-
PDF
- Split View
-
Views
-
Cite
Cite
Cyril Zakine, Rodrigue Garcia, Kumar Narayanan, Estelle Gandjbakhch, Vincent Algalarrondo, Nicolas Lellouche, Marie-Cécile Perier, Laurent Fauchier, Daniel Gras, Pierre Bordachar, Olivier Piot, Dominique Babuty, Nicolas Sadoul, Pascal Defaye, Jean-Claude Deharo, Didier Klug, Christophe Leclercq, Fabrice Extramiana, Serge Boveda, Eloi Marijon, On Behalf of DAI-PP and GPUR Investigators, Prophylactic implantable cardioverter-defibrillator in the very elderly, EP Europace, Volume 21, Issue 7, July 2019, Pages 1063–1069, https://doi.org/10.1093/europace/euz041
Close - Share Icon Share
Current guidelines do not propose any age cut-off for the primary prevention implantable cardioverter-defibrillator (ICD). However, the risk/benefit balance in the very elderly population has not been well studied.
In a multicentre French study assessing patients implanted with an ICD for primary prevention, outcomes among patients aged ≥80 years were compared with <80 years old controls matched for sex and underlying heart disease (ischaemic and dilated cardiomyopathy). A total of 300 ICD recipients were enrolled in this specific analysis, including 150 patients ≥80 years (mean age 81.9 ± 2.0 years; 86.7% males) and 150 controls (mean age 61.8 ± 10.8 years). Among older patients, 92 (75.6%) had no more than one associated comorbidity. Most subjects in the elderly group got an ICD as part of a cardiac resynchronization therapy procedure (74% vs. 46%, P < 0.0001). After a mean follow-up of 3.0 ± 2 years, 53 patients (35%) in the elderly group died, including 38.2% from non cardiovascular causes of death. Similar proportion of patients received ≥1 appropriate therapy (19.4% vs. 21.6%; P = 0.65) in the elderly group and controls, respectively. There was a trend towards more early perioperative events (P = 0.10) in the elderly, with no significant increase in late complications (P = 0.73).
Primary prevention ICD recipients ≥80 years in the real world had relatively low associated comorbidity. Rates of appropriate therapies and device-related complications were similar, compared with younger subjects. Nevertheless, the inherent limitations in interpreting observational data on this particular competing risk situation call for randomized controlled trials to provide definitive answers. Meanwhile, a careful multidisciplinary evaluation is needed to guide patient selection for ICD implantation in the elderly population.
This is the largest study thus far, comparing outcomes between implantable cardioverter-defibrillator (ICD) recipients ≥80 years and <80 years old implanted for primary prevention.
Elderly ICD recipients had relatively few comorbidities.
The overall rate of device-related complications was not different between older (24%) vs. younger subjects (26.7%; P = 0.60).
The rate of inappropriate shocks in the elderly was particularly low (1.4%) and appropriate therapy rates similar to younger subjects.
Among ICD recipients ≥80 years who died, 34.0% had received at least one appropriate ICD therapy prior to death.
Introduction
Overall efficacy of the implantable cardioverter-defibrillator (ICD) for primary prevention of sudden cardiac death (SCD) has been well established through a number of large randomized trials, providing consistent results.1,2
However, the decision of ICD implantation in specific situations, such as in elderly patients, remains much more challenging. Indeed, patients enrolled in pivotal trials have been usually much younger than patients implanted in clinical practice.3 Patients over 75 years old represented only 10% of subjects in the two groundbreaking primary prevention ICD trials (MADIT-II and SCD-HeFT), for which the median patient ages were 64 and 60 years, respectively, with study participants having relatively few comorbidities.4 In contrast, in the real world, the average age of patients hospitalized for heart failure is 70 or 80 years with frequent frailty and concomitant comorbidity. Although the absolute incidence of SCD may be higher, older patients are more likely to die from competing non-SCD causes, since the ratio of SCD to all-cause mortality decreases with advancing age.5,6 Hence, the cumulative chance of benefit from the ICD, though increasing with time elapsed from implantation, might concomitantly decrease with age, due to this competing risk situation.7 Available pooled stratified analyses from randomized controlled trials do not conclusively show that ICD as primary prevention therapy improves survival in elderly (>60 years) patients with severe left ventricular dysfunction.8
In this scenario, observational data from large, real-world registries can serve as valuable complementary information to aid decision making among the elderly. Using a large multicentre registry in a current, real-world setting, we assessed the characteristics of very old patients receiving ICDs for primary prevention and compared their outcomes with those of matched younger patients.
Methods
Population and setting
Data were retrospectively obtained for the period January 2002 to December 2014 from a large French consortium comprising 15 centres and incorporating consecutive patients from two networks (DAI-PP9–13 and GPUR; see Supplementary material online), receiving an ICD for primary prevention (no history of sustained ventricular arrhythmia) of SCD. The overall purpose of the consortium was to assess the outcomes of ICD recipients aged ≥80 years, compared with younger patients, using a case–control approach. Over the study period, the proportion of patients ≥80 years was 1.8%.
