-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Boriani, Paolo Pieragnoli, Giovanni Luca Botto, Helmut Puererfellner, Lluis Mont, Matteo Ziacchi, Antonis S Manolis, Michele Gulizia, Raymond Tukkie, Maurizio Landolina, Giuseppe Ricciardi, Manuele Cicconelli, Andrea Grammatico, Mauro Biffi, Effect of PR interval and pacing mode on persistent atrial fibrillation incidence in dual chamber pacemaker patients: a sub-study of the international randomized MINERVA trial, EP Europace, Volume 21, Issue 4, April 2019, Pages 636–644, https://doi.org/10.1093/europace/euy286
Close - Share Icon Share
Abstract
Per standard of care, dual-chamber pacemakers are programmed in DDDR mode with fixed atrioventricular (AV) delay or with long AV delay to minimize ventricular pacing. We aimed to evaluate whether the PR interval may be a specific criterion of choice between standard DDDR, to preserve AV synchrony in long PR patients, and managed ventricular pacing (MVP), to avoid ventricular desynchronization imposed by right ventricle apical pacing, in short PR patients.
In the MINERVA trial, 1166 patients were randomized to Control DDDR, MVP, or atrial anti-tachycardia pacing plus MVP (DDDRP + MVP). We evaluated the interaction of PR interval with pacing mode by comparing the risk of atrial fibrillation (AF) longer than 7 consecutive days as a function of PR interval. Out of 906 patients with available data, the median PR interval was 180 ms. The PR interval was found to significantly (P = 0.012) interact with pacing mode for AF incidence: the risk of AF > 7 days was lower [hazard ratio (HR) 0.58, 95% confidence interval (95% CI) 0.34–0.99; P = 0.047] in patients with short PR (shorter than median PR) if programmed in MVP mode compared with DDDR mode and it was lower (HR 0.65, 95% CI 0.43–0.99; P = 0.049) in patients with long PR (equal to or longer than median PR) if programmed in DDDR mode compared with MVP.
Our data show that PR interval may be used as a selection criterion to identify the optimal physiological pacing mode. Persistent AF incidence was lower in short PR patients treated by right ventricular pacing minimization and in long PR patients treated by standard dual-chamber pacing.
Physiological pacing modality in patients with sinus node disease can be identified as a function of the PR interval.
Patients with normal atrioventricular (AV) conduction benefit from algorithms which minimize apical right ventricular (RV) pacing.
Conversely patients with prolonged PR interval receive physiological pacing when treated by DDDR pacing.
Our data also suggest the importance of optimized AV sensed and paced intervals to reduce persistent AF incidence. The choice of persistent AF as the trial main endpoint, was driven by the results of several trials that showed that AF is conditioned by RV pacing-induced ventricular dyssynchrony or by AV dyssynchrony and that AF is an important prognostic marker for death, stroke, or hospital admissions in primary care.
Our results finally confirm, in a follow-up longer than previously described, that the new generation atrial anti-tachycardia pacing tested in the trial, reduces the risk of permanent or persistent atrial tachyarrhythmias.
These results have clinical importance because they explain the apparent contradiction between the knowledge that RV apical pacing is associated with adverse outcomes and the fact that a number of randomized controlled trials have not consistently shown clinical advantage of RV pacing minimization.
Introduction
Aging of the general population progressively results in increased prevalence of heart conduction disease and consequently in enhanced need for permanent pacemaker therapy.1
When introduced, more than 25 years ago, dual-chamber pacing (DDD) with atrioventricular (AV) synchrony and rate adaptive pacing (DDDR) were considered physiological pacing modes, since they mimic natural heart conditions.2 Despite these premises, the results of several trials3,4 suggested that the physiological advantage of atrial-based pacing may be offset by unnecessary apical right ventricular (RV) pacing. Therefore, algorithms to minimize ventricular pacing were added to the DDD/DDDR pacing modes. The SAVE PACe trial5 was the landmark study that associated RV pacing minimization algorithms with reduced persistent atrial fibrillation (AF) when compared with standard DDD pacing. However, recent studies and a meta-analysis6–9 did not convincingly confirm superiority of atrial pacing and sensing (AAI), or of RV pacing minimization, in improving clinical outcomes in pacemaker patients compared with standard DDD/DDDR pacing.
Presently the standard of care in dual-chamber pacemaker patients is to program the pacemaker with a long AV delay or with specific algorithms to minimize ventricular pacing in patients without conduction disease, or to program the pacemaker at a fixed AV delay in patients with high degree AV block.10,11
We hypothesized that electrocardiographic measurements, such as P wave duration, PR interval and PR segment, that are associated with atrial and AV conduction delays, may represent important screening factors to select optimal pacing modes. A long PR interval is considered an important marker, present in 40–60% of all pacemaker patients and is associated with a worse prognosis.10–15
We performed a sub-study of the MINERVA trial8,16 to evaluate the optimal pacing mode in pacemaker patients and to identify the role of PR interval, PR segment, and P wave duration, in physiological pacing.
