317 (1.9) | 0.0140 |
| Pulmonary embolismc | 16/3510 (0.5) | 11/1112 (1) | 1/1096 (0.1) | 2/985 (0.2) | 2/317 (0.6) | 0.0081 |
| Pneumothoraxd | 12/3510 (0.3) | 6/1112 (0.5) | 6/1096 (0.6) | 0/985 (0.0) | 0/317 (0.0) | 0.0687 |
| Variables . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| TLE technique, n/N (%)a | ||||||
| Locking stylets | 4360/6493 (67.15) | 1403/1987 (70.61) | 1335/2056 (64.93) | 1245/1843 (67.55) | 377/607 (62.11) | <0.0001 |
| Lead removed with traction alone | 1741/6376 (27) | 479/1925 (25) | 574/2036 (28) | 498/1812 (28) | 190/603 (32) | 0.0077 |
| Sheaths used, n/N (%)a | ||||||
| Mechanical not powered sheaths | 2359/6492 (36) | 721/1986 (36) | 796/2056 (39) | 669/1843 (36) | 173/607 (29) | <0.0001 |
| Mechanical rotational sheaths | 500/6492 (8) | 254/1986 (13) | 111/2056 (5) | 106/1843 (6) | 29/607 (5) | <0.0001 |
| Laser sheaths | 1250/6492 (19) | 372/1986 (19) | 380/2056 (19) | 370/1843 (20) | 128/607 (21) | 0.3547 |
| Alternative TLE approach, n/N (%)a | ||||||
| Femoral | 308/6492 (4.74) | 89/1986 (4.48) | 97/2056 (4.72) | 83/1843 (4.50) | 39/607 (6.43) | 0.2483 |
| Jugular | 352/6492 (5.4) | 110/1986 (5.5) | 97/2056 (5.3) | 94/1843 (5.1) | 39/607 (6.43) | 0.2483 |
| Procedural success, n/N (%)a | ||||||
| Complete radiological success | 6212/6493 (96) | 1863/1987 (94) | 1991/2056 (97) | 1764/1843 (96) | 594/607 (98) | <0.0001 |
| Partial radiological success | 184/6493 (2.83) | 81/1987 (4.08) | 39/2056 (1.90) | 52/1843 (2.82) | 12/607 (1.98) | <0.0001 |
| Failure | 97/6493 (1.49) | 43/1987 (2.16) | 26/2056 (1.26) | 27/1843 (1.47) | 1/607 (0.16) | <0.0001 |
| Acute and post-procedural complications | ||||||
| Major, n/N (%) | 95/3510 (2.7) | 30/1112 (2.7) | 28/1096 (2.6) | 26/985 (2.6) | 11/317 (3.5) | 0.8459 |
| Intra-procedural | 37/3510 (1.1) | 17/1112 (1.5) | 10/1096 (0.9) | 8/985 (0.8) | 2/317 (0.6) | 0.2945 |
| Post-procedural | 21/3510 (0.6) | 6/1112 (0.5) | 7/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.9915 |
| Procedure-related deaths | 17/3510 (0.5) | 3/1112 (0.3) | 6/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.6566 |
| In-hospital-death | 50/3510 (1.4) | 8/1112 (0.7) | 16/1096 (1.5) | 18/985 (1.8) | 8/317 (2.5) | 0.0500 |
| Cardiovascular | 28/50 (56) | 2/8 (25) | 11/16 (69) | 10/18 (56) | 5/8 (63) | 0.2294 |
| Not cardiovascular | 22/50 (44) | 6/8 (75) | 5/16 (31) | 8/18 (44) | 3/8 (37) | |
| Minor, n/N (%) | 174/3510 (5) | 50/1112 (4.5) | 53/1096 (4.8) | 53/985 (5.4) | 18/317 (5.7) | 0.7386 |
| Intra-procedural | 34/3510 (1) | 13/1112 (1.2) | 11/1096 (1.0) | 7/985 (0.7) | 3/317 (0.9) | 0.7614 |
| Post-procedural | 131/3510 (3.7) | 32/1112 (2.9) | 40/1096 (3.7) | 44/985 (4.5) | 15/317 (4.7) | 0.1999 |
| Haematoma at surgical siteb | 40/3510 (1.1) | 5/1112 (0.5) | 11/1096 (1.0) | 18/985 (1.8) | 6/317 (1.9) | 0.0140 |
| Pulmonary embolismc | 16/3510 (0.5) | 11/1112 (1) | 1/1096 (0.1) | 2/985 (0.2) | 2/317 (0.6) | 0.0081 |
| Pneumothoraxd | 12/3510 (0.3) | 6/1112 (0.5) | 6/1096 (0.6) | 0/985 (0.0) | 0/317 (0.0) | 0.0687 |
AC, anticoagulants; AP, antiplatelets; TLE, transvenous lead extraction.
