-
PDF
- Split View
-
Views
-
Cite
Cite
Gerhard Hindricks, Stanislav Weiner, Tom McElderry, Pierre Jaïs, William Maddox, Jose Ignacio Garcia-Bolao, Sang Yong Ji, Frederic Sacher, Stephan Willems, John Mounsey, Philippe Maury, Andreas Bollmann, Elizabeth Duffy, Giovanni Raciti, Roderick Tung, Tom Wong, Acute safety, effectiveness, and real-world clinical usage of ultra-high density mapping for ablation of cardiac arrhythmias: results of the TRUE HD study, EP Europace, Volume 21, Issue 4, April 2019, Pages 655–661, https://doi.org/10.1093/europace/euy191
Close - Share Icon Share
Abstract
The objective of this study was to verify acute safety, performance, and usage of a novel ultra-high density mapping system in patients undergoing ablation procedure in a real-world clinical setting.
The TRUE HD study enrolled patients undergoing catheter ablation with mapping for all arrhythmias (excluding de novo atrial fibrillation) who were followed for 1 month. Safety was determined by collecting all serious adverse events and adverse events associated with the study devices. Performance was determined as the composite of: ability to map the arrhythmia/substrate, complete the ablation applications, arrhythmia termination (where applicable), and ablation validation. Use of mapping system in the ablation validation workflow was also evaluated. Among the 519 patients who underwent a complete (504) or attempted (15) procedure, 21 (4%) serious ablation-related complications were collected, with 3 (0.57%) potentially related to the mapping catheter. Four hundred and twenty treated patients resulted in a successful procedure confirmed by arrhythmia-specific validation techniques (83.3%; 95% confidence interval: 79.8–86.5%). A total of 1419 electroanatomical maps were created with a median acquisition time of 9:23 min per map. Of these, 372 maps in 222 (44%) patients were collected for ablation validation purposes. Following validation mapping, 162/222 (73%) patients required additional ablation.
In the TRUE HD study mapping was associated with rates of acute success and complications consistent with previously published reports. Importantly, a low percentage of events (0.57%) was attributed to the mapping catheter. When performed, validation mapping was useful for identifying additional targets for ablation in the majority of patients.
This is the first prospective study to perform a systematic data collection to assess the acute safety, performance, and clinical use of a novel ultra-high density mapping technology as standard-of-care.
Acute safety of a 64 poles multi-spline basket catheter in a wide set of clinical arrhythmias and all cardiac chambers was satisfactory and showed a low rate of device-related adverse events (0.57%).
The mapping catheter performed as intended, with the percentage of cases where inability to collect a map resulted in a procedure failure was limited to 15 (2.8%) cases.
Mapping time is reasonably short, in particular for those maps collected for validation purposes.
Mapping for validation purposes after ablation was not performed in approximately half of the procedure workflows but, when performed, it identified the need for additional radio frequency lesions in the majority of patients.
Introduction
The advent of navigational and mapping technologies in the 1990s revolutionized the interventional approaches and strategies for the ablation of arrhythmias. Contemporary high-resolution mapping systems are capable of acquiring and annotating multiple electrograms (EGMs), which are processed by automated algorithms to generate activation and substrate maps to support and guide ablation procedures. More recently, a novel ultra-high density (UHD) electroanatomic mapping system enables rapid automatic acquisition of high-resolution maps through a 64 pole basket array catheter with small and closely spaced electrodes in combination with a dedicated mapping platform (Boston Scientific, Marlborough, MA, USA). The unique characteristics of rapid and automatic acquisition of maps with high spatiotemporal resolution, without the need for extensive manual annotation, has been evaluated in pre-clinical and initial experience studies.1–3 Furthermore, a number of human studies reported data in a wide range of real-world clinical settings including the use of mapping for both atrial and ventricular arrhythmias.4–14 Although most of these studies have shown that the system is safe, efficacious and clinically useful in specific settings, to date there has been no prospective study to assess the acute safety, acute effectiveness and clinical use of the system on a wide spectrum of different arrhythmias to guide ablation in real-world clinical practice. The TRUE HD study (Prospective Registry on User Experience With The Rhythmia™ Mapping System For Ablation Procedures) was conducted to address this question.
Methods
Study design
TRUE HD (NCT02698670) is a prospective, non-randomized, and observational multicenter study. The objective was to assess the acute safety, procedure success, and utility of a novel UHD mapping system, paired with a 64 pole basket array catheter with small and closely spaced electrodes (RhythmiaTM mapping system, IntellaMap OrionTM mapping catheter, Boston Scientific, Marlborough, MA, USA) in patients who underwent an ablation procedure in a real-world clinical setting. The study enrolled adults who had a clinical indication for an ablative procedure to treat the arrhythmia. All arrhythmias were included in this study with an exception of de novo atrial fibrillation (AF). Other main exclusion criteria are subjects who had undergone previous cardiac ablation within 30 days prior to enrolment and patients with unresolved adverse events from any previous invasive procedure. Patients were followed through enrolment, ablation procedure, pre-discharge, and 1 month follow-up. All sites obtained Ethics Committee/Investigation Review Boards approval of recruitment.
Study endpoints
The primary effectiveness endpoint was the acute procedural success for mapping and ablation of the primary clinical arrhythmia. A procedure was considered acutely successful if all the following criteria were met: (i) ability to map the primary arrhythmia (if ongoing) or its electro-anatomical substrate with the Rhythmia™ mapping system, (ii) completion of the necessary ablation lesions, (iii) arrhythmia termination, (when applicable), and (iv) ablation verification through a documented validation technique.
