-
PDF
- Split View
-
Views
-
Cite
Cite
Xiao-yu Liu, Peter Karl Jacobsen, Steen Pehrson, Xu Chen, Catheter ablation of incisional atrial tachycardia using remote magnetic navigation in patients after heart surgery: comparison between acquired and congenital heart disease, EP Europace, Volume 20, Issue suppl_2, May 2018, Pages ii33–ii39, https://doi.org/10.1093/europace/euy005
Close - Share Icon Share
Abstract
The objectives of this study were to assess the acute and long-term outcomes of catheter ablation in incisional atrial tachycardia (IAT) using remote magnetic navigation (RMN) in patients after heart surgery.
A total of 46 patients with IAT after heart surgery who underwent catheter ablation using RMN were included. Of these patients, 22 patients had acquired heart disease (AHD) and the remaining 24 patients had various types of congenital heart disease (CHD). In these 46 patients, 57 re-entry circuits were found in 56 procedures. The re-entry circuits were mainly distributed in right atrium (RA). Acute success of first ablation reached in 42 of 46 (91%) patients. Mean procedure duration was 115 ± 39 min, ablation duration was 678 (920.5) s, X-ray time was 4 (4.8) min, and X-ray dose was 3 (6.0) gy cm2. After a mean follow-up of 28 ± 19 months, 39 of 46 (85%) patients were free from IAT. No major complications were observed. There were no significant differences in procedure durations (AHD 113 ± 40 min vs. CHD 119 ± 38 min), ablation durations [AHD 643 (1027) s vs. CHD 712 (929) s], X-ray time [AHD 4 (4.5) min vs. CHD 4 (5.0) min], circuits in RA (AHD 85% vs. CHD 86%), acute success rates (AHD 91% vs. CHD 92%), and long-term success rates (AHD 86% vs. CHD 83%) between the two groups (P > 0.05).
Catheter ablation of IAT in patients after heart surgery using RMN is safe and effective. No significant differences related to success rates and procedure characteristics were found between patients with AHD and CHD.
What’s new?
This article summarizes the characteristics of incisional atrial tachycardia ablation using remote magnetic navigation (RMN) for acquired heart disease patients with previous heart surgeries.
Fluoroscopy time was markedly reduced by ablation with RMN compared with published data of manual ablation.
The patients with acquired and congenital heart disease presented with similar success rates and procedure characteristics.
Introduction
Incisional atrial tachycardia (IAT) commonly develops after open-heart surgery when surgical incisions create the substrate for macrore-entrant atrial tachycardia.1,2 The mechanism of macrore-entrant atrial tachycardia, described by Saoudi et al.,3 is re-entrant activation around a large (generally several centimetres in diameter) obstacle, which may be normal and abnormal structures, and have a slow conduction zone that may be targets for therapeutic action. We defined IAT as a macrore-entrant atrial tachycardia with a slow conduction zone related to scar. Re-entry circuits can be found around scars or anatomic structure or both (Figure 1). Surgical incisions are related to the development of slow conduction zones, which consequently create the substrate for macrore-entry for IAT.4,5 Incisional atrial tachycardia has been shown to contribute to high morbidity and mortality in patients after heart surgery and is often refractory to antiarrhythmic drugs.6 In recent years, catheter ablation for IAT has emerged as a promising treatment strategy, but challenges remain with respect to relatively low success rates and high recurrence rates.7 The remote magnetic navigation (RMN) system has the advantages in precise, flexible navigation, reduced fluoroscopy time, and improved safety. Several studies have described acceptable acute success rates and good long-term outcomes of supraventricular tachycardia (SVT) ablation using RMN in adult patients with congenital heart diseases (CHDs).8–10 However, none of these studies systematically demonstrated the efficacy and safety of IAT ablation using RMN in patients after open-heart surgery. To the best of our knowledge, there is no publication using RMN for IAT ablation for acquired heart diseases (AHDs) patients with previous cardiac surgery. Therefore, the goal of this study was to assess the efficacy and safety of catheter ablation using RMN for IAT in patients after open-heart surgery. Furthermore, we compared the impacts of RMN-guided catheter ablation between patients with AHD and CHD.