Data are expressed per leads.
Requiring intervention.
Not Requiring surgery.
Requiring chest tube.
TLE outcomes: technique, success and complications between patients not treated with AT, treated with AP, with AC, and both AP + AC
| Variables . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| TLE technique, n/N (%)a | ||||||
| Locking stylets | 4360/6493 (67.15) | 1403/1987 (70.61) | 1335/2056 (64.93) | 1245/1843 (67.55) | 377/607 (62.11) | <0.0001 |
| Lead removed with traction alone | 1741/6376 (27) | 479/1925 (25) | 574/2036 (28) | 498/1812 (28) | 190/603 (32) | 0.0077 |
| Sheaths used, n/N (%)a | ||||||
| Mechanical not powered sheaths | 2359/6492 (36) | 721/1986 (36) | 796/2056 (39) | 669/1843 (36) | 173/607 (29) | <0.0001 |
| Mechanical rotational sheaths | 500/6492 (8) | 254/1986 (13) | 111/2056 (5) | 106/1843 (6) | 29/607 (5) | <0.0001 |
| Laser sheaths | 1250/6492 (19) | 372/1986 (19) | 380/2056 (19) | 370/1843 (20) | 128/607 (21) | 0.3547 |
| Alternative TLE approach, n/N (%)a | ||||||
| Femoral | 308/6492 (4.74) | 89/1986 (4.48) | 97/2056 (4.72) | 83/1843 (4.50) | 39/607 (6.43) | 0.2483 |
| Jugular | 352/6492 (5.4) | 110/1986 (5.5) | 97/2056 (5.3) | 94/1843 (5.1) | 39/607 (6.43) | 0.2483 |
| Procedural success, n/N (%)a | ||||||
| Complete radiological success | 6212/6493 (96) | 1863/1987 (94) | 1991/2056 (97) | 1764/1843 (96) | 594/607 (98) | <0.0001 |
| Partial radiological success | 184/6493 (2.83) | 81/1987 (4.08) | 39/2056 (1.90) | 52/1843 (2.82) | 12/607 (1.98) | <0.0001 |
| Failure | 97/6493 (1.49) | 43/1987 (2.16) | 26/2056 (1.26) | 27/1843 (1.47) | 1/607 (0.16) | <0.0001 |
| Acute and post-procedural complications | ||||||
| Major, n/N (%) | 95/3510 (2.7) | 30/1112 (2.7) | 28/1096 (2.6) | 26/985 (2.6) | 11/317 (3.5) | 0.8459 |
| Intra-procedural | 37/3510 (1.1) | 17/1112 (1.5) | 10/1096 (0.9) | 8/985 (0.8) | 2/317 (0.6) | 0.2945 |
| Post-procedural | 21/3510 (0.6) | 6/1112 (0.5) | 7/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.9915 |
| Procedure-related deaths | 17/3510 (0.5) | 3/1112 (0.3) | 6/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.6566 |
| In-hospital-death | 50/3510 (1.4) | 8/1112 (0.7) | 16/1096 (1.5) | 18/985 (1.8) | 8/317 (2.5) | 0.0500 |
| Cardiovascular | 28/50 (56) | 2/8 (25) | 11/16 (69) | 10/18 (56) | 5/8 (63) | 0.2294 |
| Not cardiovascular | 22/50 (44) | 6/8 (75) | 5/16 (31) | 8/18 (44) | 3/8 (37) | |
| Minor, n/N (%) | 174/3510 (5) | 50/1112 (4.5) | 53/1096 (4.8) | 53/985 (5.4) | 18/317 (5.7) | 0.7386 |
| Intra-procedural | 34/3510 (1) | 13/1112 (1.2) | 11/1096 (1.0) | 7/985 (0.7) | 3/317 (0.9) | 0.7614 |
| Post-procedural | 131/3510 (3.7) | 32/1112 (2.9) | 40/1096 (3.7) | 44/985 (4.5) | 15/317 (4.7) | 0.1999 |
| Haematoma at surgical siteb | 40/3510 (1.1) | 5/1112 (0.5) | 11/1096 (1.0) | 18/985 (1.8) | 6/317 (1.9) | 0.0140 |
| Pulmonary embolismc | 16/3510 (0.5) | 11/1112 (1) | 1/1096 (0.1) | 2/985 (0.2) | 2/317 (0.6) | 0.0081 |
| Pneumothoraxd | 12/3510 (0.3) | 6/1112 (0.5) | 6/1096 (0.6) | 0/985 (0.0) | 0/317 (0.0) | 0.0687 |