Inclusion criteria for cases were: (i) De novo ICD implantation for primary prevention of SCD between January 2002 and December 2014; (ii) age ≥80 years; and (iii) underlying ischaemic cardiomyopathy (ICM) or non-ischaemic cardiomyopathy (NICM) (other causes were excluded for a more homogeneous group). Cases were compared with controls, who were primary prevention ICD recipients <80 years implanted over the same time period, and matched according to sex, underlying heart disease (ICM or NICM) and centre of implantation (to avoid sampling bias). Among 8333 screened patients, 150 cases were included and compared with 150 matched controls. The study was conducted according to the ethical principles stated in the Declaration of Helsinki, approved by the Institutional Review Boards of all participating hospitals, registered on ClinicalTrials.gov (NCT01992458), and data file declared to the French data protection committee (Commission Nationale Informatique et Liberté, CNIL, N°913203).
Patient characteristics at implantation
Patients were characterized at the time of ICD implantation. Ischaemic cardiomyopathy was defined as left ventricular ejection fraction (LVEF) <35% with a documented lesion (stenosis >70%) in at least one of the three main coronary arteries, with or without a history of myocardial infarction and/or revascularization. To classify patients as NICM, coronary artery disease had to be excluded on angiography. Primary prevention was defined as no prior history of sudden cardiac arrest and/or ventricular tachycardia/fibrillation. Exclusion criteria were age <18 years, those with an ICD implant for secondary prevention, ICD for primary prevention without structural heart disease (including Brugada, long QT syndrome among others) or structural heart disease other than ICM or NICM (hypertrophic cardiomyopathy, non-compaction cardiomyopathy, and arrhythmogenic right ventricular dysplasia).
All variables at the time of implant were defined and categorized according to literature or common practice. In addition to the New York Heart Association (NYHA) functional class, we noted the aetiology of the underlying heart disease (ICM or NICM). Glomerular filtration rate was estimated with the Cockroft–Gault formula and grouped in two categories (≥60 and <60 mL/min); QRS duration was categorized as <120 and ≥120 ms. Atrial fibrillation (AF) was defined as a history of AF (paroxysmal or persistent), documented on electrocardiogram or 24-h Holter monitoring. Comorbidities were systematically collected: cancer, chronic obstructive pulmonary disease, chronic renal failure, chronic liver disease, history of transient ischaemic neurological attack, and others (including diabetes mellitus). The type of implanted ICD device (biventricular, single chamber, or dual chamber—without manufacturer information) was recorded, and device programming was left to the treating physician’s discretion. Except in specific clinical situations, the use of two zones with high rate (ventricular tachycardia >180 b.p.m.; ventricular fibrillation >220 b.p.m.) programming was encouraged according to the prevailing consensus of the Cardiac Arrhythmias Group of the French Society of Cardiology.14 Information on medications at hospital discharge was collected, including beta-blockers, amiodarone, Class Ic anti-arrhythmics, sotalol, digoxin, calcium channel blockers, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, diuretics, anti-platelets, and vitamin K antagonists.
Patient follow-up and clinical outcomes
Outcomes assessed were (i) occurrence of appropriate therapy, including time to first appropriate therapy (anti-tachycardia pacing and/or shock), (ii) early (≤30-day post-implant) and late complications of ICD implantation, and (iii) overall as well as specific mortalities. Appropriate ICD therapy was defined as the successful termination of a sustained ventricular tachycardia or ventricular fibrillation episode by single or multiple shocks, antitachycardia pacing, or both. The date of first appropriate ICD therapy was recorded. Inappropriate shocks were documented during follow-up. Patients were monitored every 6 months in the outpatient/device clinic. Vital status data were obtained from the hospital or the general practitioner and were systematically corroborated through the French National Institute for Statistics and Economic Research. Causes of death were obtained on Medical Causes of Death (CépiDc–INSERM). The CépiDc–INSERM is an academic public institution focused on the analysis of circumstances and causes of death documented with death certificate and medical records. Two investigators adjudicated causes of deaths according to the following classification: SCD, non-sudden cardiovascular death (including progressive heart failure death and stroke), non-cardiovascular death, ICD complication related death, and ‘unknown’ when the available information did not enable the investigators to appropriately identify the cause of death. Response to cardiac resynchronization therapy (CRT) was defined as an improvement of ≥1 NYHA functional class and/or ≥5% in the LVEF in the absence of hospitalization for congestive heart failure within the first 12 months after implant.
Statistical analysis
Preparation of this report was carried out in accordance with the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) statement. Categorical variables were expressed as number and percentage, continuous variables as mean and standard deviation or median and interquartile range. Group comparisons were performed using χ2 or Fisher’s exact test as appropriate for categorical variables and Student’s t-test or Mann–Whitney for quantitative variables. Survival curves were built according to these cut-off values by the Kaplan–Meier method and compared using a log-rank test. The association between appropriate therapy and ICD groups was evaluated using Cox regression analysis and the association of early complications with ICD groups was assessed by logistic regression, considering the following covariates: sex, LVEF, NYHA class, creatinine clearance, history of AF, and the presence of a CRT device. P-values of less than 0.05 were considered statistically significant. All data were analysed at the Paris Epidemiology Unit of the Cardiovascular Research Centre of the French Institute of Health and Medical Research, using SAS programme v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline population characteristics
Three hundred ICD recipients were included in this study, including 150 cases aged ≥80 years (mean age 81.9 ± 2.0 years; 86.7% males), and 150 controls (Table 1). In the elderly group, median LVEF was 30% (25–32%), with 20% of patients having LVEF <25%, and 70% having ICM. NYHA functional class was II in 36.5% of patients, Class III in 48.7% and 75.6% of patients had no or only one comorbidity. With regard to heart failure medications, 77.1% of patients were on beta-blockers, 81.9% on angiotensin-converting enzyme inhibitors, and 34.0% with amiodarone. A majority (74.0%) of the elderly ICD recipients had CRT, which was significantly more compared with the younger group (46.3%; P < 0.0001).