Procedures where the multi-electrode basket mapping catheter entered the cardiac chamber but no electro-anatomic maps were created for either diagnosis or validation purposes were categorized as attempt regardless of whether ablation was achieved or not. Procedures with at least one utilized map for diagnosis or validation defined the treatment cases. Success with validation in treatment cases was required to count a success for the primary effectiveness endpoint.
The primary safety endpoint included all serious adverse events and study devices related adverse events within 30 days after the procedure. Seriousness and relationship with the procedure/device were determined by the investigator at each study site.
Secondary endpoints included: types of treated arrhythmias, mapping system usage data (utilized maps per patient, number of points, mapping time), ablation techniques, and acute success for the arrhythmia types.
In order to categorize mapping system data, maps were classified as diagnostic or validation (vMapTM, Boston Scientific) maps depending on their use in the context of the procedure (i.e. to determine the initial ablation strategy or to validate the results of ablation, respectively). Information on maps that were not utilized or failed due to lack of acquisition was collected in order to assess the systems technical and clinical performance.
Study devices
All patients enrolled in the study and undergoing the index ablation procedure were required to use the IntellaMap Orion 64 pole basket catheter in conjunction of the Rhythmia mapping system. Any commercially available ablation catheter could be used in the study. Additional diagnostic catheters could be used at the discretion of the physicians.
Data analysis and statistical considerations
A sample size of 500 ablation procedures to assess the rate of acute procedural success was based on the assumption of an acute procedural success rate of 77% with a two sided 95% confidence interval (CI) of 7.4% width. The anticipated rate was estimated based on expected proportion of the different arrhythmia types in the sample and success rates for single arrhythmias available from literature.15–17 Descriptive statistics were generated for the data collected at baseline, during the ablation procedure, at pre-discharge and 1 month follow-up. For continuous variables, the mean, standard deviation, median, range, and 95% CIs were reported. For discrete variables, absolute value and proportion were reported. A 95% CI based upon the Wilson-Score method was constructed for the proportion of procedures resulting in acute procedural success. To reduce potential bias in proportion of arrhythmias and ablation techniques, enrolment was limited to no more than 50 subjects at any single site. Statistical analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Population and patient enrolment
The study completed follow-up in May 2017 with a total of 572 patients enrolled at 27 global sites and resulted in a total of 504 treated cases, 15 attempted cases and the remaining not undergoing a mapping procedure. Figure 1 shows the patient flow diagram. Mapping and ablation was performed by 77 operating physicians (range: 1–6, 3 median operators per site).
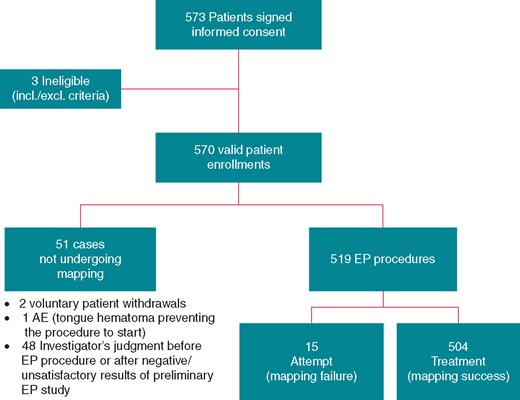
Subjects enrolment flowchart. AE, adverse event; EP, electrophysiology.
Patient clinical demographics and characteristics are summarized in Table 1. Patients withdrawn before the procedure or who did not complete the procedure were not different from those treated except for patient age and arrhythmia history/ablation history.
| Characteristics . | All patients (570) . | Treatment (504) . | Non-treated or attempted (66) . | P-value . |
|---|---|---|---|---|