| Variables . | Total (N = 3510) . | No AT (N = 1112) . | AP (N = 1096) . | AC (N = 985) . | AP + AC (N = 317) . | P-value . |
|---|---|---|---|---|---|---|
| TLE technique, n/N (%)a | ||||||
| Locking stylets | 4360/6493 (67.15) | 1403/1987 (70.61) | 1335/2056 (64.93) | 1245/1843 (67.55) | 377/607 (62.11) | <0.0001 |
| Lead removed with traction alone | 1741/6376 (27) | 479/1925 (25) | 574/2036 (28) | 498/1812 (28) | 190/603 (32) | 0.0077 |
| Sheaths used, n/N (%)a | ||||||
| Mechanical not powered sheaths | 2359/6492 (36) | 721/1986 (36) | 796/2056 (39) | 669/1843 (36) | 173/607 (29) | <0.0001 |
| Mechanical rotational sheaths | 500/6492 (8) | 254/1986 (13) | 111/2056 (5) | 106/1843 (6) | 29/607 (5) | <0.0001 |
| Laser sheaths | 1250/6492 (19) | 372/1986 (19) | 380/2056 (19) | 370/1843 (20) | 128/607 (21) | 0.3547 |
| Alternative TLE approach, n/N (%)a | ||||||
| Femoral | 308/6492 (4.74) | 89/1986 (4.48) | 97/2056 (4.72) | 83/1843 (4.50) | 39/607 (6.43) | 0.2483 |
| Jugular | 352/6492 (5.4) | 110/1986 (5.5) | 97/2056 (5.3) | 94/1843 (5.1) | 39/607 (6.43) | 0.2483 |
| Procedural success, n/N (%)a | ||||||
| Complete radiological success | 6212/6493 (96) | 1863/1987 (94) | 1991/2056 (97) | 1764/1843 (96) | 594/607 (98) | <0.0001 |
| Partial radiological success | 184/6493 (2.83) | 81/1987 (4.08) | 39/2056 (1.90) | 52/1843 (2.82) | 12/607 (1.98) | <0.0001 |
| Failure | 97/6493 (1.49) | 43/1987 (2.16) | 26/2056 (1.26) | 27/1843 (1.47) | 1/607 (0.16) | <0.0001 |
| Acute and post-procedural complications | ||||||
| Major, n/N (%) | 95/3510 (2.7) | 30/1112 (2.7) | 28/1096 (2.6) | 26/985 (2.6) | 11/317 (3.5) | 0.8459 |
| Intra-procedural | 37/3510 (1.1) | 17/1112 (1.5) | 10/1096 (0.9) | 8/985 (0.8) | 2/317 (0.6) | 0.2945 |
| Post-procedural | 21/3510 (0.6) | 6/1112 (0.5) | 7/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.9915 |
| Procedure-related deaths | 17/3510 (0.5) | 3/1112 (0.3) | 6/1096 (0.6) | 6/985 (0.6) | 2/317 (0.6) | 0.6566 |
| In-hospital-death | 50/3510 (1.4) | 8/1112 (0.7) | 16/1096 (1.5) | 18/985 (1.8) | 8/317 (2.5) | 0.0500 |
| Cardiovascular | 28/50 (56) | 2/8 (25) | 11/16 (69) | 10/18 (56) | 5/8 (63) | 0.2294 |
| Not cardiovascular | 22/50 (44) | 6/8 (75) | 5/16 (31) | 8/18 (44) | 3/8 (37) | |
| Minor, n/N (%) | 174/3510 (5) | 50/1112 (4.5) | 53/1096 (4.8) | 53/985 (5.4) | 18/317 (5.7) | 0.7386 |
| Intra-procedural | 34/3510 (1) | 13/1112 (1.2) | 11/1096 (1.0) | 7/985 (0.7) | 3/317 (0.9) | 0.7614 |
| Post-procedural | 131/3510 (3.7) | 32/1112 (2.9) | 40/1096 (3.7) | 44/985 (4.5) | 15/317 (4.7) | 0.1999 |
| Haematoma at surgical siteb | 40/3510 (1.1) | 5/1112 (0.5) | 11/1096 (1.0) | 18/985 (1.8) | 6/317 (1.9) | 0.0140 |
| Pulmonary embolismc | 16/3510 (0.5) | 11/1112 (1) | 1/1096 (0.1) | 2/985 (0.2) | 2/317 (0.6) | 0.0081 |
| Pneumothoraxd | 12/3510 (0.3) | 6/1112 (0.5) | 6/1096 (0.6) | 0/985 (0.0) | 0/317 (0.0) | 0.0687 |
AC, anticoagulants; AP, antiplatelets; TLE, transvenous lead extraction.