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Age (years) | 61.8 ± 10.8) | 81.9 ± 2.0 | ||
| Male, n (%) | 130 (86.7) | 130 (86.7) | 1.00 | |
| Ischaemic cardiomyopathy, n (%) | 105 (70.0) | 105 (70.0) | 1.00 | |
| Left ventricular ejection fraction (%) | 290 | 26.5 (25.0–30.0) | 30.0 (25.0–32.0) | 0.03 |
| QRS duration, n (%) | 243 | |||
| <120 ms | 37 (32.2) | 20 (15.6) | 0.01 | |
| 120–150 ms | 36 (31.3) | 52 (40.6) | ||
| >150 ms | 42 (36.5) | 56 (43.8) | ||
| New York Heart Association class, n (%) | 288 | |||
| I | 11 (7.9) | 15 (10.1) | 0.80 | |
| II | 57 (40.7) | 54 (36.5) | ||
| III | 64 (45.7) | 72 (48.7) | ||
| IV | 8 (5.7) | 7 (4.7) | ||
| Creatinine clearance, n (%) | 187 | |||
| <30 mL/min/1.73 m2 | 7 (9.6) | 16 (14.0) | <0.0001 | |
| 30–60 mL/min/1.73 m2 | 20 (27.4) | 66 (57.9) | ||
| >60 mL/min/1.73 m2 | 46 (63.0) | 32 (28.1) | ||
| Atrial fibrillation history, n (%) | 275 | 20 (15.4) | 60 (39.3) | <0.0001 |
| Comorbidity number, n (%) | 260 | |||
| 0 | 34 (26.4) | 28 (21.4) | 0.08 | |
| 1 | 79 (61.2) | 71 (54.2) | ||
| 2 | 13 (10.1) | 23 (17.6) | ||
| ≥3 | 3 (2.3) | 9 (6.9) | ||
| Cardiovascular medication, n (%) | 278 | |||
| Beta-blocker | 104 (77.6) | 111 (77.1) | 0.92 | |
| Amiodarone | 33 (24.6) | 49 (34.0) | 0.09 | |
| Digoxin | 8 (6.0) | 6 (4.2) | 0.49 | |
| Sotalol | 0 | 1 (0.7) | 1.00a | |
| Antiplatelet | 79 (59.0) | 90 (62.5) | 0.55 | |
| ACE inhibitor | 113 (84.3) | 118 (81.9) | 0.60 | |
| Furosemide | 112 (83.6) | 105 (72.9) | 0.03 | |
| MRA | 32 (23.9) | 23 (16.0) | 0.10 | |
| Vitamin K antagonist | 51 (38.1) | 67 (46.5) | 0.15 | |
| Calcium inhibitor | 1 (0.8) | 2 (1.4) | 1.00a | |
| Implantable cardioverter-defibrillator type | ||||
| Cardiac resynchronizarion therapy device, n (%) | 69 (46.3) | 111 (74.0) | <0.0001 |
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Age (years) | 61.8 ± 10.8) | 81.9 ± 2.0 | ||
| Male, n (%) | 130 (86.7) | 130 (86.7) | 1.00 | |
| Ischaemic cardiomyopathy, n (%) | 105 (70.0) | 105 (70.0) | 1.00 | |
| Left ventricular ejection fraction (%) | 290 | 26.5 (25.0–30.0) | 30.0 (25.0–32.0) | 0.03 |
| QRS duration, n (%) | 243 | |||
| <120 ms | 37 (32.2) | 20 (15.6) | 0.01 | |
| 120–150 ms | 36 (31.3) | 52 (40.6) | ||
| >150 ms | 42 (36.5) | 56 (43.8) | ||
| New York Heart Association class, n (%) | 288 | |||
| I | 11 (7.9) | 15 (10.1) | 0.80 | |
| II | 57 (40.7) | 54 (36.5) | ||
| III | 64 (45.7) | 72 (48.7) | ||
| IV | 8 (5.7) | 7 (4.7) | ||
| Creatinine clearance, n (%) | 187 | |||
| <30 mL/min/1.73 m2 | 7 (9.6) | 16 (14.0) | <0.0001 | |
| 30–60 mL/min/1.73 m2 | 20 (27.4) | 66 (57.9) | ||
| >60 mL/min/1.73 m2 | 46 (63.0) | 32 (28.1) | ||
| Atrial fibrillation history, n (%) | 275 | 20 (15.4) | 60 (39.3) | <0.0001 |
| Comorbidity number, n (%) | 260 | |||
| 0 | 34 (26.4) | 28 (21.4) | 0.08 | |
| 1 | 79 (61.2) | 71 (54.2) | ||
| 2 | 13 (10.1) | 23 (17.6) | ||
| ≥3 | 3 (2.3) | 9 (6.9) | ||
| Cardiovascular medication, n (%) | 278 | |||
| Beta-blocker | 104 (77.6) | 111 (77.1) | 0.92 | |
| Amiodarone | 33 (24.6) | 49 (34.0) | 0.09 | |
| Digoxin | 8 (6.0) | 6 (4.2) | 0.49 | |
| Sotalol | 0 | 1 (0.7) | 1.00a | |
| Antiplatelet | 79 (59.0) | 90 (62.5) | 0.55 | |
| ACE inhibitor | 113 (84.3) | 118 (81.9) | 0.60 | |
| Furosemide | 112 (83.6) | 105 (72.9) | 0.03 | |
| MRA | 32 (23.9) | 23 (16.0) | 0.10 | |
| Vitamin K antagonist | 51 (38.1) | 67 (46.5) | 0.15 | |
| Calcium inhibitor | 1 (0.8) | 2 (1.4) | 1.00a | |
| Implantable cardioverter-defibrillator type | ||||
| Cardiac resynchronizarion therapy device, n (%) | 69 (46.3) | 111 (74.0) | <0.0001 |
Data are expressed as mean ± standard deviation, median (1st quartile–3rd quartile), or number (%).