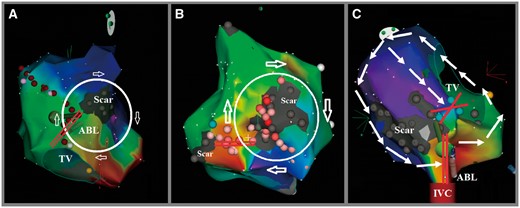
Electroanatomical activation mapping and ablation using RMN of incisional atrial tachycardia post-surgery. (A) Activation map illustrating the circuit is a clockwise loop around RA septal atriotomy scar only. The slow conduction zone of this circuit (marked as two parallel red lines) is located between the scar and tricuspid annulus. (B) Activation map illustrating the circuit is a clockwise loop around RA free wall atriotomy scar only. The slow conduction zone of this circuit (marked as two parallel red lines) is located between the scars. Some abnormal potential around and inside of the larger scar was also ablated. (C) Activation map illustrating the circuit is a counterclockwise loop around RA septal atriotomy scar and tricuspid annulus. The region between the scar and tricuspid annulus appears to be a slow conduction zone; however, there is a complete block line which marked as green dots. The slow conduction zone of this circuit (marked as two parallel red lines) is located between the scar and inferior vena cava and the tip of ablation catheter connect this zone. ABL, ablation catheter; IVC, inferior vena cava; RA, right atrium; RMN, remote magnetic navigation; TV, tricuspid annulus.
Methods
Study population
In this prospective observational study, 46 after heart surgery patients with IAT who were referred for catheter ablation at the Rigshospitalet, University of Copenhagen between April 2008 and April 2014 were included. Patients were refractory to at least one antiarrhythmic drug. Preprocedural electrocardiogram (ECG) and echocardiography imaging were acquired, and a periprocedural transoesophageal echocardiogram was performed on the day of the procedure when clinically indicated. Antiarrhythmic agents were discontinued at least five drug half-lives before the procedure. Anticoagulant medications were continued up to the day of the ablation. All patients signed an informed consent before the procedure. Remote magnetic navigation guided ablation is the preferred technique for IAT ablation in our centre. None of the procedures of IAT ablations were performed manually during this period.
Electrophysiological study
Commonly, a 6F steerable catheter (Inquiry, St Jude Medical, Inc.) was positioned within the coronary sinus (CS) and a 5F quadripolar catheter (Medtronic, Inc.) was placed at the apex of the right ventricle via the left femoral vein, respectively. In complex cases, such as patients after Mustard or Senning operations, the steerable catheter and the quadripolar catheter were placed at appropriate locations where signals of the atrium and ventricles were recorded, respectively. A SL0 sheath (St. Jude Medical, Inc.) was introduced for right sided or trans-septal procedure. Trans-septal puncture was performed in left anterior oblique view under pressure and fluoroscopic monitoring if left-sided arrhythmias were suspected. An irrigated magnetic ablation catheter (Navistar® RMT-Thermocool®, Biosense Webster Inc.) was introduced into the target atrial cavity through the SL0 sheath. In patients with intra-atrial baffle (Mustard or Senning operation), retrograde access through the aorta was performed. Intravenous heparin was administered as a bolus of 50∼100 IU/kg body weight immediately after placing the ablation catheter, followed by injection of heparin 1000 IU per hour. Surface ECG and endocardial electrograms were continuously monitored and recorded.
Remote magnetic navigation system and mapping
The magnetic ablation catheter was connected with the CARTO RMT system (Biosense Webster), the RMN Niobe® II (for patients enrolled through 30 December 2011) or Niobe ES system (for patients enrolled after 1 January 2012) (Stereotaxis Inc., St. Louis, MO, USA) and the EP-recording system (EP-Tracer, Cardio-Tek) was used to perform mapping and ablation. Remote magnetic navigation controlled the direction of the catheter tip. A catheter-advancing system (QuikCAS™, Stereotaxis Inc.) was used to control remote catheter advancement and retraction. The Odyssey® system (Stereotaxis Inc.) unified the display of the CARTO RMT system, EP-recording system, and X-ray system. Constructing an anatomical model of the target atrium was performed to define low voltage areas representing scar tissue, which could serve as boundaries for the re-entrant circuits. The scar area was defined by a bipolar electrogram ≤0.07 mV. If an arrhythmia was not present during the procedure, standard atrial pacing protocol with and without infusion of isoproterenol was performed to induce the clinical IAT. The target atrium was remapped using activation mapping in the same anatomical model. Activation mapping helped to define the majority of the cycle length of a re-entrant tachycardia in relation to the atrial anatomy. With an accurate activation map, a critical isthmus might be identified by a slow conduction zone, visualized as an area with a rapid change in colours on the CARTO map. Areas of double potentials were marked to indicate possible conduction linear blocks. Areas of highly fractionated local electrograms were marked to indicate possible slow, anisotropic conduction. Entrainment-pacing maneuvers were performed from the ablation and CS catheters at sites of interest during the entire procedure in order to confirm the position of re-entrant loops and critical isthmus.
