-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Fink, Michael Schlüter, Christian-Hendrik Heeger, Christine Lemes, Tina Lin, Tilman Maurer, Andreas Metzner, Shibu Mathew, Bruno Reissmann, Peter Wohlmuth, Andreas Rillig, Feifan Ouyang, Karl-Heinz Kuck, Roland Richard Tilz, Pulmonary vein stenosis or occlusion after catheter ablation of atrial fibrillation: long-term comparison of drug-eluting versus large bare metal stents, EP Europace, Volume 20, Issue 10, October 2018, Pages e148–e155, https://doi.org/10.1093/europace/eux291
Close - Share Icon Share
Abstract
Pulmonary vein stenosis or occlusion (PVS/O) following catheter ablation of atrial fibrillation is a rare but potentially severe complication. Treatment options include angioplasty with or without stent implantation, but data on outcome and optimal treatment strategy are limited. We report long-term results after catheter-based treatment of patients with symptomatic PVS/O.
Retrospective analysis was performed in patients undergoing pulmonary vein (PV) angiography for suspected PVS/O. All patients with PVS/O were treated with balloon angioplasty and implantation of a coronary drug-eluting stent (DES) or a peripheral large-diameter bare metal stent (LD-BMS). A total of 25 high-degree PVS/Os in 19 patients were treated. Nine PVs were treated with angioplasty and DES implantation and 16 with angioplasty and LD-BMS implantation. The ostial PV diameter was not different in the DES and LD-BMS groups (10.2 ± 2.5 mm vs. 11.1 ± 1.9 mm, P = 0.34), but the PV/stent diameter ratio was significantly lower in the former (0.43 ± 0.13 vs. 0.82 ± 0.13, P < 0.0001). Angiographic stent restenosis was observed at a median of 539 (interquartile range 99–774) days in 9 of 23 (39%) treated PVs. The restenosis rate in the LD-BMS group was only one-third of that in the DES group [3/14 (21%) vs. 6/9 (67%), respectively; P = 0.08].
The use of LD-BMS for the treatment of PVS/O was associated with an acceptable long-term outcome. Coronary DES implantation resulted in a high rate of restenosis and should therefore not be performed. Larger trials are needed to confirm our findings.
Pulmonary vein stenosis or occlusion (PVS/O) following catheter ablation of atrial fibrillation may be treated with balloon angioplasty and implantation of a coronary drug-eluting stent (DES) or a peripheral large-diameter bare metal stent (LD-BMS).
In 19 patients with a total of 25 PVS/O lesions, angiographic stent restenosis was observed at a median of 539 days in 9 of 23 (39%) treated PVs.
The restenosis rate in the LD-BMS group (21%) was only one-third of that in the DES group (67%; P = 0.08).
Treatment of PVS/O with peripheral LD-BMS was associated with an acceptable long-term outcome, whereas the use of coronary (small-diameter) DES resulted in a high rate of restenosis.
Introduction
Pulmonary vein stenosis or occlusion (PVS/O) is a rare but potentially severe complication following catheter ablation of atrial fibrillation (AF). Symptoms include dyspnoea, haemoptysis, recurrent pneumonia, and pulmonary hypertension. It is caused by the application of radiofrequency current (RFC) energy inside the pulmonary veins (PVs) rather than outside at the PV antra.1 The incidence of PVS/O requiring an interventional or surgical treatment has decreased over the years with the improvement in ablation techniques and operator experience; it is estimated to be currently less than 1%.2 The optimal treatment strategy is still unknown, and available data regarding the outcome after invasive treatment are limited.3 Most often, percutaneous transluminal angioplasty (PTA) with or without stent implantation is performed. We report on the long-term results after catheter-based treatment of symptomatic PVS/O with PTA and subsequent implantation of either balloon-expanding coronary drug-eluting stents (DES) with a maximum diameter of 5 mm or self-expanding peripheral large-diameter bare metal stents (LD-BMS) up to 10 mm in diameter.
Methods
Inclusion criteria and study population
All patients who underwent treatment for symptomatic PVS/O from 2001 to 2017 at our institution were included in this study. Data were analysed in a retrospective fashion. The study was approved by the local ethics committee (file number WF-15/16). The study is in line with the declaration of Helsinki. All patient information was anonymized. Significant PVS/O was defined as a stenosis >75% of the vessel lumen or a total occlusion as confirmed by interventional angiography or symptoms suggestive of PVS/O (dyspnoea, haemoptysis, recurrent pneumonia, and pulmonary hypertension) with angiographically confirmed PVS >50% and <75%. All patients underwent PTA and stent implantation.
Initially, DES implantation was used. Due to the high restenosis rate and the non-physiological small diameter of the largest available DES (5 mm), implantation of a LD-BMS was used after November 2011 for vessels >5 mm in diameter.
Angioplasty and stenting procedures
Before the procedures, all patients underwent transoesophageal echocardiography (TOE) to exclude intracardiac thrombi and visualize the left atrium (LA) and pulmonary vein (PV) anatomy. All procedures were performed under deep sedation using midazolam, propofol, and sufentanil. Left atrial (LA) access was obtained via trans-septal puncture. One or two 8.5-Fr trans-septal sheaths (SL1, St. Jude Medical, Inc., St Paul, MN, USA) were inserted into the LA. After trans-septal puncture, intravenous heparin was administered targeting an activated clotting time of ≥300 s. Pulmonary vein angiography was performed in left anterior oblique 40° and right anterior oblique 30° projections. In case of verification of a high-degree PVS/O, stepwise balloon predilatation in 1-mm increments was conducted with balloon diameters of 2.5–9 mm and lengths of 15–40 mm.