| Age (years) | 62 ± 14 | 63 ± 13 | 57 ± 16 | 0.001 |
| Male gender, N (%) | 350 (61.3) | 310 (61.4) | 40 (60.6) | 0.90 |
| Valvular heart disease | 142 (24.9) | 129 (25.6) | 13 (19.7) | 0.30 |
| Coronary artery disease | 92 (16.1) | 79 (15.7) | 13 (19.7) | 0.40 |
| Congestive heart failure | 61 (10.7) | 57 (11.3) | 4 (6.1) | 0.19 |
| Stroke | 50 (8.8) | 46 (9.1) | 4 (6.1) | 0.41 |
| Clinical arrhythmia history | ||||
| Ventricular tachycardia | 123 (21.6) | 104 (20.6) | 19 (28.8) | 0.13 |
| Atrial fibrillation | 316 (55.7) | 298 (59.1) | 18 (28.6) | <0.001 |
| Atrial tachycardia | 130 (23.1) | 117 (23.4) | 13 (20.3) | 0.58 |
| CTI dependent atrial flutter | 252 (44.9) | 230 (46.2) | 22 (34.9) | 0.09 |
| Non-macroreentrant atrial tachycardia | 33 (5.9) | 28 (5.6) | 5 (7.9) | 0.46 |
| Cardiac procedure history | ||||
| PTCA | 34 (6.0) | 33 (6.6) | 1 (1.5) | 0.10 |
| CABG | 28 (4.9) | 25 (5.0) | 3 (4.5) | 0.88 |
| Pacemaker | 46 (8.1) | 41 (8.1) | 5 (7.6) | 0.88 |
| ICD | 45 (7.9) | 41 (8.1) | 4 (6.1) | 0.56 |
| Cardiac valve | 45 (7.9) | 43 (8.6) | 2 (3.0) | 0.12 |
| Previous catheter ablation | 327 (57.4) | 304 (60.3) | 23 (34.8) | <0.001 |
| Comorbidities | ||||
| COPD | 36 (6.3) | 31 (6.2) | 5 (7.6) | 0.66 |
| Diabetes | 93 (16.3) | 80 (15.9) | 13 (19.7) | 0.43 |
| Hepatic disease | 11 (1.9) | 9 (1.8) | 2 (3.0) | 0.49 |
| Neurological disease | 16 (2.8) | 15 (3.0) | 1 (1.5) | 0.50 |
| Renal disease | 41 (7.2) | 36 (7.1) | 5 (7.6) | 0.90 |
| Gastrointestinal bleeding | 13 (2.3) | 13 (2.6) | 0 (0.0) | 0.19 |
| Hyperlipidaemia | 237 (41.6) | 213 (42.3) | 24 (36.4) | 0.36 |
| Sleep-disordered breathing | 72 (12.7) | 64 (12.7) | 8 (12.1) | 0.89 |
| Other comorbidities | 177 (31.1) | 162 (32.1) | 15 (22.7) | 0.12 |
| Characteristics . | All patients (570) . | Treatment (504) . | Non-treated or attempted (66) . | P-value . |
|---|---|---|---|---|
| Age (years) | 62 ± 14 | 63 ± 13 | 57 ± 16 | 0.001 |
| Male gender, N (%) | 350 (61.3) | 310 (61.4) | 40 (60.6) | 0.90 |
| Valvular heart disease | 142 (24.9) | 129 (25.6) | 13 (19.7) | 0.30 |
| Coronary artery disease | 92 (16.1) | 79 (15.7) | 13 (19.7) | 0.40 |
| Congestive heart failure | 61 (10.7) | 57 (11.3) | 4 (6.1) | 0.19 |
| Stroke | 50 (8.8) | 46 (9.1) | 4 (6.1) | 0.41 |
| Clinical arrhythmia history | ||||
| Ventricular tachycardia | 123 (21.6) | 104 (20.6) | 19 (28.8) | 0.13 |
| Atrial fibrillation | 316 (55.7) | 298 (59.1) | 18 (28.6) | <0.001 |
| Atrial tachycardia | 130 (23.1) | 117 (23.4) | 13 (20.3) | 0.58 |
| CTI dependent atrial flutter | 252 (44.9) | 230 (46.2) | 22 (34.9) | 0.09 |
| Non-macroreentrant atrial tachycardia | 33 (5.9) | 28 (5.6) | 5 (7.9) | 0.46 |
| Cardiac procedure history | ||||
| PTCA | 34 (6.0) | 33 (6.6) | 1 (1.5) | 0.10 |
| CABG | 28 (4.9) | 25 (5.0) | 3 (4.5) | 0.88 |
| Pacemaker | 46 (8.1) | 41 (8.1) | 5 (7.6) | 0.88 |
| ICD | 45 (7.9) | 41 (8.1) | 4 (6.1) | 0.56 |
| Cardiac valve | 45 (7.9) | 43 (8.6) | 2 (3.0) | 0.12 |
| Previous catheter ablation | 327 (57.4) | 304 (60.3) | 23 (34.8) | <0.001 |
| Comorbidities | ||||
| COPD | 36 (6.3) | 31 (6.2) | 5 (7.6) | 0.66 |
| Diabetes | 93 (16.3) | 80 (15.9) | 13 (19.7) | 0.43 |
| Hepatic disease | 11 (1.9) | 9 (1.8) | 2 (3.0) | 0.49 |
| Neurological disease | 16 (2.8) | 15 (3.0) | 1 (1.5) | 0.50 |
| Renal disease | 41 (7.2) | 36 (7.1) | 5 (7.6) | 0.90 |
| Gastrointestinal bleeding | 13 (2.3) | 13 (2.6) | 0 (0.0) | 0.19 |
| Hyperlipidaemia | 237 (41.6) | 213 (42.3) | 24 (36.4) | 0.36 |
| Sleep-disordered breathing | 72 (12.7) | 64 (12.7) | 8 (12.1) | 0.89 |
| Other comorbidities | 177 (31.1) | 162 (32.1) | 15 (22.7) | 0.12 |
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CTI, cavo-tricuspid isthmus; ICD, implantable cardioverter-defibrillator; PTCA, percutaneous transluminal coronary angioplasty.