Radiofrequency catheter ablation for incisional atrial tachycardia using remote magnetic navigation system
Radiofrequency catheter ablation was performed during IAT. Ablation targets were chosen in critical isthmus of re-entry circuits. Radiofrequency energy was delivered in a point-by-point fashion in the temperature control mode with a target temperature of <45°C. Radiofrequency power output was set at 30–40 W based on different locations in the atrium with a catheter irrigation rate of 10–30 mL/min. After termination of IAT by ablation, programmed atrial stimulation was applied to re-induce tachycardia. Any other newly induced IAT was targeted, and programmed atrial stimulation was repeated until no further IAT was inducible. If the IAT could not be terminated by ablation, electrical cardioversion was performed. Acute success was defined as non-inducibility and bi-directional block in the critical isthmus for all targeted IATs during the first procedure.
Follow-up
All patients were routinely evaluated in outpatient clinics 2–3 months after discharge and then followed by their local cardiologists. A 12-lead ECG, event recorder, and Holter recordings were performed in patients suffering symptomatic palpitations. Recurrence was defined as symptomatic and/or asymptomatic episodes of atrial tachycardia(s) confirmed by ECG, event recorder, or Holter recordings. Long-term success was defined as freedom from atrial tachycardia(s). A second procedure was recommended for patients with recurrence. The distinction between recurrence of an ablated IAT and occurrence of a new IAT was confirmed by comparing the electroanatomic maps.
Complications
Complications were divided into two categories: major and minor. Major complications consisted of acute myocardial infarction, stroke, major bleeding, cardiac tamponade, and atrial-oesophageal fistulae. Minor complications included pericarditis and inguinal haematoma.
Statistical analysis
Normally distributed continuous variables are expressed as mean ± standard deviation and skewed continuous variables as median (interquartile range). Categorical variables were presented as a percentage. Patients were divided into two groups according to the underlying heart diseases: one group for AHD and the other for CHD. An unpaired Student’s t-test or non-parametric test (Mann–Whitney test) was used to compare the continuous variables between the two groups. Categorical data were analysed using the χ2 test or the Fisher’s exact test. For the long-term outcomes, survival functions were estimated by the Kaplan–Meier analysis. A value of P <0.05 was considered statistically significant. SPSS 19.0 statistical package was used for analysis. For purpose of analysis, multiple procedures within the same patient were assumed to be independent. In such cases, only the period after the last procedure was assessed for freedom from arrhythmia.
Results
Baseline characteristics of patients
The baseline patient characteristics are listed in Table 1 and previous surgeries are presented in Figure 2. Four patients had mitral valve replacement with MAZE operation during same procedure.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Gender (female/male) | 2/20 | 7/17 | 9/37 | 0.14 |
| Age (years) | 63 ± 15 | 49 ± 11 | 56 ± 13 | <0.01 |
| Age at surgery (years) | 56 ± 10 | 29 ± 10 | 42 ± 10 | <0.01 |
| Duration from surgery to ablation (months) | 75 ± 52 | 347 ± 118 | 217 ± 93 | <0.01 |
| EF (%) | 46 ± 8 | 48 ± 6 | 47 ± 7 | 0.34 |
| Antiarrhythmic drug | ||||
| Amiodarone | 10 | 14 | 24 | – |
| β-Blocking agent | 13 | 11 | 24 | – |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Gender (female/male) | 2/20 | 7/17 | 9/37 | 0.14 |
| Age (years) | 63 ± 15 | 49 ± 11 | 56 ± 13 | <0.01 |
| Age at surgery (years) | 56 ± 10 | 29 ± 10 | 42 ± 10 | <0.01 |
| Duration from surgery to ablation (months) | 75 ± 52 | 347 ± 118 | 217 ± 93 | <0.01 |