ACE, angiotensin-converting enzyme inhibitor; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist.
The Fisher’s exact test.
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Age (years) | 61.8 ± 10.8) | 81.9 ± 2.0 | ||
| Male, n (%) | 130 (86.7) | 130 (86.7) | 1.00 | |
| Ischaemic cardiomyopathy, n (%) | 105 (70.0) | 105 (70.0) | 1.00 | |
| Left ventricular ejection fraction (%) | 290 | 26.5 (25.0–30.0) | 30.0 (25.0–32.0) | 0.03 |
| QRS duration, n (%) | 243 | |||
| <120 ms | 37 (32.2) | 20 (15.6) | 0.01 | |
| 120–150 ms | 36 (31.3) | 52 (40.6) | ||
| >150 ms | 42 (36.5) | 56 (43.8) | ||
| New York Heart Association class, n (%) | 288 | |||
| I | 11 (7.9) | 15 (10.1) | 0.80 | |
| II | 57 (40.7) | 54 (36.5) | ||
| III | 64 (45.7) | 72 (48.7) | ||
| IV | 8 (5.7) | 7 (4.7) | ||
| Creatinine clearance, n (%) | 187 | |||
| <30 mL/min/1.73 m2 | 7 (9.6) | 16 (14.0) | <0.0001 | |
| 30–60 mL/min/1.73 m2 | 20 (27.4) | 66 (57.9) | ||
| >60 mL/min/1.73 m2 | 46 (63.0) | 32 (28.1) | ||
| Atrial fibrillation history, n (%) | 275 | 20 (15.4) | 60 (39.3) | <0.0001 |
| Comorbidity number, n (%) | 260 | |||
| 0 | 34 (26.4) | 28 (21.4) | 0.08 | |
| 1 | 79 (61.2) | 71 (54.2) | ||
| 2 | 13 (10.1) | 23 (17.6) | ||
| ≥3 | 3 (2.3) | 9 (6.9) | ||
| Cardiovascular medication, n (%) | 278 | |||
| Beta-blocker | 104 (77.6) | 111 (77.1) | 0.92 | |
| Amiodarone | 33 (24.6) | 49 (34.0) | 0.09 | |
| Digoxin | 8 (6.0) | 6 (4.2) | 0.49 | |
| Sotalol | 0 | 1 (0.7) | 1.00a | |
| Antiplatelet | 79 (59.0) | 90 (62.5) | 0.55 | |
| ACE inhibitor | 113 (84.3) | 118 (81.9) | 0.60 | |
| Furosemide | 112 (83.6) | 105 (72.9) | 0.03 | |
| MRA | 32 (23.9) | 23 (16.0) | 0.10 | |
| Vitamin K antagonist | 51 (38.1) | 67 (46.5) | 0.15 | |
| Calcium inhibitor | 1 (0.8) | 2 (1.4) | 1.00a | |
| Implantable cardioverter-defibrillator type | ||||
| Cardiac resynchronizarion therapy device, n (%) | 69 (46.3) | 111 (74.0) | <0.0001 |
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Age (years) | 61.8 ± 10.8) | 81.9 ± 2.0 | ||
| Male, n (%) | 130 (86.7) | 130 (86.7) | 1.00 | |
| Ischaemic cardiomyopathy, n (%) | 105 (70.0) | 105 (70.0) | 1.00 | |
| Left ventricular ejection fraction (%) | 290 | 26.5 (25.0–30.0) | 30.0 (25.0–32.0) | 0.03 |
| QRS duration, n (%) | 243 | |||
| <120 ms | 37 (32.2) | 20 (15.6) | 0.01 | |
| 120–150 ms | 36 (31.3) | 52 (40.6) | ||
| >150 ms | 42 (36.5) | 56 (43.8) | ||
| New York Heart Association class, n (%) | 288 | |||
| I | 11 (7.9) | 15 (10.1) | 0.80 | |
| II | 57 (40.7) | 54 (36.5) | ||
| III | 64 (45.7) | 72 (48.7) | ||
| IV | 8 (5.7) | 7 (4.7) | ||
| Creatinine clearance, n (%) | 187 | |||
| <30 mL/min/1.73 m2 | 7 (9.6) | 16 (14.0) | <0.0001 | |
| 30–60 mL/min/1.73 m2 | 20 (27.4) | 66 (57.9) | ||
| >60 mL/min/1.73 m2 | 46 (63.0) | 32 (28.1) | ||
| Atrial fibrillation history, n (%) | 275 | 20 (15.4) | 60 (39.3) | <0.0001 |
| Comorbidity number, n (%) | 260 | |||
| 0 | 34 (26.4) | 28 (21.4) | 0.08 | |
| 1 | 79 (61.2) | 71 (54.2) | ||
| 2 | 13 (10.1) | 23 (17.6) | ||
| ≥3 | 3 (2.3) | 9 (6.9) | ||
| Cardiovascular medication, n (%) | 278 | |||
| Beta-blocker | 104 (77.6) | 111 (77.1) | 0.92 | |
| Amiodarone | 33 (24.6) | 49 (34.0) | 0.09 | |
| Digoxin | 8 (6.0) | 6 (4.2) | 0.49 | |
| Sotalol | 0 | 1 (0.7) | 1.00a | |
| Antiplatelet | 79 (59.0) | 90 (62.5) | 0.55 | |
| ACE inhibitor | 113 (84.3) | 118 (81.9) | 0.60 | |
| Furosemide | 112 (83.6) | 105 (72.9) | 0.03 | |
| MRA | 32 (23.9) | 23 (16.0) | 0.10 | |
| Vitamin K antagonist | 51 (38.1) | 67 (46.5) | 0.15 | |
| Calcium inhibitor | 1 (0.8) | 2 (1.4) | 1.00a | |
| Implantable cardioverter-defibrillator type | ||||
| Cardiac resynchronizarion therapy device, n (%) | 69 (46.3) | 111 (74.0) | <0.0001 |
Data are expressed as mean ± standard deviation, median (1st quartile–3rd quartile), or number (%).