| Characteristics . | All patients (570) . | Treatment (504) . | Non-treated or attempted (66) . | P-value . |
|---|---|---|---|---|
| Age (years) | 62 ± 14 | 63 ± 13 | 57 ± 16 | 0.001 |
| Male gender, N (%) | 350 (61.3) | 310 (61.4) | 40 (60.6) | 0.90 |
| Valvular heart disease | 142 (24.9) | 129 (25.6) | 13 (19.7) | 0.30 |
| Coronary artery disease | 92 (16.1) | 79 (15.7) | 13 (19.7) | 0.40 |
| Congestive heart failure | 61 (10.7) | 57 (11.3) | 4 (6.1) | 0.19 |
| Stroke | 50 (8.8) | 46 (9.1) | 4 (6.1) | 0.41 |
| Clinical arrhythmia history | ||||
| Ventricular tachycardia | 123 (21.6) | 104 (20.6) | 19 (28.8) | 0.13 |
| Atrial fibrillation | 316 (55.7) | 298 (59.1) | 18 (28.6) | <0.001 |
| Atrial tachycardia | 130 (23.1) | 117 (23.4) | 13 (20.3) | 0.58 |
| CTI dependent atrial flutter | 252 (44.9) | 230 (46.2) | 22 (34.9) | 0.09 |
| Non-macroreentrant atrial tachycardia | 33 (5.9) | 28 (5.6) | 5 (7.9) | 0.46 |
| Cardiac procedure history | ||||
| PTCA | 34 (6.0) | 33 (6.6) | 1 (1.5) | 0.10 |
| CABG | 28 (4.9) | 25 (5.0) | 3 (4.5) | 0.88 |
| Pacemaker | 46 (8.1) | 41 (8.1) | 5 (7.6) | 0.88 |
| ICD | 45 (7.9) | 41 (8.1) | 4 (6.1) | 0.56 |
| Cardiac valve | 45 (7.9) | 43 (8.6) | 2 (3.0) | 0.12 |
| Previous catheter ablation | 327 (57.4) | 304 (60.3) | 23 (34.8) | <0.001 |
| Comorbidities | ||||
| COPD | 36 (6.3) | 31 (6.2) | 5 (7.6) | 0.66 |
| Diabetes | 93 (16.3) | 80 (15.9) | 13 (19.7) | 0.43 |
| Hepatic disease | 11 (1.9) | 9 (1.8) | 2 (3.0) | 0.49 |
| Neurological disease | 16 (2.8) | 15 (3.0) | 1 (1.5) | 0.50 |
| Renal disease | 41 (7.2) | 36 (7.1) | 5 (7.6) | 0.90 |
| Gastrointestinal bleeding | 13 (2.3) | 13 (2.6) | 0 (0.0) | 0.19 |
| Hyperlipidaemia | 237 (41.6) | 213 (42.3) | 24 (36.4) | 0.36 |
| Sleep-disordered breathing | 72 (12.7) | 64 (12.7) | 8 (12.1) | 0.89 |
| Other comorbidities | 177 (31.1) | 162 (32.1) | 15 (22.7) | 0.12 |
| Characteristics . | All patients (570) . | Treatment (504) . | Non-treated or attempted (66) . | P-value . |
|---|---|---|---|---|
| Age (years) | 62 ± 14 | 63 ± 13 | 57 ± 16 | 0.001 |
| Male gender, N (%) | 350 (61.3) | 310 (61.4) | 40 (60.6) | 0.90 |
| Valvular heart disease | 142 (24.9) | 129 (25.6) | 13 (19.7) | 0.30 |
| Coronary artery disease | 92 (16.1) | 79 (15.7) | 13 (19.7) | 0.40 |
| Congestive heart failure | 61 (10.7) | 57 (11.3) | 4 (6.1) | 0.19 |
| Stroke | 50 (8.8) | 46 (9.1) | 4 (6.1) | 0.41 |
| Clinical arrhythmia history | ||||
| Ventricular tachycardia | 123 (21.6) | 104 (20.6) | 19 (28.8) | 0.13 |
| Atrial fibrillation | 316 (55.7) | 298 (59.1) | 18 (28.6) | <0.001 |
| Atrial tachycardia | 130 (23.1) | 117 (23.4) | 13 (20.3) | 0.58 |
| CTI dependent atrial flutter | 252 (44.9) | 230 (46.2) | 22 (34.9) | 0.09 |
| Non-macroreentrant atrial tachycardia | 33 (5.9) | 28 (5.6) | 5 (7.9) | 0.46 |
| Cardiac procedure history | ||||
| PTCA | 34 (6.0) | 33 (6.6) | 1 (1.5) | 0.10 |
| CABG | 28 (4.9) | 25 (5.0) | 3 (4.5) | 0.88 |
| Pacemaker | 46 (8.1) | 41 (8.1) | 5 (7.6) | 0.88 |
| ICD | 45 (7.9) | 41 (8.1) | 4 (6.1) | 0.56 |
| Cardiac valve | 45 (7.9) | 43 (8.6) | 2 (3.0) | 0.12 |
| Previous catheter ablation | 327 (57.4) | 304 (60.3) | 23 (34.8) | <0.001 |
| Comorbidities | ||||
| COPD | 36 (6.3) | 31 (6.2) | 5 (7.6) | 0.66 |
| Diabetes | 93 (16.3) | 80 (15.9) | 13 (19.7) | 0.43 |
| Hepatic disease | 11 (1.9) | 9 (1.8) | 2 (3.0) | 0.49 |
| Neurological disease | 16 (2.8) | 15 (3.0) | 1 (1.5) | 0.50 |
| Renal disease | 41 (7.2) | 36 (7.1) | 5 (7.6) | 0.90 |
| Gastrointestinal bleeding | 13 (2.3) | 13 (2.6) | 0 (0.0) | 0.19 |
| Hyperlipidaemia | 237 (41.6) | 213 (42.3) | 24 (36.4) | 0.36 |
| Sleep-disordered breathing | 72 (12.7) | 64 (12.7) | 8 (12.1) | 0.89 |
| Other comorbidities | 177 (31.1) | 162 (32.1) | 15 (22.7) | 0.12 |
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CTI, cavo-tricuspid isthmus; ICD, implantable cardioverter-defibrillator; PTCA, percutaneous transluminal coronary angioplasty.