| EF (%) | 46 ± 8 | 48 ± 6 | 47 ± 7 | 0.34 |
| Antiarrhythmic drug | ||||
| Amiodarone | 10 | 14 | 24 | – |
| β-Blocking agent | 13 | 11 | 24 | – |
P-values listed represent differences between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease; EF, ejection fraction.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Gender (female/male) | 2/20 | 7/17 | 9/37 | 0.14 |
| Age (years) | 63 ± 15 | 49 ± 11 | 56 ± 13 | <0.01 |
| Age at surgery (years) | 56 ± 10 | 29 ± 10 | 42 ± 10 | <0.01 |
| Duration from surgery to ablation (months) | 75 ± 52 | 347 ± 118 | 217 ± 93 | <0.01 |
| EF (%) | 46 ± 8 | 48 ± 6 | 47 ± 7 | 0.34 |
| Antiarrhythmic drug | ||||
| Amiodarone | 10 | 14 | 24 | – |
| β-Blocking agent | 13 | 11 | 24 | – |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Gender (female/male) | 2/20 | 7/17 | 9/37 | 0.14 |
| Age (years) | 63 ± 15 | 49 ± 11 | 56 ± 13 | <0.01 |
| Age at surgery (years) | 56 ± 10 | 29 ± 10 | 42 ± 10 | <0.01 |
| Duration from surgery to ablation (months) | 75 ± 52 | 347 ± 118 | 217 ± 93 | <0.01 |
| EF (%) | 46 ± 8 | 48 ± 6 | 47 ± 7 | 0.34 |
| Antiarrhythmic drug | ||||
| Amiodarone | 10 | 14 | 24 | – |
| β-Blocking agent | 13 | 11 | 24 | – |
P-values listed represent differences between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease; EF, ejection fraction.
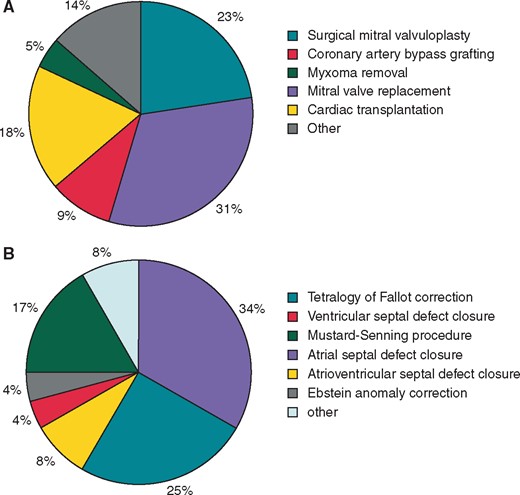
Types of cardiac surgery. (A) Acquired heart disease and (B) congenital heart disease.
Ablation procedures and complications
Procedure-related data including total procedure duration (from puncture to introducer withdrawal), radiofrequency duration (from mapping conclusion to two-way blocking line created), X-ray time, and dose are listed in Table 2. There were no significant differences found in procedure parameters between the AHD and CHD group. There were no major complications. Three patients had minor complications, which were all groin haematomas.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Procedure duration (min) | 113 ± 40 | 119 ± 38 | 115 ± 39 | 0.55 |
| Radiofrequency duration (s) | 643 (1027) | 712 (929) | 678 (920.5) | 0.68 |
| X-ray time (min) | 4 (4.5) | 4 (5.0) | 4 (4.8) | 0.94 |
| X-ray dose (gy cm2) | 3 (5.0) | 3 (7.0) | 3 (6.0) | 0.61 |
| Complications (minor) | 2 | 1 | 3 | 0.60 |
| Complications (major) | 0 | 0 | 0 | – |
| Cycle length (ms) | 305 ± 83 | 331 ± 109 | 319 ± 96 | 0.30 |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Procedure duration (min) | 113 ± 40 | 119 ± 38 | 115 ± 39 | 0.55 |
| Radiofrequency duration (s) | 643 (1027) | 712 (929) | 678 (920.5) | 0.68 |
| X-ray time (min) | 4 (4.5) | 4 (5.0) | 4 (4.8) | 0.94 |
| X-ray dose (gy cm2) | 3 (5.0) | 3 (7.0) | 3 (6.0) | 0.61 |
| Complications (minor) | 2 | 1 | 3 | 0.60 |
| Complications (major) | 0 | 0 | 0 | – |
| Cycle length (ms) | 305 ± 83 | 331 ± 109 | 319 ± 96 | 0.30 |
P-values listed represent differences between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Procedure duration (min) | 113 ± 40 | 119 ± 38 | 115 ± 39 | 0.55 |
| Radiofrequency duration (s) | 643 (1027) | 712 (929) | 678 (920.5) | 0.68 |
| X-ray time (min) | 4 (4.5) | 4 (5.0) | 4 (4.8) | 0.94 |
| X-ray dose (gy cm2) | 3 (5.0) | 3 (7.0) | 3 (6.0) | 0.61 |
| Complications (minor) | 2 | 1 | 3 | 0.60 |
| Complications (major) | 0 | 0 | 0 | – |
| Cycle length (ms) | 305 ± 83 | 331 ± 109 | 319 ± 96 | 0.30 |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Procedure duration (min) | 113 ± 40 | 119 ± 38 | 115 ± 39 | 0.55 |
| Radiofrequency duration (s) | 643 (1027) | 712 (929) | 678 (920.5) | 0.68 |
| X-ray time (min) | 4 (4.5) | 4 (5.0) | 4 (4.8) | 0.94 |
| X-ray dose (gy cm2) | 3 (5.0) | 3 (7.0) | 3 (6.0) | 0.61 |
| Complications (minor) | 2 | 1 | 3 | 0.60 |
| Complications (major) | 0 | 0 | 0 | – |
| Cycle length (ms) | 305 ± 83 | 331 ± 109 | 319 ± 96 | 0.30 |
P-values listed represent differences between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease.