Data are expressed per leads.
Requiring intervention.
Not Requiring surgery.
Requiring chest tube.
Procedural outcomes
The radiological procedural success rate was 96%, and was a bit higher in the AT subgroups (94% vs. 97% vs. 96% vs. 98%, for No-AT, AP, AC, and AP/AC, respectively, P < 0.0001). Regarding major complications, the overall incidence (2.7%) was not statistically different among groups (Figure 2). Nevertheless, the incidence of in-hospital death for any cause was 1.4% and resulted statistically higher in the AT subgroups (0.7% vs. 1.5% vs. 1.8% vs. 2.5%, for No-AT, AP, AC, and AP/AC, respectively, P = 0.0500), while no statistically significant differences in procedure-related death were observed among groups (0.3% vs. 0.6% vs. 0.6% vs. 0.6%, for No-AT, AP, AC, and AP/AC, respectively, P = 0.0656) (Figure 3).
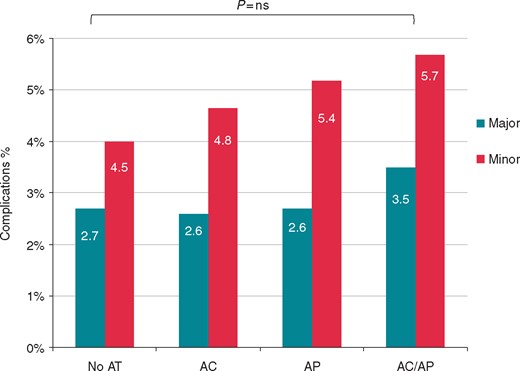
Comparison of major and minor complication rate between patients not treated with AT, treated with AP, with AC, and both AP + AC. AC, anticoagulants; AP, antiplatelets.
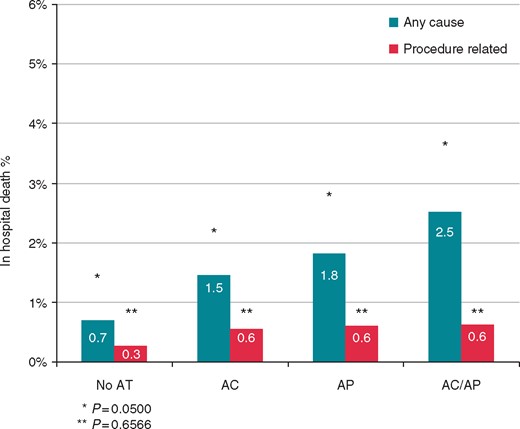
Comparison of all-cause and procedure-related death rates between patients not treated with AT, treated with AP, with AC, and both AP + AC. AC, anticoagulants; AP, antiplatelets.
Regarding minor complications, the overall incidence (5%) was not statistically different among groups (Figure 2). Of note, the sub-analysis showed a statistically significant difference in the occurrence of haematoma requiring reintervention, which were more frequent in the AT subgroups (0.5% vs. 1% vs. 1.8% vs. 1.9%, for No-AT, AP, AC, and AP/AC, respectively, P = 0.0140), and of pulmonary embolism not requiring surgery, which was more often observed in the No-AT group (1% vs. 0.1% vs. 0.2% vs. 0.6%, for No-AT, AP, AC, and AP/AC, respectively, P = 0.0081) (Figure 4).