Safety results
During the 30-day period following the procedure, there were 61 serious adverse events in 49 patients. Twenty-one of these were ablation procedure related and represented 4% of the 519 patients undergoing or attempting mapping (Table 2). Among these, three adverse events were classified as either possibly or causally related to the basket mapping catheter (two dislodgment of pre-existing pacemaker lead and one cardiac tamponade), resulting in a rate of 0.57% adverse events associated with the TRUE HD study devices.
| Adverse events . | Ablation-related serious adverse events . | Device-related serious adverse events . | ||
|---|---|---|---|---|
| N events . | Patients, N (%) . | N events . | Patients, N (%) . | |
| Total . | 21 . | 21 (4) . | 3 . | 3 (0.57) . |
| Myocardial perforation with tamponade | 5 | 5 (1) | 0 | 0 (0) |
| Puncture site haematoma | 2 | 2 (0.4) | 0 | 0 (0) |
| Implanted devices dislodgementa | 2 | 2 (0.4) | 2 | 2 (0.4) |
| Asystole, vagal origin (after last sheath removal) | 1 | 1 (0.2) | 0 | 0 (0) |
| 3rd degree AV block—permanent (during PVC ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericardial effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Pleural effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse drug reaction | 1 | 1 (0.2) | 0 | 0 (0) |
| Hypotension/haemodynamic instability | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericarditis | 1 | 1 (0.2) | 0 | 0 (0) |
| Pseudoaneurysm | 1 | 1 (0.2) | 0 | 0 (0) |
| Junctional bradycardia (>20 days after ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Cardiac tamponade | 1 | 1 (0.2) | 1 | 1 (0.2) |
| CO2 retention requiring intubation | 1 | 1 (0.2) | 0 | 0 (0) |
| Oesophageal thermal injury after PVI treated with stent | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse events . | Ablation-related serious adverse events . | Device-related serious adverse events . | ||
|---|---|---|---|---|
| N events . | Patients, N (%) . | N events . | Patients, N (%) . | |
| Total . | 21 . | 21 (4) . | 3 . | 3 (0.57) . |
| Myocardial perforation with tamponade | 5 | 5 (1) | 0 | 0 (0) |
| Puncture site haematoma | 2 | 2 (0.4) | 0 | 0 (0) |
| Implanted devices dislodgementa | 2 | 2 (0.4) | 2 | 2 (0.4) |
| Asystole, vagal origin (after last sheath removal) | 1 | 1 (0.2) | 0 | 0 (0) |
| 3rd degree AV block—permanent (during PVC ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericardial effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Pleural effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse drug reaction | 1 | 1 (0.2) | 0 | 0 (0) |
| Hypotension/haemodynamic instability | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericarditis | 1 | 1 (0.2) | 0 | 0 (0) |
| Pseudoaneurysm | 1 | 1 (0.2) | 0 | 0 (0) |
| Junctional bradycardia (>20 days after ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Cardiac tamponade | 1 | 1 (0.2) | 1 | 1 (0.2) |
| CO2 retention requiring intubation | 1 | 1 (0.2) | 0 | 0 (0) |
| Oesophageal thermal injury after PVI treated with stent | 1 | 1 (0.2) | 0 | 0 (0) |
AV, atrio-ventricular; CO2, carbon dioxide; PVC, premature ventricular complexes; PVI, pulmonary vein isolation.
Dislodgement of other permanent pacemaker leads due to catheter manipulation during the procedure.
| Adverse events . | Ablation-related serious adverse events . | Device-related serious adverse events . | ||
|---|---|---|---|---|
| N events . | Patients, N (%) . | N events . | Patients, N (%) . | |
| Total . | 21 . | 21 (4) . | 3 . | 3 (0.57) . |
| Myocardial perforation with tamponade | 5 | 5 (1) | 0 | 0 (0) |
| Puncture site haematoma | 2 | 2 (0.4) | 0 | 0 (0) |
| Implanted devices dislodgementa | 2 | 2 (0.4) | 2 | 2 (0.4) |
| Asystole, vagal origin (after last sheath removal) | 1 | 1 (0.2) | 0 | 0 (0) |
| 3rd degree AV block—permanent (during PVC ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericardial effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Pleural effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse drug reaction | 1 | 1 (0.2) | 0 | 0 (0) |
| Hypotension/haemodynamic instability | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericarditis | 1 | 1 (0.2) | 0 | 0 (0) |
| Pseudoaneurysm | 1 | 1 (0.2) | 0 | 0 (0) |
| Junctional bradycardia (>20 days after ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Cardiac tamponade | 1 | 1 (0.2) | 1 | 1 (0.2) |
| CO2 retention requiring intubation | 1 | 1 (0.2) | 0 | 0 (0) |
| Oesophageal thermal injury after PVI treated with stent | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse events . | Ablation-related serious adverse events . | Device-related serious adverse events . | ||
|---|---|---|---|---|
| N events . | Patients, N (%) . | N events . | Patients, N (%) . | |
| Total . | 21 . | 21 (4) . | 3 . | 3 (0.57) . |
| Myocardial perforation with tamponade | 5 | 5 (1) | 0 | 0 (0) |
| Puncture site haematoma | 2 | 2 (0.4) | 0 | 0 (0) |
| Implanted devices dislodgementa | 2 | 2 (0.4) | 2 | 2 (0.4) |
| Asystole, vagal origin (after last sheath removal) | 1 | 1 (0.2) | 0 | 0 (0) |
| 3rd degree AV block—permanent (during PVC ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericardial effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Pleural effusion | 1 | 1 (0.2) | 0 | 0 (0) |
| Adverse drug reaction | 1 | 1 (0.2) | 0 | 0 (0) |
| Hypotension/haemodynamic instability | 1 | 1 (0.2) | 0 | 0 (0) |
| Pericarditis | 1 | 1 (0.2) | 0 | 0 (0) |
| Pseudoaneurysm | 1 | 1 (0.2) | 0 | 0 (0) |
| Junctional bradycardia (>20 days after ablation) | 1 | 1 (0.2) | 0 | 0 (0) |
| Cardiac tamponade | 1 | 1 (0.2) | 1 | 1 (0.2) |
| CO2 retention requiring intubation | 1 | 1 (0.2) | 0 | 0 (0) |
| Oesophageal thermal injury after PVI treated with stent | 1 | 1 (0.2) | 0 | 0 (0) |
AV, atrio-ventricular; CO2, carbon dioxide; PVC, premature ventricular complexes; PVI, pulmonary vein isolation.