ACE, angiotensin-converting enzyme inhibitor; ICD, implantable cardioverter-defibrillator; MRA, mineralocorticoid receptor antagonist.
The Fisher’s exact test.
Compared with the 150 matched controls (Table 1), elderly patients had higher median LVEF (30.0% vs. 26.5%; P = 0.03), longer QRS duration (P = 0.01), higher prevalence of chronic kidney disease (P < 0.0001), and AF (39.3% vs. 15.4%; P < 0.0001).
Follow-up and survival analysis
Appropriate/inappropriate therapies and complications
The mean follow-up was 3.1 ± 2.4 years in ICD recipients ≥80 years and 2.9 ± 2.0 years in younger controls (P = 0.34) (Table 2). At least one appropriate therapy occurred in 26 (19.4%) ICD recipients ≥80 years vs. 32 (21.6%) patients in the younger group (P = 0.65). Survival curves showed that the probability of appropriate therapy was not significantly different between elderly patients and controls (log-rank test, P = 0.48) (Figure 1). In patients with CRT, the proportion of appropriate therapy was 19% in the elderly group and 25% in the control group (P = 0.33). Even among patients without a CRT device, the proportion of appropriate therapy was not statistically different between older patients and controls (21% vs. 18%, respectively; P = 0.70). Moreover, among elderly patients the proportion of appropriate therapy was very similar between CRT responders and CRT non-responders (20% vs. 21%; P = 0.81). In multivariate analysis, accounting for sex, LVEF, NYHA class, creatinine clearance, AF history, and the presence of CRT device, older age (≥80 years) was not more associated with the occurrence of appropriate therapy compared with younger age group (<80 years) [hazard ratio 1.14, 95% confidence interval (CI) 0.67–1.79; P = 0.59]. Median duration from implantation to first therapy was 1.8 (0.3–3.4) years in ICD recipients ≥80 years and 1.1 (0.5–2.5) years in the younger control group (P = 0.55).
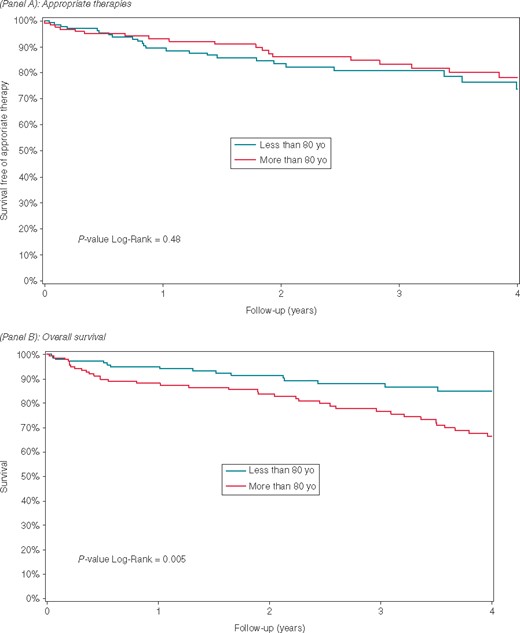
The Kaplan–Meier curve illustrating freedom from appropriate therapies (A) and overall survival (B).