Location of re-entry circuits and slow conduction zones
A total of 57 IATs were identified during the 56 procedures (Table 3), mean cycle length (Table 2) was 319 ± 96 ms. Nine patients underwent multiple procedures (eight patients underwent two procedures and one patient underwent three procedures).
| . | AHD . | CHD . | All . |
|---|---|---|---|
| Patients with multiple circuits | 2 | 4 | 6 |
| Patients with trans-septal puncture | 4 | 4 | 8 |
| Circuits | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Around mitral valve | 2 | 2 | 4 |
| Around pulmonary veins | 2 | 1 | 3 |
| Around intracardiac baffle | 0 | 1 | 1 |
| RA | 23 | 26 | 49 |
| Around tricuspid valve | 6 | 5 | 11 |
| Around vena cava | 4 | 5 | 9 |
| Around scar only | 13 | 16 | 29 |
| Slow conduction zone | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Roof | 1 | 1 | 2 |
| Septum | 2 | 1 | 3 |
| Free wall | 1 | 2 | 3 |
| RA | 23 | 26 | 49 |
| Septum | 3 | 5 | 8 |
| Anterior wall | 6 | 7 | 13 |
| Lateral wall | 9 | 9 | 18 |
| Posterior wall | 5 | 5 | 10 |
| . | AHD . | CHD . | All . |
|---|---|---|---|
| Patients with multiple circuits | 2 | 4 | 6 |
| Patients with trans-septal puncture | 4 | 4 | 8 |
| Circuits | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Around mitral valve | 2 | 2 | 4 |
| Around pulmonary veins | 2 | 1 | 3 |
| Around intracardiac baffle | 0 | 1 | 1 |
| RA | 23 | 26 | 49 |
| Around tricuspid valve | 6 | 5 | 11 |
| Around vena cava | 4 | 5 | 9 |
| Around scar only | 13 | 16 | 29 |
| Slow conduction zone | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Roof | 1 | 1 | 2 |
| Septum | 2 | 1 | 3 |
| Free wall | 1 | 2 | 3 |
| RA | 23 | 26 | 49 |
| Septum | 3 | 5 | 8 |
| Anterior wall | 6 | 7 | 13 |
| Lateral wall | 9 | 9 | 18 |
| Posterior wall | 5 | 5 | 10 |
AHD, acquired heart disease; CHD, congenital heart disease; LA, left atrium; RA, right atrium.