Dislodgement of other permanent pacemaker leads due to catheter manipulation during the procedure.
Procedure success
Procedure success with validation occurred in 420 patients (83.3%; 95% CI: 79.8–86.5%). Proportion of arrhythmia types, procedure success for the 504 treated patients and for the different arrhythmia types is presented in Figure 2 and Table 3.
| Group . | N . | Success with verification, N (%; 95% CI) . | Success without verification, N (%; 95% CI) . | Failure, N (%; 95% CI) . |
|---|---|---|---|---|
| Study group | 504 | 420 (83.3; 79.8–86.5) | 52 (10.3; 7.8–13.3) | 32 ( 6.3; 4.4–8.8) |
| Patients with ongoing or induced arrhythmia | 336 | 272 (80.9; 76.3–85) | 32 (9.5; 6.6– 13.2) | 32 (9.5; 6.6–13.2) |
| Patients without ongoing arrhythmia and not induced | 168 | 148 (88.1; 82.2–92.6) | 20 (11.9; 7.4–17.8) | 0 |
| Group . | N . | Success with verification, N (%; 95% CI) . | Success without verification, N (%; 95% CI) . | Failure, N (%; 95% CI) . |
|---|---|---|---|---|
| Study group | 504 | 420 (83.3; 79.8–86.5) | 52 (10.3; 7.8–13.3) | 32 ( 6.3; 4.4–8.8) |
| Patients with ongoing or induced arrhythmia | 336 | 272 (80.9; 76.3–85) | 32 (9.5; 6.6– 13.2) | 32 (9.5; 6.6–13.2) |
| Patients without ongoing arrhythmia and not induced | 168 | 148 (88.1; 82.2–92.6) | 20 (11.9; 7.4–17.8) | 0 |
CI, confidence intervals.
| Group . | N . | Success with verification, N (%; 95% CI) . | Success without verification, N (%; 95% CI) . | Failure, N (%; 95% CI) . |
|---|---|---|---|---|
| Study group | 504 | 420 (83.3; 79.8–86.5) | 52 (10.3; 7.8–13.3) | 32 ( 6.3; 4.4–8.8) |
| Patients with ongoing or induced arrhythmia | 336 | 272 (80.9; 76.3–85) | 32 (9.5; 6.6– 13.2) | 32 (9.5; 6.6–13.2) |
| Patients without ongoing arrhythmia and not induced | 168 | 148 (88.1; 82.2–92.6) | 20 (11.9; 7.4–17.8) | 0 |
| Group . | N . | Success with verification, N (%; 95% CI) . | Success without verification, N (%; 95% CI) . | Failure, N (%; 95% CI) . |
|---|---|---|---|---|
| Study group | 504 | 420 (83.3; 79.8–86.5) | 52 (10.3; 7.8–13.3) | 32 ( 6.3; 4.4–8.8) |
| Patients with ongoing or induced arrhythmia | 336 | 272 (80.9; 76.3–85) | 32 (9.5; 6.6– 13.2) | 32 (9.5; 6.6–13.2) |
| Patients without ongoing arrhythmia and not induced | 168 | 148 (88.1; 82.2–92.6) | 20 (11.9; 7.4–17.8) | 0 |
CI, confidence intervals.
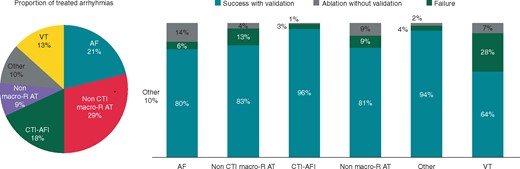
Arrhythmia proportions and success per group. AF, atrial fibrillation; AT, atrial tachycardia; CTI, cavo-tricuspid isthmus; macro-R, macro-reentrant; Other, other supraventricular tachycardias or accessory pathways; VT, ventricular tachycardia.
Device technical and clinical performance
Out of 504 Treatment patients, 9 (1.8%) cases had the 64 pole basket catheter replaced during the procedure due to: absence of signals/software messages from the workstation (5), mechanical—breaking or kinking (3), and noise (2). None of these cases resulted in patient complications or adverse events.
When analysing the ability to acquire maps as intended, the electrophysiologist was unable to collect any maps for diagnostic purposes in 15 ‘attempted’ patients out of 519 undergoing the electrophysiology (EP) procedure (2.6%) due to: cardiac rhythm instability (10), insufficient electro-anatomical information (4), or patient hemodynamic instability (1). In the remaining 504 treated patients, having at least one utilized map to support the procedure, out of 1419 utilized maps, 134 were not utilized due to: anatomical characteristics preventing complete map collection in 24 patients, rapid changes in rhythm in 69, tissue susceptibility to frequent premature ventricular complexes or arrhythmias at contact with the mapping catheter in 6, and acquisition interruption due to haemodynamic instability in 2. The ablation failure rate in patients with at least one unused map did not differ significantly from the failure rate in those subjects where all the maps were utilized (8/125; 6.4% vs. 32/375; 6.7%; P = 0.48).