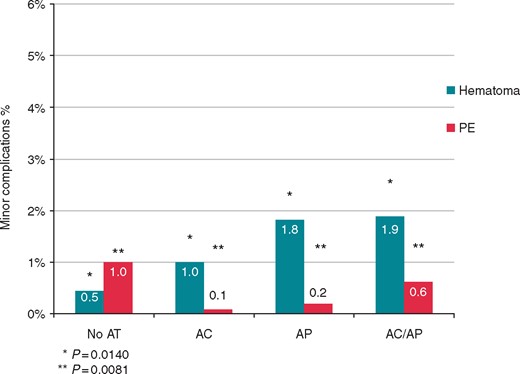
Comparison of haematoma (requiring revision) and pulmonary embolism (PE) not requiring surgery rates between patients not treated with AT, treated with AP, with AC, and both AP + AC. AC, anticoagulants; AP, antiplatelets.
At the sub-analysis of AC patients according to the pre-procedural AC management strategy, no differences were observed in terms of major complications, including death. Only AC ‘continued’ showed a more frequent pocket haematoma requiring reintervention (4.6%, P = 0.0253).
Procedural outcomes are shown in Table 3.
Predictors of transvenous lead extraction complications
Predictors of overall major and minor complications were also investigated. Several clinical and procedural variables were tested in the analysis, including AT regimen type (No-AT, AP, AC, and AP/AC) and AC management (interruption < or > 3 days, with or without bridging). Low (<30 TLE procedures/year) volume centre [hazard ratio (HR) 2.56, 95% confidence interval (CI) 1.16–5.5; P = 0.02], lead implantation time > 5 years (HR 2.69, 95% CI 1.14–6.37, P = 0.02), and use of laser sheath (HR 2.64, 95% CI 1.29–5.59, P = 0.01) resulted independent predictors of overall in-hospital complications in the uni- and multi-variate analyses. Regarding in-hospital death for any cause, only age >65 years (HR 2.85, 95% CI 1.16–7.14; P = 0.02) and New York Heart Association (NYHA) Class III/IV (NYHA III: HR 2.82, 95% CI 1.16–6.82; P = 0.02; NYHA IV: HR 6.59, 95% CI 1.78–24.46; P > 0.005) were independent predictors in the uni- and multivariate analyses.
Discussion
Although, PM or ICD implantation are classified as interventions with low bleeding risk,11,12 lead extraction differs significantly from other CIED procedures and peri-procedural management of AT therapy in candidates for TLE deserves separate discussion.
Data from registries and RCTs document a cumulative rate of complications >10%. Although rare (2%), major complications are mainly represented by haemorrhagic events from direct and/or indirect trauma on the vascular and cardiac wall, such as venous laceration, cardiac perforation, tamponade, and haemothorax, which can be fatal in 0.3–0.7% of cases.13–18 Similar data were confirmed in the ELECTRa Registry.7 Our sub-analysis showed that, AT subgroups, (AP, AC, AP/AC) had an high success rate, also higher than No-AT (97% vs. 96% vs. 98% vs. 94% respectively, P < 0.0001). With the prevalent AT management observed in the registry (i.e. AP continued in 74% and AC interrupted in 93% of patients about 3 days before TLE) complication rate was low, with a non-statistically different incidence of overall major (2.7% vs. 2.6% vs. 2.6% vs. 3.5% for No-AT, AP, AC, and AP/AC, respectively, P = 0.845) and minor events (4.5% vs. 4.8% vs. 5.4% vs. 5.7% for No-AT, AP, AC, and AP/AC, respectively, P = 0.738), even if a negative trend cannot be completely excluded (Figure 1). At the analysis of predictors, overall complications were predicted by technical/procedural factors, as old leads removed with laser sheaths in low-volume centres, but neither by clinical factors or AT regimens. Among TLE major complications, only in-hospital mortality (but not intra-procedural death, which was comparable among groups), resulted higher in AT patients, especially in the subgroup under combined AP/AC therapy (Figure 2). Of note, in-hospital mortality was predicted only by clinical factors, as old age and low NYHA class, but, also in this case, not by AT regimens. This finding seems to support the concept that, also minimizing pre-procedural AP and AC therapy, patient complexity and fragility, often observed in patients under AT, are likely to be responsible for the poor in-hospital outcome.