Methods
Study design, patient population, and follow-up
The MINERVA trial8,16 was an international multi-centre, randomized, single-blind controlled study involving 63 cardiology centres in 15 countries. The study was conducted in compliance with the Declaration of Helsinki and approved by the Ethics Committees of all participating centres. All patients provided written informed consent. Inclusion criteria were standard indications for permanent dual-chamber pacing10,11 and history of atrial tachyarrhythmias (AT/AF). The main exclusion criteria were history of long-standing persistent AF and third-degree AV block. After implantation of a dual-chamber pacemaker, eligible patients were randomly assigned to standard dual-chamber pacing (Control DDDR), DDDR with managed ventricular pacing (MVP) or atrial preventive pacing, atrial antitachycardia pacing (ATP), or MVP (DDDRP + MVP).
Randomization was stratified by the presence or absence of documented AV block and by left ventricular ejection fraction. Patients underwent follow-up examination at 3 and 6 months after implantation and every 6 months thereafter, until study closure. Further information about the study is described in the Supplementary material online.
Managed ventricular pacing
The MVP mode provides atrial-based pacing to promote intrinsic ventricular conduction and reduce unnecessary RV pacing. It also warrants ventricular backup pacing if loss of AV conduction is detected for at least two out of four atrial depolarizations.
Reactive ATP™
Reactive ATP™ is an algorithm that monitors the atrial rhythm and can respond to a sustained AT/AF episode by delivering a special set of atrial ATP therapies according to the AT/AF cycle length and regularity.8,16
Analysis objectives and endpoints
The primary objective of this secondary analysis was to compare the incidence of persistent AF in the three randomized study arms as a function of the PR interval. We also aimed to evaluate if the PR interval, P wave duration, or PR segment interacts with pacing mode, DDD/DDDR or MVP, in terms of persistent AF incidence. The main endpoint of the analysis was persistent AF (i.e. AF lasting > 7 days) since several trials have shown AF to be conditioned by RV pacing-induced ventricular dyssynchrony or by AV dyssynchrony.3–8
Expert electrophysiologists measured P wave duration, PR interval (estimated as the time between P wave onset and QRS onset), and PR segment (estimated as the time between P wave end and QRS onset), evaluating electrocardiography (ECG) lead II derivation at a velocity of 50 mm/s.
Statistical analysis
The analysis set included all patients randomized in the MINERVA trial with information about the PR interval. Patients were considered in the randomization arm, according to the intention-to-treat principle.
Patient characteristics were summarized via descriptive statistics, including mean and standard deviation, or median with the interquartile range (IQR), for continuous variables, and counts and percentages for categorical variables, as appropriate.
The actuarial incidence of persistent AF was estimated by the Kaplan–Meier methods and compared by randomization arms and by PR interval. Atrial fibrillation actuarial incidence has been described, rather than AF actual incidence, since we wanted to outline how pacemaker programming or patient characteristics affect AF risk taking into account the time to the event and the actual number of patients under observation.
Risk of persistent AF was analysed through the Cox proportional hazard method, reporting hazard ratios (HR) with 95% confidence intervals (CI). The proportional hazard assumptions were tested using Schoenfeld residuals. After checking for collinearity, all variables that were significant at the 0.10 level were analysed in a multivariable backwards elimination model.
Forest plots were reported to show effect modifications by the PR interval, the P wave duration and the PR segment according to the relationship between randomized pacing mode and persistent AF.
All tests were two-sided and a P-value <0.05 was considered statistically significant.
The SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Results
Out of 1166 patients randomized in the main study, information about PR intervals at pre-implant electrocardiography (ECG) was collected in 906 (78%) patients—303 Control DDDR, 318 MVP, and 285 DDDRP + MVP patients—who were included in the following analyses.
Patient baseline characteristics are shown in Table 1. Approximately half of the patients were female, and mean age was 73 ± 9 years. All patients had a history of AT/AF. Atrial fibrillation was documented in 84% of patients, and atrial flutter or atrial tachycardia in the remaining patients.
| Baseline characteristics . | Overall . |