Mapping workflow during the ablation procedure
A total of 1419 electroanatomical used maps were created in 504 patients with a median of 2 maps per patient [interquartile range (IQR): 1–4] and 6983 points per map (IQR: 3253–13 005).
The median time required to create one map was 9:23 min (IQR: 4:58–16:25 min). Overall, each patient spent a median time of 29:41 min (IQR: 6:15–49:08 min) during the case strictly dedicated to mapping time. The median procedure time (from first sheath entered in the patient to patient leaving the EP lab) was 183 min (IQR: 129–264 min); accordingly the median percentage of time spent during the procedure for mapping was 19% (IQR: 13–25%).
When analysing the acquisition of the utilized maps in respect to the ablation workflow, 632 maps were collected in 493 patients before the first ablation, 661 (297 patients) between first and last ablation and 126 (120 patients) after last ablation. When considering ablation validation, 372 vMaps were collected in 222 (44%) patients. vMaps were collected in a median time of 5:28 min, accounting for a median of 4015 points (EGM) with 259 maps only mapping the area of interest. Following vMaps, 162/222 (73%) patients required additional ablation(s) (Table 4).
| Arrhythmias . | Pts (treatment) . | at least one vMap, (% treatment pts) . | vMap before last ablation (% all vMaps) . |
|---|---|---|---|
| All | 504 | 216 (42.9) | 157 (72.7) |
| AF | 108 | 53 (49.1) | 43 (81.1) |
| Non-CTI re-entrant AT | 144 | 81 (56.3) | 57 (70.4) |
| Focal AT | 43 | 8 (18.6) | 8 (100) |
| Typical AFl (CTI) | 92 | 68 (73.9) | 46 (67.6) |
| Ventricular | 66 | 4 (6) | 1 (25) |
| Other | 51 | 2 (3.9) | 2 (100) |
| Arrhythmias . | Pts (treatment) . | at least one vMap, (% treatment pts) . | vMap before last ablation (% all vMaps) . |
|---|---|---|---|
| All | 504 | 216 (42.9) | 157 (72.7) |
| AF | 108 | 53 (49.1) | 43 (81.1) |
| Non-CTI re-entrant AT | 144 | 81 (56.3) | 57 (70.4) |
| Focal AT | 43 | 8 (18.6) | 8 (100) |
| Typical AFl (CTI) | 92 | 68 (73.9) | 46 (67.6) |
| Ventricular | 66 | 4 (6) | 1 (25) |
| Other | 51 | 2 (3.9) | 2 (100) |
AFl, atrial flutter; AT, atrial tachycardia; CTI, cavo-tricuspid isthmus; pts, patients.
| Arrhythmias . | Pts (treatment) . | at least one vMap, (% treatment pts) . | vMap before last ablation (% all vMaps) . |
|---|---|---|---|
| All | 504 | 216 (42.9) | 157 (72.7) |
| AF | 108 | 53 (49.1) | 43 (81.1) |
| Non-CTI re-entrant AT | 144 | 81 (56.3) | 57 (70.4) |
| Focal AT | 43 | 8 (18.6) | 8 (100) |
| Typical AFl (CTI) | 92 | 68 (73.9) | 46 (67.6) |
| Ventricular | 66 | 4 (6) | 1 (25) |
| Other | 51 | 2 (3.9) | 2 (100) |
| Arrhythmias . | Pts (treatment) . | at least one vMap, (% treatment pts) . | vMap before last ablation (% all vMaps) . |
|---|---|---|---|
| All | 504 | 216 (42.9) | 157 (72.7) |
| AF | 108 | 53 (49.1) | 43 (81.1) |
| Non-CTI re-entrant AT | 144 | 81 (56.3) | 57 (70.4) |
| Focal AT | 43 | 8 (18.6) | 8 (100) |
| Typical AFl (CTI) | 92 | 68 (73.9) | 46 (67.6) |
| Ventricular | 66 | 4 (6) | 1 (25) |
| Other | 51 | 2 (3.9) | 2 (100) |
AFl, atrial flutter; AT, atrial tachycardia; CTI, cavo-tricuspid isthmus; pts, patients.
Discussion
This is the first prospective multi-centre study to perform systematic data collection in order to describe the safety, performance, and clinical use of a novel UHD mapping technology as used in a wide sample of different arrhythmias. Previous studies published on this system included smaller cohorts of patients,3,4,7,8,11–13 focused on specific mapping capabilities or concentrated on specific arrhythmia types.10 Importantly, the prior studies also demonstrated that the system is safe and capable of rapid electro-anatomical map collection with no need for manual annotation. Moreover, these studies demonstrated the clinical advantages in the management of particular cases such as complex atrial11 or ventricular tachycardias (VTs).14 The low noise level in the system (0.01 mV), allowing recording of very low-amplitude potentials, has the advantage of detecting EGMs within the scarred areas of the myocardium critical to the VT circuit2 or conduction gaps essential for re-entry from previous procedures such as in the setting of redo AF.10
The present study has similar characteristics of any large multicenter standard-of-care study, as the collected information came from a large group of heterogeneous sites with a large number of operating physicians (77) resulting in different arrhythmia types and a wide spectrum of ablation strategies. Moreover, in addition to determining the acute success of the procedure, this study focused on the mapping part of the process insofar as mapping information helps to determine the ablation strategy or verify previous ablative applications.