| . | AHD . | CHD . | All . |
|---|---|---|---|
| Patients with multiple circuits | 2 | 4 | 6 |
| Patients with trans-septal puncture | 4 | 4 | 8 |
| Circuits | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Around mitral valve | 2 | 2 | 4 |
| Around pulmonary veins | 2 | 1 | 3 |
| Around intracardiac baffle | 0 | 1 | 1 |
| RA | 23 | 26 | 49 |
| Around tricuspid valve | 6 | 5 | 11 |
| Around vena cava | 4 | 5 | 9 |
| Around scar only | 13 | 16 | 29 |
| Slow conduction zone | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Roof | 1 | 1 | 2 |
| Septum | 2 | 1 | 3 |
| Free wall | 1 | 2 | 3 |
| RA | 23 | 26 | 49 |
| Septum | 3 | 5 | 8 |
| Anterior wall | 6 | 7 | 13 |
| Lateral wall | 9 | 9 | 18 |
| Posterior wall | 5 | 5 | 10 |
| . | AHD . | CHD . | All . |
|---|---|---|---|
| Patients with multiple circuits | 2 | 4 | 6 |
| Patients with trans-septal puncture | 4 | 4 | 8 |
| Circuits | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Around mitral valve | 2 | 2 | 4 |
| Around pulmonary veins | 2 | 1 | 3 |
| Around intracardiac baffle | 0 | 1 | 1 |
| RA | 23 | 26 | 49 |
| Around tricuspid valve | 6 | 5 | 11 |
| Around vena cava | 4 | 5 | 9 |
| Around scar only | 13 | 16 | 29 |
| Slow conduction zone | 27 | 30 | 57 |
| LA | 4 | 4 | 8 |
| Roof | 1 | 1 | 2 |
| Septum | 2 | 1 | 3 |
| Free wall | 1 | 2 | 3 |
| RA | 23 | 26 | 49 |
| Septum | 3 | 5 | 8 |
| Anterior wall | 6 | 7 | 13 |
| Lateral wall | 9 | 9 | 18 |
| Posterior wall | 5 | 5 | 10 |
AHD, acquired heart disease; CHD, congenital heart disease; LA, left atrium; RA, right atrium.
In the AHD group, 85% (23/27) of the circuits and slow conduction zones were located in the right atrium (RA). There were 48% (13/27) of the circuits found around scars only and 52% (14/27) around scar and anatomic structure. Thirty percent (8/27) of the slow conduction zones were located between scars and 70% (19/27) located between scar and anatomic structure.
In the CHD group, 86% (26/30) of the circuits and slow conduction zones were located in the RA. There were 53% (16/30) of the circuits found around scars only and 47% (14/30) around scar and anatomic structure. Thirty-three percent (10/30) of the slow conduction zones were located between scars and 66% (20/30) were located between scar and anatomic structure.
Both CHD and AHD groups had a significant number of IATs located in the RA (26/30 vs. 23/27, respectively), but no difference was found between the two groups (P = 1.0). There were no significant differences in the percentages of re-entry circuits around scar only (P = 0.79). There were also no significant differences in the percentages of slow conduction zones located between scars (P = 0.78).
In the CHD group, four patients with multiple circuits were identified, of which two presented with three circuits and two with two circuits. In the AHD group, two patients with multiple circuits were identified, of which one presented with three circuits and one with four circuits. There were no significant differences in patients with multiple circuits between the two groups (P = 0.67).
Acute results and long-term follow-up
Acute success and recurrence rates are listed in Table 4 and presented in Figure 3. Acute success in the initial procedure was obtained in 91% (42/46) of all patients. There were no significant differences in acute success rates (AHD 91% vs. CHD 92%) between the two groups (P > 0.05). Four patients were acute failures, two of whom were in the AHD group and two of whom were in the CHD group. One patient in the AHD group without acute success underwent a redo procedure 1 week later, resulted in acute and long-term success. The remaining three patients declined a redo procedure.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Follow-up (month) | 26 ± 18 | 29 ± 19 | 28 ± 19 | 0.49 |
| Acute success after first ablation | 20 | 22 | 42 | 1.00 |
| Recurrence after first ablation | 4 | 8 | 12 | 0.41 |
| Duration of first recurrence (month) | 11 ± 11 | 7 ± 6 | 8 ± 8 | 0.41 |
| Long-term success | 19 | 20 | 39 | 1.00 |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Follow-up (month) | 26 ± 18 | 29 ± 19 | 28 ± 19 | 0.49 |
| Acute success after first ablation | 20 | 22 | 42 | 1.00 |
| Recurrence after first ablation | 4 | 8 | 12 | 0.41 |
| Duration of first recurrence (month) | 11 ± 11 | 7 ± 6 | 8 ± 8 | 0.41 |
| Long-term success | 19 | 20 | 39 | 1.00 |
P-values listed were calculated between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease.