|---|---|
| (n = 906) . | |
| Demographics | |
| Age (years), mean ± SD | 73 ± 9 |
| Gender (male), n (%) | 444 (49.0%) |
| Medical history | |
| AT/AF history n (%) | 906 (100%) |
| Paroxysmal AT/AF, n (%) | 737 (81.4%) |
| Persistent AT/AF, n (%) | 169 (18.6%) |
| Atrial cardioversions, n (%) | 215 (24.7%) |
| Hypertension, n (%) | 623 (71.2%) |
| Stroke/TIA, n (%) | 83 (9.3%) |
| Diabetes, n (%) | 150 (17.0%) |
| Cardiovascular hospitalizations history, n (%) | 296 (34.7%) |
| AT/AF hospitalizations history, n (%) | 219 (25.7%) |
| LVEF, mean ± SD | 57 ± 11 |
| LVEF ≤ 50, n (%) | 183 (30.6%) |
| LVESV, mean ± SD | 57 ± 44 |
| PR interval (ms), median (IQR range) | 180 (160–205) |
| CHADS2, mean ± SD | 1.7 ± 1.1 |
| CHADS2 > 2, n (%) | 153 (18.7%) |
| Beta-blocker, n (%) | 334 (37.1%) |
| Class III antiarrhythmic agents, n (%) | 254 (28.2%) |
| Class IC antiarrhythmic agents, n (%) | 139 (15.4%) |
| Anticoagulants, n (%) | 388 (43.1%) |
| Antiplatelets, n (%) | 359 (39.8%) |
| Diuretics, n (%) | 308 (34.2%) |
| Baseline characteristics . | Overall . |
|---|---|
| (n = 906) . | |
| Demographics | |
| Age (years), mean ± SD | 73 ± 9 |
| Gender (male), n (%) | 444 (49.0%) |
| Medical history | |
| AT/AF history n (%) | 906 (100%) |
| Paroxysmal AT/AF, n (%) | 737 (81.4%) |
| Persistent AT/AF, n (%) | 169 (18.6%) |
| Atrial cardioversions, n (%) | 215 (24.7%) |
| Hypertension, n (%) | 623 (71.2%) |
| Stroke/TIA, n (%) | 83 (9.3%) |
| Diabetes, n (%) | 150 (17.0%) |
| Cardiovascular hospitalizations history, n (%) | 296 (34.7%) |
| AT/AF hospitalizations history, n (%) | 219 (25.7%) |
| LVEF, mean ± SD | 57 ± 11 |
| LVEF ≤ 50, n (%) | 183 (30.6%) |
| LVESV, mean ± SD | 57 ± 44 |
| PR interval (ms), median (IQR range) | 180 (160–205) |
| CHADS2, mean ± SD | 1.7 ± 1.1 |
| CHADS2 > 2, n (%) | 153 (18.7%) |
| Beta-blocker, n (%) | 334 (37.1%) |
| Class III antiarrhythmic agents, n (%) | 254 (28.2%) |
| Class IC antiarrhythmic agents, n (%) | 139 (15.4%) |
| Anticoagulants, n (%) | 388 (43.1%) |
| Antiplatelets, n (%) | 359 (39.8%) |
| Diuretics, n (%) | 308 (34.2%) |
AT/AF, atrial tachyarrhythmias; CHADS2, stroke risk factor based on congestive heart failure, hypertension, age, diabetes, and stroke; IQR, interquartile range; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; SD, standard deviation; TIA, transient ischaemic attack.
| Baseline characteristics . | Overall . |
|---|---|
| (n = 906) . | |
| Demographics | |
| Age (years), mean ± SD | 73 ± 9 |
| Gender (male), n (%) | 444 (49.0%) |
| Medical history | |
| AT/AF history n (%) | 906 (100%) |
| Paroxysmal AT/AF, n (%) | 737 (81.4%) |
| Persistent AT/AF, n (%) | 169 (18.6%) |
| Atrial cardioversions, n (%) | 215 (24.7%) |
| Hypertension, n (%) | 623 (71.2%) |
| Stroke/TIA, n (%) | 83 (9.3%) |
| Diabetes, n (%) | 150 (17.0%) |
| Cardiovascular hospitalizations history, n (%) | 296 (34.7%) |
| AT/AF hospitalizations history, n (%) | 219 (25.7%) |
| LVEF, mean ± SD | 57 ± 11 |
| LVEF ≤ 50, n (%) | 183 (30.6%) |
| LVESV, mean ± SD | 57 ± 44 |
| PR interval (ms), median (IQR range) | 180 (160–205) |
| CHADS2, mean ± SD | 1.7 ± 1.1 |
| CHADS2 > 2, n (%) | 153 (18.7%) |
| Beta-blocker, n (%) | 334 (37.1%) |
| Class III antiarrhythmic agents, n (%) | 254 (28.2%) |
| Class IC antiarrhythmic agents, n (%) | 139 (15.4%) |
| Anticoagulants, n (%) | 388 (43.1%) |
| Antiplatelets, n (%) | 359 (39.8%) |
| Diuretics, n (%) | 308 (34.2%) |
| Baseline characteristics . | Overall . |
|---|---|
| (n = 906) . | |
| Demographics | |
| Age (years), mean ± SD | 73 ± 9 |
| Gender (male), n (%) | 444 (49.0%) |
| Medical history | |
| AT/AF history n (%) | 906 (100%) |
| Paroxysmal AT/AF, n (%) | 737 (81.4%) |
| Persistent AT/AF, n (%) | 169 (18.6%) |
| Atrial cardioversions, n (%) | 215 (24.7%) |
| Hypertension, n (%) | 623 (71.2%) |
| Stroke/TIA, n (%) | 83 (9.3%) |
| Diabetes, n (%) | 150 (17.0%) |
| Cardiovascular hospitalizations history, n (%) | 296 (34.7%) |
| AT/AF hospitalizations history, n (%) | 219 (25.7%) |
| LVEF, mean ± SD | 57 ± 11 |
| LVEF ≤ 50, n (%) | 183 (30.6%) |
| LVESV, mean ± SD | 57 ± 44 |
| PR interval (ms), median (IQR range) | 180 (160–205) |
| CHADS2, mean ± SD | 1.7 ± 1.1 |
| CHADS2 > 2, n (%) | 153 (18.7%) |
| Beta-blocker, n (%) | 334 (37.1%) |
| Class III antiarrhythmic agents, n (%) | 254 (28.2%) |
| Class IC antiarrhythmic agents, n (%) | 139 (15.4%) |
| Anticoagulants, n (%) | 388 (43.1%) |
| Antiplatelets, n (%) | 359 (39.8%) |
| Diuretics, n (%) | 308 (34.2%) |
AT/AF, atrial tachyarrhythmias; CHADS2, stroke risk factor based on congestive heart failure, hypertension, age, diabetes, and stroke; IQR, interquartile range; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; SD, standard deviation; TIA, transient ischaemic attack.