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Mean follow-up (years) | 2.9 ± 2 | 3.1 ± 2.4 | 0.34 | |
| ≥1 appropriate therapy, n (%) | 282 | 32 (21.6) | 26 (19.4) | 0.65 |
| Implantation-first therapy delay (years), median (IQR) | 50 | 1.1 (0.5–2.5) | 1.8 (0.3–3.4) | 0.55 |
| Early complications (≤30 days), n (%) | 296 | 21 (14) | 31 (21.2) | 0.10 |
| Lead dysfunction | 5 (3.3) | 6 (4.1) | 0.72 | |
| Bleeding/haematoma | 5 (3.3) | 15 (10.3) | 0.02 | |
| Sepsis | 2 (1.3) | 0 | 0.50a | |
| Tamponade | 0 | 1 (0.7) | 0.49a | |
| Pneumothorax | 1 (0.7) | 0 | 1.00a | |
| Death | 0 | 0 | – | |
| Other | 8 (5.3) | 9 (6.2) | 0.76 | |
| Late complications (>30 days), n (%) | 287 | 19 (12.9) | 16 (11.5) | 0.73 |
| Lead dysfunction | 8 (5.4) | 10 (7.2) | 0.53 | |
| Sepsis | 5 (3.4) | 2 (1.4) | 0.45a | |
| Inappropriate shock | 5 (3.4) | 2 (1.4) | 0.45a | |
| Other | 1 (0.7) | 2 (1.4) | 0.61a |
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Mean follow-up (years) | 2.9 ± 2 | 3.1 ± 2.4 | 0.34 | |
| ≥1 appropriate therapy, n (%) | 282 | 32 (21.6) | 26 (19.4) | 0.65 |
| Implantation-first therapy delay (years), median (IQR) | 50 | 1.1 (0.5–2.5) | 1.8 (0.3–3.4) | 0.55 |
| Early complications (≤30 days), n (%) | 296 | 21 (14) | 31 (21.2) | 0.10 |
| Lead dysfunction | 5 (3.3) | 6 (4.1) | 0.72 | |
| Bleeding/haematoma | 5 (3.3) | 15 (10.3) | 0.02 | |
| Sepsis | 2 (1.3) | 0 | 0.50a | |
| Tamponade | 0 | 1 (0.7) | 0.49a | |
| Pneumothorax | 1 (0.7) | 0 | 1.00a | |
| Death | 0 | 0 | – | |
| Other | 8 (5.3) | 9 (6.2) | 0.76 | |
| Late complications (>30 days), n (%) | 287 | 19 (12.9) | 16 (11.5) | 0.73 |
| Lead dysfunction | 8 (5.4) | 10 (7.2) | 0.53 | |
| Sepsis | 5 (3.4) | 2 (1.4) | 0.45a | |
| Inappropriate shock | 5 (3.4) | 2 (1.4) | 0.45a | |
| Other | 1 (0.7) | 2 (1.4) | 0.61a |
Data are expressed as mean ± standard deviation, median (1st quartile–3rd quartile), or number (%).
ICD, implantable cardioverter-defibrillator; IQR, interquartile range.
The Fisher’s exact test.
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Mean follow-up (years) | 2.9 ± 2 | 3.1 ± 2.4 | 0.34 | |
| ≥1 appropriate therapy, n (%) | 282 | 32 (21.6) | 26 (19.4) | 0.65 |
| Implantation-first therapy delay (years), median (IQR) | 50 | 1.1 (0.5–2.5) | 1.8 (0.3–3.4) | 0.55 |
| Early complications (≤30 days), n (%) | 296 | 21 (14) | 31 (21.2) | 0.10 |
| Lead dysfunction | 5 (3.3) | 6 (4.1) | 0.72 | |
| Bleeding/haematoma | 5 (3.3) | 15 (10.3) | 0.02 | |
| Sepsis | 2 (1.3) | 0 | 0.50a | |
| Tamponade | 0 | 1 (0.7) | 0.49a | |
| Pneumothorax | 1 (0.7) | 0 | 1.00a | |
| Death | 0 | 0 | – | |
| Other | 8 (5.3) | 9 (6.2) | 0.76 | |
| Late complications (>30 days), n (%) | 287 | 19 (12.9) | 16 (11.5) | 0.73 |
| Lead dysfunction | 8 (5.4) | 10 (7.2) | 0.53 | |
| Sepsis | 5 (3.4) | 2 (1.4) | 0.45a | |
| Inappropriate shock | 5 (3.4) | 2 (1.4) | 0.45a | |
| Other | 1 (0.7) | 2 (1.4) | 0.61a |
| . | N . | ICD recipients <80 years old (N = 150) . | ICD recipients ≥80 years old (N = 150) . | P-value . |
|---|---|---|---|---|
| Mean follow-up (years) | 2.9 ± 2 | 3.1 ± 2.4 | 0.34 | |
| ≥1 appropriate therapy, n (%) | 282 | 32 (21.6) | 26 (19.4) | 0.65 |
| Implantation-first therapy delay (years), median (IQR) | 50 | 1.1 (0.5–2.5) | 1.8 (0.3–3.4) | 0.55 |
| Early complications (≤30 days), n (%) | 296 | 21 (14) | 31 (21.2) | 0.10 |
| Lead dysfunction | 5 (3.3) | 6 (4.1) | 0.72 | |
| Bleeding/haematoma | 5 (3.3) | 15 (10.3) | 0.02 | |
| Sepsis | 2 (1.3) | 0 | 0.50a | |
| Tamponade | 0 | 1 (0.7) | 0.49a | |
| Pneumothorax | 1 (0.7) | 0 | 1.00a | |
| Death | 0 | 0 | – | |
| Other | 8 (5.3) | 9 (6.2) | 0.76 | |
| Late complications (>30 days), n (%) | 287 | 19 (12.9) | 16 (11.5) | 0.73 |
| Lead dysfunction | 8 (5.4) | 10 (7.2) | 0.53 | |
| Sepsis | 5 (3.4) | 2 (1.4) | 0.45a | |
| Inappropriate shock | 5 (3.4) | 2 (1.4) | 0.45a | |
| Other | 1 (0.7) | 2 (1.4) | 0.61a |
Data are expressed as mean ± standard deviation, median (1st quartile–3rd quartile), or number (%).