Regarding TLE minor complications, they usually include both bleeding or thrombotic complications, such as pocket haematoma, low-risk pulmonary thromboembolism, and post-extraction deep vein thrombosis at the venous entry site.19 In our sub-analysis, the incidence of minor complications was comparable (5%) among groups. Of note, a high rate of minor bleedings (i.e. pocket haematoma requiring surgery), but with a lower incidence of pulmonary thromboembolism, were observed in AT subgroups (Table 3), especially in AP/AC subgroup (Figure 3), and when AC was ‘continued’ (Table 4). We could argue that AP with/without continued or bridged AC increases the bleeding risk, but potentially reducing the embolic one, invariably associated with any surgery.
Comparison of complications and deaths between AC patients according to the pre-procedural management AC strategy
| Complications . | Total AC Pts (N = 1287) . | Interrupted AC ‘with bridging’ (N = 504) . | Interrupted AC ‘without bridging’ (N = 696) . | Continued AC (N = 87) . | P-value . |
|---|---|---|---|---|---|
| Major, n/N (%) | 36/1287 (2.80) | 16/504 (3.17) | 18/696 (2.59) | 2/87 (2.30) | 0.7955 |
| Intra-procedural death | 5/1287 (0.39) | 2/504 (0.40) | 3/696 (0.43) | 0/87 (0.00) | 0.8299 |
| Post-procedural death | 3/1287 (0.23) | 2/504 (0.40) | 0/696 (0.00) | 1/87 (1.15) | 0.0689 |
| Minor, n/N (%) | 70/1287 (5.44) | 31/504 (6.15) | 35/696 (5.03) | 4/87 (4.60) | 0.6557 |
| Intra-procedural | 10/1287 (0.78) | 0/504 (0.00) | 10/696 (1.44)a | 0/87 (0.00) | 0.0138 |
| Post-procedural | 60/1287 (4.66) | 30/504 (5.95) | 26/696 (3.74) | 4/87 (4.60) | 0.1986 |
| Haematoma at surgical site req. reintervention | 23/1287 (1.79) | 12/504 (2.38) | 7/696 (1.01) | 4/87 (4.60)b | 0.0253 |
| Blood transfusion | 9/1287 (0.70) | 7/504 (1.39) | 2/696 (0.29) | 0/87 (0.00) | 0.0559 |
| Pulmonary embolism not req. surgery | 3/1287 (0.23) | 2/504 (0.40) | 1/696 (0.14) | 0/87 (0.00) | 0.5994 |
| Vascular repair near the implant site | 1/1287 (0.08) | 1/504 (0.20) | 0/696 (0.00) | 0/87 (0.00) | 0.4596 |
| Haemotorax without chest tube | 1/1287 (0.08) | 0/504 (0.00) | 1/696 (0.14) | 0/87 (0.00) | 0.6538 |
| Complications . | Total AC Pts (N = 1287) . | Interrupted AC ‘with bridging’ (N = 504) . | Interrupted AC ‘without bridging’ (N = 696) . | Continued AC (N = 87) . | P-value . |
|---|---|---|---|---|---|
| Major, n/N (%) | 36/1287 (2.80) | 16/504 (3.17) | 18/696 (2.59) | 2/87 (2.30) | 0.7955 |
| Intra-procedural death | 5/1287 (0.39) | 2/504 (0.40) | 3/696 (0.43) | 0/87 (0.00) | 0.8299 |
| Post-procedural death | 3/1287 (0.23) | 2/504 (0.40) | 0/696 (0.00) | 1/87 (1.15) | 0.0689 |
| Minor, n/N (%) | 70/1287 (5.44) | 31/504 (6.15) | 35/696 (5.03) | 4/87 (4.60) | 0.6557 |
| Intra-procedural | 10/1287 (0.78) | 0/504 (0.00) | 10/696 (1.44)a | 0/87 (0.00) | 0.0138 |
| Post-procedural | 60/1287 (4.66) | 30/504 (5.95) | 26/696 (3.74) | 4/87 (4.60) | 0.1986 |
| Haematoma at surgical site req. reintervention | 23/1287 (1.79) | 12/504 (2.38) | 7/696 (1.01) | 4/87 (4.60)b | 0.0253 |
| Blood transfusion | 9/1287 (0.70) | 7/504 (1.39) | 2/696 (0.29) | 0/87 (0.00) | 0.0559 |
| Pulmonary embolism not req. surgery | 3/1287 (0.23) | 2/504 (0.40) | 1/696 (0.14) | 0/87 (0.00) | 0.5994 |
| Vascular repair near the implant site | 1/1287 (0.08) | 1/504 (0.20) | 0/696 (0.00) | 0/87 (0.00) | 0.4596 |
| Haemotorax without chest tube | 1/1287 (0.08) | 0/504 (0.00) | 1/696 (0.14) | 0/87 (0.00) | 0.6538 |
AC, anticoagulants.