The median (IQR) follow-up duration was 34 (24–46) months.
In the DDDR arm, study investigators programmed moderately prolonged AV intervals to avoid unnecessary ventricular pacing. The median (IQR) sensed AV delay was 200 (150–280) ms and paced AV delay was 230 (180–280) ms.
Persistent atrial fibrillation incidence
Persistent AF incidence is shown in Figure 1. At 42 months follow-up, persistent AF occurred in 81 Control DDDR patients [actuarial incidence 34.2%, 95% CI 27.9–41.5%), in 79 MVP patients (actuarial incidence 37.2%, 95% CI 29.7–45.8%), and in 42 DDDRP + MVP patients (actuarial incidence 22.8%, 95% CI 16.8–30.5%), (HR 0.543, 95% CI 0.378–0.780; P < 0.001 vs. control DDDR and HR 0.540, 95% CI 0.375–0.778; P < 0.001 vs. MVP). Multivariate analysis showed that DDDRP + MVP was associated with a reduced risk of AF longer than 7 days (HR 0.54, 95% CI 0.38–0.76; P < 0.001) compared with the other pacing modes.
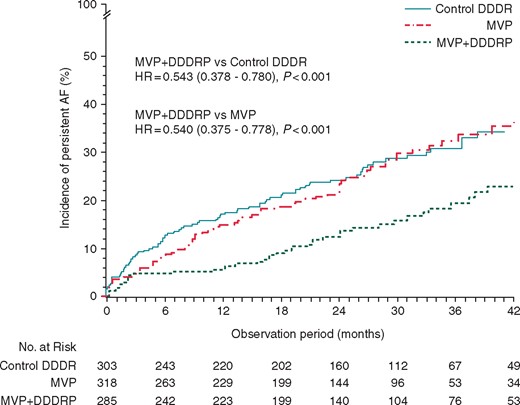
Incidence of persistent AF in the three randomized study arms. AF, atrial fibrillation; HR, hazard ratio; MVP, managed ventricular pacing.
PR interval, P wave duration, and PR segment
The median PR interval was 180 ms (IQR 160–205 ms), the median P wave duration was 80 ms (IQR 60–100 ms), and the median PR segment was 80 ms (IQR 60–120 ms). The patient cohort was divided in subgroups, according to median values of ECG derived variables, e.g. short PR (<180 ms) and long PR (≥180 ms).
Actuarial incidence of persistent AF at 42 months is shown in Figure 2 for the three randomization groups for patients with short and long PR intervals, respectively. Control DDDR and MVP pacing modes showed a characteristic cross behaviour; Control DDDR was associated with higher AF risk for short PR and lower AF risk for long PR, while exactly the opposite occurred for MVP (lower AF risk for short PR and higher AF risk for long PR higher).
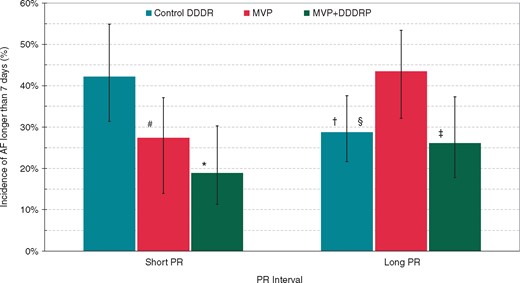
Incidence of AF longer than 7 days in the three study arms as a function of short and long PR interval. Whisker lines on the graph represent AF incidence standard errors. Comparisons within short PR patient subgroup: #HR (95% CI) = 0.58 (0.34–0.99) P = 0.047 vs. Control DDDR; *HR (95% CI) = 0.39 (0.22–0.71) P = 0.002 vs. Control DDDR. Comparisons within long PR patient subgroup: §HR (95% CI) = 0.65 (0.43–1.00) P = 0.049 vs. MVP; ‡HR (95% CI) = 0.51 (0.31–0.81) P = 0.005 vs. MVP. Comparisons within short and long PR patient subgroups: †HR (95% CI) = 0.61 (0.38–0.98) P = 0.043 vs. control DDDDR short PR. AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; MVP, managed ventricular pacing.
PR interval significantly (P = 0.012) interacted with pacing mode (MVP vs. Control DDDR) in terms of persistent AF as shown in Figure 3. Also, PR segment was associated with a significant (P = 0.046) interaction with pacing mode (MVP vs. Control DDDR), while P wave duration showed no significant interaction.
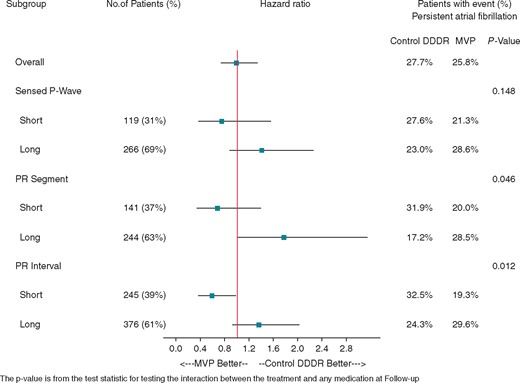
Hazard ratios of persistent AF for short and long PR interval, P segment, and sensed P wave. AF, atrial fibrillation; MVP, managed ventricular pacing.