ICD, implantable cardioverter-defibrillator; IQR, interquartile range.
The Fisher’s exact test.
Overall, early complications tended to be greater in the older group, but it did not reach statistical significance: [31 (21.2%) early complications in ≥80 years vs. 21 (14.0%) in controls (P = 0.10)], with a higher occurrence of haematoma among elderly (10.3% vs. 3.3%; P = 0.02). By multivariate logistic regression, being an ICD recipient ≥80 years was not independently associated with the occurrence of early complications (odds ratio 1.66, 95% CI 0.90–3.04; P = 0.10). There were no deaths related to ICD implantation in either group. There were no differences with regard to frequency of lead dysfunction, sepsis, tamponade, and pneumothorax. The overall late complication rate as well as frequency of individual late complications after ICD implantation, were not significantly different between the two groups. Two patients (1.4%) received inappropriate shocks in the ≥80-year group, related to AF.
The total rate of combined early and late complications, was not different between the two groups: 24.0% and 26.7% in younger and older patients, respectively (P = 0.60).
Mortality and cause of death
Overall death rate was higher in ICD recipients ≥80 years compared with younger controls (36.3% vs. 12.9%; P < 0.0001); this was also reflected in the Kaplan–Meier curves and log-rank test (Figure 1). Fifty three patients (36.3%) died during follow-up among the elderly group, giving an annual mortality incidence of 11.6 per 100 patient-years (95% CI 8.4–14.8); the younger group had an annual mortality rate about 4.5 per 100 patient-years (95% CI 2.5–6.5). Even among patients with creatinine clearance ≥30 mL/min/1.73 m2, mortality was higher in the elderly compared with the younger group (36% vs. 13%, respectively; P < 0.0001). Mortality among ischaemic aetiology patients was 42% in the elderly vs. 15% in the younger group (P < 0.0001); among the non-ischaemic patients, mortality was 24% in the older group and 9% in the control group (P = 0.05). Among the elderly with cause of death identified (64.1%), 21 patients (61.8%) died from cardiovascular and 13 (38.2%) from non-cardiovascular causes. Among elderly patients who died, 18 patients (34.0%) had received at least one appropriate ICD therapy prior to death.
The delay between first ICD therapy and death was not different between ICD recipients ≥80 years and younger controls (2.7 ± 2.6 vs. 2.1 ± 2.8 years, respectively; P = 0.79).
Discussion
This study provides useful data on real-world outcomes in very elderly ICD recipients implanted for primary prevention. To the best of our knowledge, this is the largest study systematically comparing ICD recipients ≥80 years to younger controls matched for sex and underlying cardiomyopathy. Of note, the elderly ICD recipients in our study had relatively few associated comorbidities, likely reflecting careful selection by treating clinicians, of appropriate candidates for implantation in this potentially high-risk group. In this context, our data show comparable outcomes, both in terms of appropriate therapies as well as complications, in the elderly cases when compared with younger controls.
Safety of implantable cardioverter-defibrillator implantation in elderly patients
The a priori potential for increased complications as well overall higher mortality may discourage physicians from implanting ICD in elderly patients. Indeed, in our study, early complications tended to be more frequent (although not statistically significant), among ICD recipients ≥80 years old, with especially more haematoma compared with younger controls. In contrast, haematoma was not seen to be more frequent in a previous study.15 The higher observed rate of cardiac resynchronization therapy-defibrillator (CRT-D) in the elderly group may also have confounded this association. Elderly patients with an indication for CRT may be more likely to get a primary prevention ICD as part of a CRT-D, whereas treating cardiologists might be reluctant to implant single- or dual-chamber ICDs per se for primary prevention in the older population. This relatively higher proportion of CRT was also described in another study using 75 years as age cut-off.16 The high prevalence of AF in the elderly, with attendant need for anticoagulation may also contribute to the higher rate of haematoma.
During long-term follow-up, the rate of inappropriate shocks, mainly due to supra-ventricular arrhythmias, was extremely low. This rate was similar to other previous studies, which have shown an inverse relationship between age and the likelihood of inappropriate therapies, in view of a less physically active older population.17 The high cut-off rate programming in accordance with MADIT-RIT and the Cardiac Arrhythmias Group of the French Society of Cardiology internal consensus also likely contributed to minimizing inappropriate therapies.18
Beyond safety: potential relevance of implantable cardioverter-defibrillator in elderly patients
The 2017 AHA/ACC/HRS clinical guidelines and the 2015 European Society of Cardiology (ESC) guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of SCD advise ICD implantation with a Class IIa recommendation in older populations if ‘survival of greater than 1 year is expected’, but do not mention any specific age cut-off or frailty criteria.19,20 The benefit of ICD in the elderly population is debated and it is generally acknowledged that the evidence base is not as strong in this age group. In SCD primary prevention trials, patients ≥75 years old constituted less than 10% of those enrolled, although 28% of Americans eligible for ICD implantation are ≥80 years.21 In a subgroup analysis from MADIT II, the population aged ≥75 years appeared to benefit from ICD therapy, with a 44% reduction in all-cause mortality compared with medical treatment, especially when patients did not have risk factors associated with non-SCD (factors proposed by Goldenberg et al.22 from MADIT II).23 However, the other large pivotal randomized trial, SCD-HeFT, failed to demonstrate a benefit of implanting ICD in very old patients.2 When looking at observational studies, patients ≥75 years had similar appropriate shock rates compared with younger subjects, but without evidence of prolonging survival.24,25 Also, a pooled analysis of secondary prevention ICD trials (with patients at much higher risk than the regular primary prevention group) reported that there was no benefit of the ICD beyond 75 years of age, likely due to a higher rate of non-arrhythmic death.26 Here, in the largest study assessing ICD recipients ≥80 years, 34% of patients received at least one appropriate therapy. Although appropriate therapy is not a good surrogate of aborted SCD, this figure suggests that the ICD is of potential relevance to prevent SCD in selected older individuals.