Comparison between ‘interrupted with bridging’ vs. ‘without’ (P = 0.018).
Comparison between ‘interrupted without bridging’ vs. ‘continued’ (P = 0.028).
Comparison of complications and deaths between AC patients according to the pre-procedural management AC strategy
| Complications . | Total AC Pts (N = 1287) . | Interrupted AC ‘with bridging’ (N = 504) . | Interrupted AC ‘without bridging’ (N = 696) . | Continued AC (N = 87) . | P-value . |
|---|---|---|---|---|---|
| Major, n/N (%) | 36/1287 (2.80) | 16/504 (3.17) | 18/696 (2.59) | 2/87 (2.30) | 0.7955 |
| Intra-procedural death | 5/1287 (0.39) | 2/504 (0.40) | 3/696 (0.43) | 0/87 (0.00) | 0.8299 |
| Post-procedural death | 3/1287 (0.23) | 2/504 (0.40) | 0/696 (0.00) | 1/87 (1.15) | 0.0689 |
| Minor, n/N (%) | 70/1287 (5.44) | 31/504 (6.15) | 35/696 (5.03) | 4/87 (4.60) | 0.6557 |
| Intra-procedural | 10/1287 (0.78) | 0/504 (0.00) | 10/696 (1.44)a | 0/87 (0.00) | 0.0138 |
| Post-procedural | 60/1287 (4.66) | 30/504 (5.95) | 26/696 (3.74) | 4/87 (4.60) | 0.1986 |
| Haematoma at surgical site req. reintervention | 23/1287 (1.79) | 12/504 (2.38) | 7/696 (1.01) | 4/87 (4.60)b | 0.0253 |
| Blood transfusion | 9/1287 (0.70) | 7/504 (1.39) | 2/696 (0.29) | 0/87 (0.00) | 0.0559 |
| Pulmonary embolism not req. surgery | 3/1287 (0.23) | 2/504 (0.40) | 1/696 (0.14) | 0/87 (0.00) | 0.5994 |
| Vascular repair near the implant site | 1/1287 (0.08) | 1/504 (0.20) | 0/696 (0.00) | 0/87 (0.00) | 0.4596 |
| Haemotorax without chest tube | 1/1287 (0.08) | 0/504 (0.00) | 1/696 (0.14) | 0/87 (0.00) | 0.6538 |
| Complications . | Total AC Pts (N = 1287) . | Interrupted AC ‘with bridging’ (N = 504) . | Interrupted AC ‘without bridging’ (N = 696) . | Continued AC (N = 87) . | P-value . |
|---|---|---|---|---|---|
| Major, n/N (%) | 36/1287 (2.80) | 16/504 (3.17) | 18/696 (2.59) | 2/87 (2.30) | 0.7955 |
| Intra-procedural death | 5/1287 (0.39) | 2/504 (0.40) | 3/696 (0.43) | 0/87 (0.00) | 0.8299 |
| Post-procedural death | 3/1287 (0.23) | 2/504 (0.40) | 0/696 (0.00) | 1/87 (1.15) | 0.0689 |
| Minor, n/N (%) | 70/1287 (5.44) | 31/504 (6.15) | 35/696 (5.03) | 4/87 (4.60) | 0.6557 |
| Intra-procedural | 10/1287 (0.78) | 0/504 (0.00) | 10/696 (1.44)a | 0/87 (0.00) | 0.0138 |
| Post-procedural | 60/1287 (4.66) | 30/504 (5.95) | 26/696 (3.74) | 4/87 (4.60) | 0.1986 |
| Haematoma at surgical site req. reintervention | 23/1287 (1.79) | 12/504 (2.38) | 7/696 (1.01) | 4/87 (4.60)b | 0.0253 |
| Blood transfusion | 9/1287 (0.70) | 7/504 (1.39) | 2/696 (0.29) | 0/87 (0.00) | 0.0559 |
| Pulmonary embolism not req. surgery | 3/1287 (0.23) | 2/504 (0.40) | 1/696 (0.14) | 0/87 (0.00) | 0.5994 |
| Vascular repair near the implant site | 1/1287 (0.08) | 1/504 (0.20) | 0/696 (0.00) | 0/87 (0.00) | 0.4596 |
| Haemotorax without chest tube | 1/1287 (0.08) | 0/504 (0.00) | 1/696 (0.14) | 0/87 (0.00) | 0.6538 |
AC, anticoagulants.