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Follow-up (month) | 26 ± 18 | 29 ± 19 | 28 ± 19 | 0.49 |
| Acute success after first ablation | 20 | 22 | 42 | 1.00 |
| Recurrence after first ablation | 4 | 8 | 12 | 0.41 |
| Duration of first recurrence (month) | 11 ± 11 | 7 ± 6 | 8 ± 8 | 0.41 |
| Long-term success | 19 | 20 | 39 | 1.00 |
| . | AHD (n = 22) . | CHD (n = 24) . | Total (n = 46) . | P-value . |
|---|---|---|---|---|
| Follow-up (month) | 26 ± 18 | 29 ± 19 | 28 ± 19 | 0.49 |
| Acute success after first ablation | 20 | 22 | 42 | 1.00 |
| Recurrence after first ablation | 4 | 8 | 12 | 0.41 |
| Duration of first recurrence (month) | 11 ± 11 | 7 ± 6 | 8 ± 8 | 0.41 |
| Long-term success | 19 | 20 | 39 | 1.00 |
P-values listed were calculated between the AHD group and CHD group.
AHD, acquired heart disease; CHD, congenital heart disease.
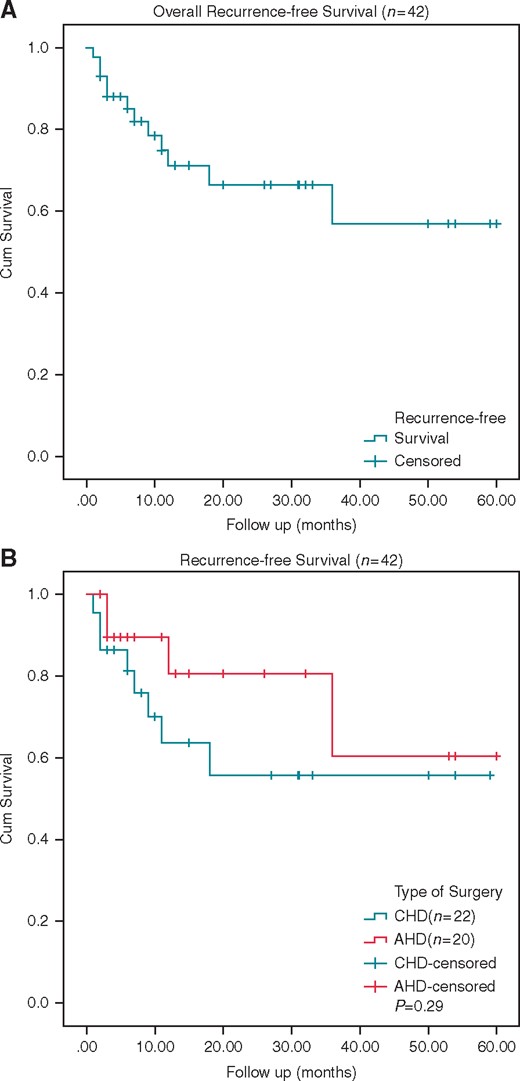
The Kaplan–Meier curve of scar-related atrial flutter patients with acute success. (A) Overall recurrence-free survival of all the patients. (B) Recurrence-free survival of AHD and CHD groups. AHD, acquired heart disease; CHD, congenital heart disease.
Of the patients with acute success after primary ablation, 30 of 42 (71%) patients were free from any atrial tachycardia recurrence during follow-up, 16 in the AHD group and 14 in the CHD group. Atrial tachycardia recurred in 12 of 42 (29%) patients, four in the AHD group and eight in the CHD group.
Of the four patients with recurrence in the AHD group, two received a repeat ablation procedure. Both patients had acute and long-term success. Confirmed by electrophysiology studies, one of these patients was identified to have a recurrence of the previously ablated IAT and the other patient had new re-entrant circuits.
Of eight patients in the CHD group with recurrent arrhythmias, six underwent repeat procedures and all these patients resulted in acute success. Two of the six patients were identified to have a recurrence of the previously ablated IAT, three had new circuits and the remaining one patient had both. During follow-up, one of the six patients had a recurrence of arrhythmia. This patient had a third time ablation, which resulted in both acute and long-term success. Confirmed by electrophysiology studies, a new onset IAT was diagnosed in this patient.
At the end of follow-up, 85% (39/46) of the patients were free of recurrence of IAT without any antiarrrhythmic drugs, the other seven patients were treated with antiarrhythmic therapy. There were no significant differences in long-term success rates (AHD 86% vs. CHD 83%) between the two groups (P > 0.05).
Discussion
Main findings
This is the first study focusing on the acute and long-term outcomes of IAT ablation using RMN in patients after heart surgery. In this study, our main findings are as follows. First, IAT ablation using RMN was safe and provided acceptable acute and long-term outcomes. Second, fluoroscopy time was markedly reduced by ablation with RMN compared with published data of manual ablation.2 Third, the patients with AHD and the patients with CHD presented with similar success rates and procedure characteristics.