Univariate and multivariate Cox regression analyses, performed on the cohort of patients randomized to MVP or to Control DDDR arms, confirmed that MVP, compared with DDDR, was associated with lower risk of persistent AF in short PR patients (Table 2) while DDDR, compared with MVP, was associated with lower risk of persistent AF in long PR (Table 3).
Cox Regression analyses of persistent atrial fibrillation (AF >7 days) risk in patients with short PR
| Short PR patients . | Persistent AF risk . | Persistent AF risk . | ||
|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | |||
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| MVP | 0.59 (0.35–0.98) | 0.043 | 0.58 (0.34–0.99) | 0.047 |
| Age ≥ 75 years | 1.05 (0.65–1.72) | 0.834 | ||
| Gender (male) | 0.82 (0.50–1.35) | 0.434 | ||
| Atrial cardioversions | 1.39 (0.79–2.45) | 0.254 | ||
| Hypertension | 0.97 (0.57–1.67) | 0.921 | ||
| Stroke/TIA | 0.89 (0.36–2.22) | 0.800 | ||
| Diabetes | 1.24 (0.67–2.30) | 0.490 | ||
| LVEF ≤ 50 | 1.01 (0.53–1.93) | 0.979 | ||
| Beta-blocker | 1.16 (0.70–1.90) | 0.568 | ||
| Class III antiarrhythmic agents | 0.85 (0.49–1.48) | 0.558 | ||
| Class IC antiarrhythmic agents | 0.76 (0.33–1.78) | 0.533 | ||
| Short PR patients . | Persistent AF risk . | Persistent AF risk . | ||
|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | |||
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| MVP | 0.59 (0.35–0.98) | 0.043 | 0.58 (0.34–0.99) | 0.047 |
| Age ≥ 75 years | 1.05 (0.65–1.72) | 0.834 | ||
| Gender (male) | 0.82 (0.50–1.35) | 0.434 | ||
| Atrial cardioversions | 1.39 (0.79–2.45) | 0.254 | ||
| Hypertension | 0.97 (0.57–1.67) | 0.921 | ||
| Stroke/TIA | 0.89 (0.36–2.22) | 0.800 | ||
| Diabetes | 1.24 (0.67–2.30) | 0.490 | ||
| LVEF ≤ 50 | 1.01 (0.53–1.93) | 0.979 | ||
| Beta-blocker | 1.16 (0.70–1.90) | 0.568 | ||
| Class III antiarrhythmic agents | 0.85 (0.49–1.48) | 0.558 | ||
| Class IC antiarrhythmic agents | 0.76 (0.33–1.78) | 0.533 | ||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
The variables outlined in bold characters are those associated with a significant (P > 0.05) risk of persistent atrial fibrillation.
Cox Regression analyses of persistent atrial fibrillation (AF >7 days) risk in patients with short PR
| Short PR patients . | Persistent AF risk . | Persistent AF risk . | ||
|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | |||
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| MVP | 0.59 (0.35–0.98) | 0.043 | 0.58 (0.34–0.99) | 0.047 |
| Age ≥ 75 years | 1.05 (0.65–1.72) | 0.834 | ||
| Gender (male) | 0.82 (0.50–1.35) | 0.434 | ||
| Atrial cardioversions | 1.39 (0.79–2.45) | 0.254 | ||
| Hypertension | 0.97 (0.57–1.67) | 0.921 | ||
| Stroke/TIA | 0.89 (0.36–2.22) | 0.800 | ||
| Diabetes | 1.24 (0.67–2.30) | 0.490 | ||
| LVEF ≤ 50 | 1.01 (0.53–1.93) | 0.979 | ||
| Beta-blocker | 1.16 (0.70–1.90) | 0.568 | ||
| Class III antiarrhythmic agents | 0.85 (0.49–1.48) | 0.558 | ||
| Class IC antiarrhythmic agents | 0.76 (0.33–1.78) | 0.533 | ||
| Short PR patients . | Persistent AF risk . | Persistent AF risk . | ||
|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | |||
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| MVP | 0.59 (0.35–0.98) | 0.043 | 0.58 (0.34–0.99) | 0.047 |
| Age ≥ 75 years | 1.05 (0.65–1.72) | 0.834 | ||
| Gender (male) | 0.82 (0.50–1.35) | 0.434 | ||
| Atrial cardioversions | 1.39 (0.79–2.45) | 0.254 | ||
| Hypertension | 0.97 (0.57–1.67) | 0.921 | ||
| Stroke/TIA | 0.89 (0.36–2.22) | 0.800 | ||
| Diabetes | 1.24 (0.67–2.30) | 0.490 | ||
| LVEF ≤ 50 | 1.01 (0.53–1.93) | 0.979 | ||
| Beta-blocker | 1.16 (0.70–1.90) | 0.568 | ||
| Class III antiarrhythmic agents | 0.85 (0.49–1.48) | 0.558 | ||
| Class IC antiarrhythmic agents | 0.76 (0.33–1.78) | 0.533 | ||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
The variables outlined in bold characters are those associated with a significant (P > 0.05) risk of persistent atrial fibrillation.