Comparison between ‘interrupted with bridging’ vs. ‘without’ (P = 0.018).
Comparison between ‘interrupted without bridging’ vs. ‘continued’ (P = 0.028).
To date, there are no controlled clinical data on AT therapy management associated with extraction procedures and recommendations about AT management remain poor.19 The role of AT therapy was not included as primary or secondary endpoint in the ELECTRa Registry and recommendation on the optimal management of AT therapy would be beyond the purpose of the sub-analysis. Nevertheless, some considerations are due. Management of AT therapy in candidates for any surgery should be based on the concept of balancing the thrombotic risk with the bleeding risk. The potential risk of injury to cardiovascular structures with fatal or disabling complications, and the possible need for emergency percutaneous or surgical procedures, are legitimate reasons to consider such procedures as interventions with a high haemorrhagic risk.20 Nevertheless, the thrombo-embolic risk should not be underestimated. Post-procedural embolic risk remains invariably associated with the patient profile, the traumatic nature of the procedure, and the duration of confinement to bed. In the registry, No-AT patients showed an high rate of thrombo-embolic events. It could be argued that a strategy of an AT therapy minimization, including AP dose reduction and AC interruption with or without bridging, could be beneficial, improving the peri-surgical TLE outcome. Regarding DOAC patients, no recommendations may be given at the present. The favourable pharmacokinetics of DOAC and the emerging possibility to rapidly reverse their effects, may further optimize peri-procedural AT management. A potential pre-procedural AT management strategy was summarized in the Supplementary material online, Appendix S1 (Supplementary material online, Table S1).
At present, our sub-analysis shows that AT does not seem to predict death before TLE but identifies a subset of fragile patients with a worse in-hospital TLE outcome. Antiplatelet and/or AC treatment, alone or in combination, should alert physicians to the potentially high-risk TLE profile of this population. Considering the elective nature of TLE, AT therapy minimization, including partial or complete discontinuation depending on the thrombo-embolic risk, seems to be a reasonable approach.
Conclusions
In the ESC-EHRA ELECTRa Registry, AT patients showed a high TLE success rate. Not intra-procedural, but only in-hospital overall mortality, was increased in AT subgroups and it was predicted by clinical factors, such as age and NYHA class. Minor complications, such as pocket bleeding, were also more frequent in AT, whereas pulmonary thromboembolism appeared reduced in comparison with no-AT patients. Therefore, in patients under chronic AT therapy who undergo TLE, AT therapy should be minimized. Further data are warranted from prospectively designed studies.
Acknowledgements
Registry Executive Committee of the EURObservational Research Programme (EORP). Data collection was conducted by the EORP department from the ESC by Myriam Glemot as Project Officer, Maryna Andarala as Data Manager. Statistical analyses were performed by Cécile Laroche. Overall activities were co-ordinated and supervised by Doctor Aldo P. Maggioni (EORP Scientific Coordinator). All investigators are listed in the Supplementary material online, Appendix S1.
Funding
Medtronic, Cook Medical, Boston Scientific, Spectranetics, and Zoll.
Conflict of interest: M.G.B. reports grants from Boston Scientific, Medtronic, and Zoll. A.D.C., C.B.-L., C.B., J.-C.D., A.K., C.L., P.G.G., A.M.T.: no conflict of interest declared. A.A. reports being consultant to Biosense Webster, Boston Scientific, Corvia, Daiichi-Sankyo, EBR Systems, Medtronic, Microport CRM. He receives speaker fees from Boston Scientific, Medtronic, Microport CRM. F.R. receives minor consultation and speaker fees from Daiichi-Sankyo, Boston Scientific, Microport, and Bayer. N.D. reports research grants from Abbott, Biotronik, Boston Scientific and Medtronic to the institution without personal financial benefits. A.M. received honoraria for participation in committees of studies sponsored by Bayer, Novartis, and Fresenius. C.K. reports being member of the speaker’s bureau for Medtronic, Boston Scientific, Spectranetics/Philips. C.R. reports being member of the speaker’s bureau for Spectranetics/Philips.
References
Author notes
A complete list of the ELECTRa Investigators is provided in the Supplementary material online, Appendix S1.