Comparison of the present study with previous studies
Incisional atrial tachycardia belongs to a kind of atypical atrial flutter, which is becoming an increasingly prevalent arrhythmia.2,7,11–16 However, the studies which focus only on IATs mediated by surgical scars are limited.
Kalman et al.,1 ablated IAT using entrainment to define critical isthmus of conduction. This study included 18 CHD patients with a reported 83% acute success rate. During follow-up nine patients were asymptomatic and required no antiarrhythmic therapy, which indicated the recurrence rate was 50%. Leonelli et al.,2 ablated IATs using a three-dimensional mapping system by manual catheter navigation. Their study included 4 CHD patients and 16 AHD patients with a reported 100% acute success rate and 10% recurrence rate. Their mean fluoroscopy time was 42.3 ± 26.4 min. In the current study, the acute success rate of IAT ablation reached 91% and the recurrence rate of patients with successful primary procedure was approximately 29%. Including multiple procedures, the long-term success rate was 85%. However, the X-ray time was only 4 (4.8) min, which is much less compared with manually navigated ablation procedures.2 There were no major complications using RMN in this study population despite the complex anatomy of these patients.
Incisional atrial tachycardia ablation using remote magnetic navigation in patients with congenital heart disease
The RMN system has been used to treat a variety of arrhythmias in CHD patients. According to these previous reports, the RMN system has been shown to be safe and effective treating SVTs in patients with complex anatomies.7,8,17–20 However, these reports included IAT ablation outcomes with other intra-atrial re-entrant tachycardia ablation outcomes and did not analyse them separately. No detailed information regarding acute and long-term outcomes, procedural duration, and X-ray time of IAT ablation have been reported.
To the best of our knowledge, this current study is the largest-scale study of catheter ablation using RMN to treat IAT patients with CHD post-surgery. The acute success rate of CHD group was 92%, the long-term success rate was 83%, the procedural duration was 119 ± 38 min, and the X-ray time was only 4 (5.0) min. These results suggest that RMN ablation can achieve a high success rate with low X-ray time and dose. These results also demonstrate that RMN could be used safely for complex anatomy arrhythmia ablation. The advantage of RMN in IAT ablation is the high maneuverability of the magnetic catheter. It makes mapping of critical circuits easier, and constant catheter–tissue contacting during the ablation results in effective lesion formation.
As X-ray time had a great difference among the values in the CHD group, it was mainly because angiography was used during the procedure in order to define the very complex anatomy in select patients in the CHD group. If it weren’t for having to do angiography, the X-ray time might have greater reductions.
Incisional atrial tachycardia ablation using remote magnetic navigation in patients with acquired heart disease
To the best of our knowledge there has not been a dedicated publication to date using RMN for IAT ablation in AHD patients with previous heart surgeries. In the current study, the acute success rate of IAT is 91% with the X-ray time of 4 (4.5) min. The recurrence rate is 20% during the follow-up. After redo procedures, the long-term success rate reached 86%. The distribution of circuits and location of ablation lines were similar between the CHD and AHD groups. There were no significant differences in acute success rates, recurrence rates, long-term success rates, procedure duration, radiofrequency application ablation time, and X-ray exposure time between the two groups. The results suggest that catheter ablation of IATs using RMN is safe and efficacious in AHD patients with prior surgery. It seems that the underlying disease does not impact the procedural characteristics and the outcomes of IAT ablation using RMN.
Limitations
The sample size of this study is small. The grouping of post-surgical substrates into two categories may be an over simplification, as the underlying anatomic and haemodynamic differences within the groups might play a role in arrhythmogenesis. In the CHD group, a minority of the patients had very complex CHD. This could potentially influence our high success rates with low recurrence rates. Although it seems that the use of the RMN system offers advantages in complex anatomic situations, future large and randomized clinical trials in patients undergoing complex catheter ablation procedures are needed to demonstrate these potential benefits. We hope that future prospective and possibly multicentre studies in more well-defined patient groups will provide further insights to select the optimal mapping and ablation techniques for individual patients.
Conclusion
This study has demonstrated that catheter ablation of IAT after surgery using RMN is safe and effective. No significant differences related to success rates and procedure characteristics were found between patients with AHD and CHD.
Conflict of interest: none declared.
Supplement: This paper was published as part of a supplement supported by an educational grant from Society for Cardiac Robotic Navigation and unrestricted educational grant from Stereotaxis, Inc.