Cox Regression analyses of persistent atrial fibrillation (AF > 7 days) risk in patients with long PR
| Long PR patients . | Persistent AF risk Univariate analysis . | Persistent AF risk Multivariate analysis . | ||
|---|---|---|---|---|
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Control DDDR | 0.73 (0.49–1.09) | 0.124 | 0.65 (0.43–1.00) | 0.049 |
| Age ≥ 75 years | 1.54 (1.03–2.29) | 0.035 | ||
| Gender (male) | 1.22 (0.82–1.81) | 0.320 | ||
| Atrial cardioversions | 2.02 (1.33–3.07) | 0.001 | 2.13 (1.39–3.27) | <0.001 |
| Hypertension | 1.56 (0.98–2.48) | 0.059 | 1.62 (0.99–2.66) | 0.055 |
| Stroke/TIA | 1.11 (0.59–2.08) | 0.739 | ||
| Diabetes | 1.03 (0.62–1.72) | 0.897 | ||
| LVEF ≤ 50 | 1.45 (0.91–2.32) | 0.122 | ||
| Beta-blocker | 1.12 (0.75–1.66) | 0.580 | ||
| Class III antiarrhythmic agents | 1.08 (0.71–1.65) | 0.725 | ||
| Class IC antiarrhythmic agents | 0.63 (0.35–1.13) | 0.119 | ||
| Long PR patients . | Persistent AF risk Univariate analysis . | Persistent AF risk Multivariate analysis . | ||
|---|---|---|---|---|
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Control DDDR | 0.73 (0.49–1.09) | 0.124 | 0.65 (0.43–1.00) | 0.049 |
| Age ≥ 75 years | 1.54 (1.03–2.29) | 0.035 | ||
| Gender (male) | 1.22 (0.82–1.81) | 0.320 | ||
| Atrial cardioversions | 2.02 (1.33–3.07) | 0.001 | 2.13 (1.39–3.27) | <0.001 |
| Hypertension | 1.56 (0.98–2.48) | 0.059 | 1.62 (0.99–2.66) | 0.055 |
| Stroke/TIA | 1.11 (0.59–2.08) | 0.739 | ||
| Diabetes | 1.03 (0.62–1.72) | 0.897 | ||
| LVEF ≤ 50 | 1.45 (0.91–2.32) | 0.122 | ||
| Beta-blocker | 1.12 (0.75–1.66) | 0.580 | ||
| Class III antiarrhythmic agents | 1.08 (0.71–1.65) | 0.725 | ||
| Class IC antiarrhythmic agents | 0.63 (0.35–1.13) | 0.119 | ||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
The variables outlined in bold characters are those associated with a significant (P > 0.05) risk of persistent atrial fibrillation.
Cox Regression analyses of persistent atrial fibrillation (AF > 7 days) risk in patients with long PR
| Long PR patients . | Persistent AF risk Univariate analysis . | Persistent AF risk Multivariate analysis . | ||
|---|---|---|---|---|
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Control DDDR | 0.73 (0.49–1.09) | 0.124 | 0.65 (0.43–1.00) | 0.049 |
| Age ≥ 75 years | 1.54 (1.03–2.29) | 0.035 | ||
| Gender (male) | 1.22 (0.82–1.81) | 0.320 | ||
| Atrial cardioversions | 2.02 (1.33–3.07) | 0.001 | 2.13 (1.39–3.27) | <0.001 |
| Hypertension | 1.56 (0.98–2.48) | 0.059 | 1.62 (0.99–2.66) | 0.055 |
| Stroke/TIA | 1.11 (0.59–2.08) | 0.739 | ||
| Diabetes | 1.03 (0.62–1.72) | 0.897 | ||
| LVEF ≤ 50 | 1.45 (0.91–2.32) | 0.122 | ||
| Beta-blocker | 1.12 (0.75–1.66) | 0.580 | ||
| Class III antiarrhythmic agents | 1.08 (0.71–1.65) | 0.725 | ||
| Class IC antiarrhythmic agents | 0.63 (0.35–1.13) | 0.119 | ||
| Long PR patients . | Persistent AF risk Univariate analysis . | Persistent AF risk Multivariate analysis . | ||
|---|---|---|---|---|
| Baseline characteristics . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Control DDDR | 0.73 (0.49–1.09) | 0.124 | 0.65 (0.43–1.00) | 0.049 |
| Age ≥ 75 years | 1.54 (1.03–2.29) | 0.035 | ||
| Gender (male) | 1.22 (0.82–1.81) | 0.320 | ||
| Atrial cardioversions | 2.02 (1.33–3.07) | 0.001 | 2.13 (1.39–3.27) | <0.001 |
| Hypertension | 1.56 (0.98–2.48) | 0.059 | 1.62 (0.99–2.66) | 0.055 |
| Stroke/TIA | 1.11 (0.59–2.08) | 0.739 | ||
| Diabetes | 1.03 (0.62–1.72) | 0.897 | ||
| LVEF ≤ 50 | 1.45 (0.91–2.32) | 0.122 | ||
| Beta-blocker | 1.12 (0.75–1.66) | 0.580 | ||
| Class III antiarrhythmic agents | 1.08 (0.71–1.65) | 0.725 | ||
| Class IC antiarrhythmic agents | 0.63 (0.35–1.13) | 0.119 | ||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
The variables outlined in bold characters are those associated with a significant (P > 0.05) risk of persistent atrial fibrillation.
PR segment
Persistent AF evaluated as a function of the PR segment—time between P wave end and QRS onset—showed a linear increase in the MVP arm and a stepwise decrease in the Control DDDR arm (Figure 4).