Competing risk and appropriate patient selection
Non-arrhythmic mortality is a competing risk to arrhythmic sudden cardiac death. Indeed, the main cause of death in a primary prevention ICD population remains progressive heart failure. According to Koplan, the average survival of an octogenarian with ejection fraction <30% and renal failure (creatinine clearance <40 mL/min) is an estimated 19 months.27 However, it is obvious that there is considerable heterogeneity even among the elderly population and careful patient selection through overall health assessment is therefore the key. In the CeRtiTuDe cohort study for example, older patients with greater comorbidity were observed to be more likely to receive cardiac resynchronization therapy-pacemaker (CRT-P) rather than CRT-D therapy, with finally no significant excess of SCD mortality in the CRT-P group, reflecting good clinical judgement.28
Multidisciplinary evaluation involving a geriatrician and other specialists, taking into account biological (not chronological) age, autonomy and cognitive abilities would help ascertain the best candidates for ICD implantation among the elderly.14,29 Another way to improve screening is the use of clinical scores aimed at determining overall risk of death. Several scores have been developed but remain to be prospectively tested.30
Limitations
Although we have reported real-world data from a large multicentre cohort of elderly patients with specific cause of death analysis, we need to acknowledge some limitations. We did not compare ICD recipients with non-implanted patients, and therefore, cannot definitively assess the extent to which ICD may reduce mortality in this group; only a randomized trial design can achieve this. Rather we aimed to provide an indirect assessment by looking at specific causes of mortality. Secondly, because only the date of first appropriate therapy is known, we were unable to evaluate the extent to which early post shock mortality differs between the elderly vs. younger patients as we did not have that information. Thirdly, as mentioned earlier, the extent to which appropriate therapies may reflect truly life-saving events is debatable. Moreover, the proportion of CRT and renal failure were much higher in the elderly group compared with controls, which could be a source of potential residual bias, even after adjustment, and our results should be viewed in this perspective. Lastly, it appears that the ICD recipients ≥80 years in the current study were particularly well selected by treating physicians in terms of overall health status, as they had very few comorbidities. On the other hand, these data likely reflect the actual way of selection in real-life, less prone to potential selection bias found in randomized trial populations and therefore relevant to daily clinical practice.
Conclusion
This large, population-based real-world assessment shows that among patients ≥80 years undergoing primary prevention ICD implantation, one-third received at least one appropriate therapy prior to death. Although there was a trend towards more early complications compared with younger recipients, late complications and inappropriate therapies were similar. Our results suggest that among well selected older subjects with relatively few comorbidities, as reflected in our study population, the primary prevention ICD may be of relevance. Nevertheless, the inherent limitations in interpreting observational data in this particular competing risk situation call for randomized controlled trials to provide definitive answers. Meanwhile, a careful multidisciplinary evaluation is needed to guide patient selection for ICD implantation in the elderly population.
Acknowledgements
We thank the investigators of the enrolling medical centres for participating in the study and sharing their data, the Research Associates Nicolas Estrugo, Sandrine Hervouet, Nathalie de Carsin, Radu Mosei, Juliette Tennenbaum, Raoul Hubac-Coupet, Alexandre Bendavid, and Marine Sroussi for collecting the data, and the Arrhythmia Group of the French Society of Cardiology for supporting this project.
Funding
This work was supported by the following independent institutions: the French Institute of Health and Medical Research, the Toulouse Association for the Study of Rhythm Disturbances, and the French Society of Cardiology.
Conflict of interest: R.G. received consulting fees from Abbott and Boston Scientific. N.L. received consulting fees from Medtronic, Abott and Boston Scientific. D.G. received consulting fees from Biotronik, Medtronic, Abbott and Boston Scientific. O.P. received consulting fees and research grants from Abbott, Medtronic and Microport. D.B. received research grants from Medtronic and Boston Scientific. N.S. received consulting fees from Biotronik, Medtronic, Abbott and Microport. P.D. received consulting fees and research grants from Boston Scientific, Medtronic, Abbott and Microport. D.K. received consulting fees from Abbott, Medtronic, Microport, Boston Scientific, and Biotronik. C.L. research grants from Biotronik, Medtronic, Abbott and Boston Scientific. S.B. has received consulting fees from Medtronic, Boston Scientific and Microport. E.M. received consulting fees and research grants from Zoll, Bristol Myers Squibb, Bayer, Medtronic, Abbott, Biotronik, Boston Scientific and Microport. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
Van
Author notes
Cyril Zakine and Rodrigue Garcia authors are co-first authors.