This study confirms that the 64 pole multi-electrode basket catheter is safe to map arrhythmias in all the cardiac chambers. At 1 month follow-up after the index procedure the procedural related events of 4% aligns with contemporary experience. In-depth analysing the root cause of the adverse events in respect to the study devices, revealed a very low event rate of events (0.57%) associated with the basket mapping catheter, limited to two cases of dislodgments of other leads (without entrapment) and one cardiac tamponade resolved with pericardial drainage.
The primary effectiveness endpoint was acute procedural success based on the pre-defined criteria that reflected mapping, ablation and validation. In this study, the overall success rate was 83.3% and the success rate of subgroups ranged from 64% in ventricular arrhythmias to 96% in typical atrial flutter. The majority of cases in this study included non-cavo-tricuspid isthmus (CTI) macro-reentrant atrial tachycardias (ATs) (144) and re-do AF (108), with success >80%, in line with existing literature data. Notably, the treated cases included arrhythmia types different from those traditionally considered complex such as VTs or certain left-sided ATs: the study included 92 (18%) CTI dependent right atrial flutters and 37 (7%) atrioventricular nodal reentrant tachycardia (AVNRT). Although these arrhythmias do not strictly require 3D mapping and a traditional approach can be followed, advanced mapping may nevertheless have specific advantages. In typical atrial flutter, the use of high-resolution mapping can help the detection of residual slow CTI conduction18 or determine complex patterns around the tricuspid valve annulus. For AVNRT, although a stepwise approach based on anatomic and EGM findings are routine practice for ablation, voltage mapping has been proposed for direct visualization of the slow pathway.19 In our study, 3D mapping of AVNRT and CTI dependent AFl led to a very high success rate with only one case of failure.
In the context of the utility, this study also assessed the ability of the mapping catheter to perform as intended, i.e. the capability of the catheter to collect the necessary data points for diagnostic or validation purposes. The percentage of cases where inability to collect a map resulted in a procedure failure was limited to 15 (2.8%) cases. Moreover, among these cases, the reasons for not colleting a successful map were mostly related with unstable cardiac rhythm, in terms of circuitry or consequential haemodynamic, rather than catheter itself (e.g. difficulties to reach an area, noise, or too low signal) with only four cases resulting in catheter-related difficulties preventing collection of a satisfactory map for the procedure purpose, (0.8% of the cases). Accordingly, from the specific perspective of the mapping system, this performance data together with the safety results collected in the TRUE HD provides an excellent profile for the mapping system and the mapping catheter together.
Differences in ablation practice and workflow data in this study not only encompass the heterogeneous spectrum of arrhythmias but also the high number of operators involved.
Mapping purpose was also collected in order to investigate if and when the system was used also as a supportive tool for ablation validation. In this study, repeat electroanatomical mapping for validation purposes was used in approximately half of the cases. Amongst the cases where validation were performed, the far majority were helpful to identify the need for additional ablation to complete the procedure. Although reasons for adding ablation applications after validation mapping were not detailed in the study, these results suggest that validation mapping often proves to be a valuable tool that should be included in the workflow routinely. Data collected in this study also showed that mapping itself did not add substantial time to the procedure. The system allows quick and automatic acquisition of maps without the need for manual annotation, with very limited or no need for post-processing. The TRUE HD study showed that mapping time is reasonable for different arrhythmias. In particular, the acquisition of validation maps was even faster when compared with initial diagnostic mapping, because data collection for validation often occurs only in a limited part of interest of the chamber in the majority of the cases.
Limitations
The main limitations of the present study are the lack of a control group and the limited follow-up period, that did not allow to assess clinical effectiveness of ablation through freedom from recurrences of primary arrhythmia in the treated patients. The absence of this follow-up is essentially justified by the inclusion of a large set of arrhythmias and by the main objective to evaluate usage and acute profile of a new mapping system. Another limitation is the lack of a standardized workflow for mapping which may have led to a better evaluation of the acute result in perspective of an optimized use of the UHD mapping system, although this reflects the diverse practice between operators and centres in the real-world.
Conclusions
The TRUE HD study prospectively evaluated the safety and acute effectiveness of using a novel UHD mapping system in a wide spectrum of clinical arrhythmias as standard-of-care by a variety of electrophysiologists worldwide. This is the first real-world data acquired at such scale that confirm that the system performs well and with a very low rate of patient device-related complications. When performed, validation mapping was useful for identifying additional targets for ablation in the majority of patients.
Acknowledgements
The authors thank Rami Guirguis, (Boston Scientific Corporation) for support in the study.
Funding
This work was supported by Boston Scientific Corp. Steering Committee: T.M. (USA), G.H. (Germany), P.J. (France), S.W. (USA), and T.W. (UK).
Conflict of interest: G.H.: research grants to the institution from Abbott, Biotronik, Boston Scientific, and Medtronic without personal financial benefits. S.W., T.W. and P.J.: have received consultant fees from Boston Scientific Corp.; T.M.: has received consulting fees from St Jude Medical, Biosense Webster, and Boston Scientific Corp.; W.M.: has received consulting fees from Biosense Webster, St Jude Medical, Boston Scientific Corp., Medtronic, and Biotronik; J.I.G.-B. has received consultant, proctoring and speaker's fees from Boston Scientific Corporation and St. Jude Medical; and G.R. and E.D. are employees of Boston Scientific Corp. All remaining authors have declared no conflicts of interest.