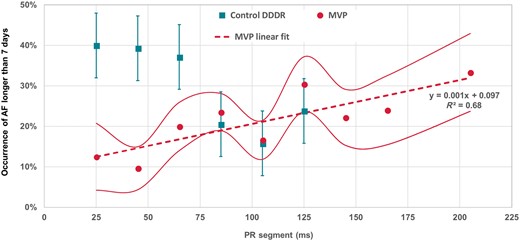
Incidence of AF longer than 7 days as a function of PR segment for patients randomized to DDDR or MVP pacing mode. In the graph, the teal whisker lines represent AF occurrence standard errors for the Control DDDR patients, while red continuous lines represent AF occurrence standard errors for the MVP patients. AF, atrial fibrillation; MVP, managed ventricular pacing.
Atrial and ventricular pacing
The median (IQR) percentage of atrial pacing was 70% (39–90%) in the Control DDDR arm, 73% (42–92%) in the MVP arm, and 93% (81–97%) in the DDDRP + MVP arm (P < 0.001 vs. other groups). The median (IQR) percentage of ventricular pacing was 53% (15–84%) in the Control DDDR arm, 1% (0–9%) in the MVP arm, and 2% (0–11%) in the DDDRP + MVP arm (P < 0.001 comparing Control DDDR with both arms with MVP). The median (IQR) percentage of ventricular pacing was 34% (6–72%) in Control DDDR patients with short PR, 65% (27–90%) in Control DDDR patients with long PR, 1% (0–8%) in MVP patients with short PR, 1% (0–9%) in MVP patients with long PR, 1% (0–4%) in DDDRP + MVP patients with short PR, and 3% (0–14%) in DDDRP + MVP patients with long PR.
Discussion
Current ESC guidelines10 on cardiac pacing indicate dual-chamber pacing and AV management to minimize RV apical pacing in patients with sinus node disease and in patients with intermittent AV block, and indicate dual-chamber pacing with fixed AV interval in patients with persistent AV block to guarantee AV synchrony. Similarly, Heart Rhythm Society, American College of Cardiology, and American Heart Association guidelines11 on cardiac pacing indicate atrial pacing in patients without impaired AV conduction, and dual-chamber pacing in patients with impaired, or future concern of, AV conduction with a desire for AV synchrony. Patients are not sharply divided in two separate groups according to presence or absence of AV conduction disease, rather we observe a continuum from prolonged PR to third-degree AV block. Therefore, the decision between MVP and DDD may be difficult due to the trade-off between re-establishing a favourable AV synchrony by ventricular pacing with optimal transmitral left ventricular (LV) filling and inducing ventricular dyssynchrony and LV dysfunction by pacing.
In the MINERVA trial,8 we showed that the incidence of the main endpoint, composed by death, cardiovascular hospitalizations, and permanent AF, was significantly reduced by DDDRP + MVP programming. In the ancillary study here reported, we chose persistent AF as the main endpoint since AF has been shown to be associated with RV pacing and AV dyssynchrony3–8 and because we wanted to improve statistical power in evaluating of the interaction between pacing mode and PR interval.
Main results
Our study found that, (i) in patients with short PR interval, ventricular pacing minimization is associated with lower incidence of persistent AF compared with standard DDDR pacing, (ii) in patients with long PR interval, DDDR pacing is associated with a lower incidence persistent AF when compared with MVP mode and (iii) atrial anti-tachycardia pacing prevents persistent AF.
Short PR patients
The clinical benefit of reducing RV pacing has been suggested by both observational and randomized studies4,5 and has been described in pacing guidelines,10 but it has been recently questioned.6–9 Our data, through interaction analysis (Figure 3) and multivariate Cox regression analysis (Table 2), convincingly show that RV pacing minimization is indeed the right clinical choice in patients with sinus node disease without prolonged PR interval. This finding reinforces the results of the SAVE PACe trial5 where the significant reduction of persistent AF risk in the RV pacing minimization arm was attributable to prevention of RV pacing in normal PR patients. In the RV minimization arm of that trial, most patients with intrinsic PR longer than 215 ms after sensed atrial events and longer than 245 ms after paced atrial events were actually receiving DDD pacing.
Long PR patients
The fact that patients with long PR intervals were associated with a lower persistent AF if paced in standard DDDR mode compared with MVP mode is a second important finding of our study and represents the first clinical evidence showing a statistically significant interaction (P = 0.012) between PR interval and pacing mode (Figure 3). The PREFER MVP trial12 showed a higher incidence of persistent AF in MVP vs. DDD pacing in patients with PR interval ≥230 ms. The DANPACE trial14 showed a higher AF incidence in AAI vs. DDD pacing in patients with PQ interval >180 ms. In the MVP trial,17 a higher incidence of death or heart failure hospitalizations was observed in implantable cardioverter-defibrillator patients with PR ≥230 ms treated by MVP vs. ventricular pacing and sensing (VVI) pacing.
Long AV delay may be deleterious in patients with long PR or in general with conduction disturbances, because left atrial (LA) contraction is anticipated or because LV contraction is delayed, mitral valve closure may occur too early, E and A waves may be fused causing a reduction of the left ventricle filling time, and possibly mitral regurgitation.18
PR interval—an effect modifier of the pacing therapy
Prolonged PR interval was once thought to be a predominantly benign finding. There is now growing evidence suggesting that long PR is associated with increased AF risk and poor prognosis.12–15
The observation that PR interacts with MVP and DDDR therapy (Figure 3) means that PR interval is an effect modifier of the pacing therapy, which is something not reported in previous studies. This finding suggests that dual-chamber pacing might be an effective way to combat the adverse effects associated with prolonged PR intervals through shortening of the atrial-ventricular delay.
Clinical implications
Our results contribute to the design of a physiological pacing model by showing apical RV pacing has some adverse effects, like being associated with AF, in patients with preserved cardiac conduction, while DDD pacing may be preferred to ventricular pacing minimization for patients with long PR intervals.
Interestingly, in the DDDR arm, long PR patients were associated with lower AF incidence, despite a median ventricular pacing percentage of 65%, compared with short PR patients who had a median pacing percentage of 34%. This confirms that ventricular pacing is not bad ‘per se’ depending on patient characteristics such as atrial conduction delays and AV conduction.
Pacing guidelines for patients with sinus node disease may leverage the PR interval as a screening factor to choose the optimal pacing mode between DDD pacing with ventricular pacing minimization algorithms and standard DDD pacing with fixed AV intervals. An alternative option may be to program DDD pacing with a long AV interval, but so far neither our study, where investigators used moderately prolonged AV intervals, nor other studies have identified the ‘optimally long’ AV delay.
Our data also show the importance of the PR segment, which is shown to interact with pacing mode and to influence AF incidence (Figure 3). In particular, Figure 4 shows that in the MVP arm AF risk, as a function of PR segment, increases linearly—by 1% for every 10 ms increase of PR segment. Figure 4 also shows that AF risk has a step-like behaviour in DDDR patients with higher risk if the PR segment is too short. It is well recognized that DDD with a fixed AV delay cannot be the optimal choice for all patients since, by nature, optimal AV delay has wide inter- and intra-patient variability. If the AV interval is too short, either because LA is delayed (eventually for interatrial conduction delays) or because ventricular contraction is anticipated, the outcomes may be insufficient time for atrial emptying, reduced atrial contribution, and haemodynamic issues due to anticipated mitral valve closure.18 The physiologic importance of the temporal relation between atrial and ventricular contraction has long been recognized. Chirife et al.19 showed that programming of the AV interval, which actually determines AV synchrony in the right heart, may result in AV asynchrony in the left heart. Our data may suggest that even modern pacemakers cannot always provide physiological pacing and may explain the contradictory results of several prospective trials that compared VVI, AAI/MVP, and DDD modes.3,5–9 While not all pacemaker patients will suffer serious consequences with programming of the AV delay slightly shorter or longer than the optimal AV delay, the AV optimization should be warranted in many patients, especially those with long PR intervals or left ventricle dysfunction, to improve haemodynamics and quality of life. The continuous AV interval optimization, already available in some cardiac resynchronization therapy devices,20 is warranted to all pacemaker patients, especially those with prolonged AV conduction.
Limitations
PR interval was not collected in 260 (22%) of the randomized patients; however, we verified that the cohorts of patients with and without PR interval information were not different, showing no selection bias.
We did not specifically assess the effects of AV intervals on AF occurrence. Since we recognize that AV intervals and interatrial conduction delays interact in a complex way in determining pacing-induced interventricular dyssynchrony and ventricular filling, we evaluated the association between AF and PR segments that take into account the AV intervals and the P wave durations. ECG measurements were performed based on a single lead configuration (lead II derivation). This could have limited our capability to establish the interaction of P wave duration with the pacing mode.
We divided our population in six subgroups according to median PR and to the three treatment arms. The numbers of studied patients and the number of persistent AF events did not allow us to divide our patient population according to many PR interval ranges while maintaining sufficient statistical power. For this reason, we cannot definitely estimate the precise value PR interval that becomes too long and ventricular pacing becomes beneficial.
Conclusions
Our data show that PR interval significantly interacts with the association between pacing mode and persistent AF incidence and consequently it may be used as a selection criterion to identify optimal physiological pacing modes in patients with AF history. Progression to persistent AF is prevented by RV pacing minimization in normal PR patients and by standard dual-chamber pacing in long PR patients. Future studies hopefully will confirm and expand our suggestions.
The observation that anti-tachycardia pacing therapies prevent the natural progression to persistent or permanent AF, regardless PR interval, indicates that Reactive ATP™ should be programmed in addition to the optimal pacing mode.
Acknowledgements
We gratefully remember Professor Luigi Padeletti who was the MINERVA trial Principal Investigator and a scientific guide for all of us. The current manuscript represents one of his last intellectual legacies.
The MINERVA trial has been registered at ClinicalTrials.gov (Identifier: NCT00262119).
Funding
The study was sponsored by Medtronic plc., which performed study management, data management, and statistical analysis. The sponsor had no role in the collection of clinical data, in the interpretation of data, and in the decision to submit the article for publication.
Conflict of interest: The following authors received modest speaker fees or modest consultant/advisory board grants: G. B. from Boston Scientific, Medtronic, Abbott and Boehringer Ingelheim, L.M. from Biotronik, Boston Scientific, Medtronic, Sanofi Sorin and Abbott, M.L. from Medtronic, LivaNova and Boston Scientific. H.P. and R.T. from Medtronic. M.C. and A.G. are employees of Medtronic plc. The other authors have no conflicts of interest.