Stenting was performed using balloon-expandable coronary DES and self-expanding [n = 13 (81%)] or balloon-expandable (n = 3) LD-BMS. Stent dimensions were 3.0–5.0 mm (diameter) × 8.0–32.0 mm (length) for DES [TAXUS 3.0–5.0 × 8–32 mm (Boston Scientific, Marlborough, MA, USA) and Endeavor Resolute 4.0 × 9 mm (Medtronic, Minneapolis, MN, USA)] and 7.0–10.0 mm (diameter) × 18.0–21.0 mm (length) for LD-BMS [Epic 7.0–10.0 × 19–21 mm (Boston Scientific), Scuba 9.0 × 18.0 mm (Medtronic) and Omnilink Elite 8.0 × 19 mm (Abbott, Lake Bluff, IL, USA)]. After stenting, oral anticoagulation and/or platelet inhibition therapy was initiated at the discretion of the operator, based on the angiographic results, the CHADS2/CHA2DS2-VASc score and the HASBLED score.
All procedures except one (Patient 7) were treated by the same operator with extensive experience in LA catheter ablation and coronary artery angioplasty procedures (K.-H.K.).
Clinical follow-up
After the index procedure, an interventional follow-up with PV angiography was recommended to all patients after 3, 6, and 12 months and in case of symptom recurrence. Additional TOE or computed tomography angiography examinations were undertaken on an individual basis for each patient. In cases of documented restenosis, repeat intervention was conducted on an individual decision for each case. Restenosis was defined as a >75% narrowing of the vessel lumen documented at the end of the index procedure for the affected PV.
Statistical analysis
Continuous variables are described as means with standard deviations or as medians with interquartile range (IQR; first and third quartiles), as appropriate. Categorical data are presented as counts and proportions. Differences between continuous variables are examined with t-tests or Wilcoxon tests, as appropriate. Categorical variables were compared with the Fisher’s exact test. The Kaplan–Meier product-limit estimator was used to estimate recurrence-free survival. The Kaplan–Meier curves and life tables were stratified by the number of procedures. The value of P < 0.05 was considered statistically significant.
Results
Patients
In this study, 19 patients (51 ± 14 years; 13 men) were included. All patients were symptomatic with dyspnoea and/or haemoptysis; two patients suffered additionally from recurrent pneumonia. All patients had previously undergone ablation at our centre (n = 8) or at tertiary ablation centres, using RFC energy applied either via a focal ablation catheter (n = 17) or a circular multi-electrode catheter (n = 2, PVAC; Medtronic Inc.). A median of 1 [IQR 1, 2] catheter ablation procedures had been performed to treat paroxysmal [n = 16 (84%)] or persistent [n = 3 (16%)] AF at a median of 7 [IQR 3, 10] months prior to PV stenting. Baseline patient characteristics are shown in Table 1.
| . | All . | DES only . | LD-BMS only . |
|---|---|---|---|
| Patients | 19 | 6 | 11 |
| Age (years) | 51 ± 14 | 55 ± 12 | 52 ± 13 |
| <50 years | 7 (37) | 2 (33) | 3 (27) |
| Male gender | 13 (68) | 5 (83) | 7 (64) |
| Persistent AF | 3 (16) | 1 (17) | 1 (9) |
| CHA2DS2-VASc score | 1 [0, 1] | 1 [0, 1] | 1 [0, 1] |
| Hypertension | 6 (32) | 4 (67) | 2 (18) |
| Diabetes mellitus | 1 (5) | 0 (0) | 1 (9) |
| HASBLED score | 0 [0, 1] | 1 [0, 1] | 0 [0, 0] |
| HLP | 2 (11) | 1 (17) | 1 (9) |
| Valvular heart disease | 2 (11) | 1 (17) | 0 (0) |
| CMP | 1 (5) | 0 (0) | 1 (9) |
| Pacemaker or ICD | 2 (11) | 1 (17) | 1 (9) |
| Months from ablation to PTA | 7 [3, 10] | 6 [1, 8] | 8 [3, 10] |
| EP procedures before PTA | 1 [1, 2] | 2 [1, 2] | 1 [1, 2] |
| LA diameter | 41 [40, 42] | 41 [39, 43] | 40 [40, 40] |
| . | All . | DES only . | LD-BMS only . |
|---|---|---|---|
| Patients | 19 | 6 | 11 |
| Age (years) | 51 ± 14 | 55 ± 12 | 52 ± 13 |
| <50 years | 7 (37) | 2 (33) | 3 (27) |
| Male gender | 13 (68) | 5 (83) | 7 (64) |
| Persistent AF | 3 (16) | 1 (17) | 1 (9) |
| CHA2DS2-VASc score | 1 [0, 1] | 1 [0, 1] | 1 [0, 1] |
| Hypertension | 6 (32) | 4 (67) | 2 (18) |
| Diabetes mellitus | 1 (5) | 0 (0) | 1 (9) |
| HASBLED score | 0 [0, 1] | 1 [0, 1] | 0 [0, 0] |
| HLP | 2 (11) | 1 (17) | 1 (9) |
| Valvular heart disease | 2 (11) | 1 (17) | 0 (0) |
| CMP | 1 (5) | 0 (0) | 1 (9) |
| Pacemaker or ICD | 2 (11) | 1 (17) | 1 (9) |
| Months from ablation to PTA | 7 [3, 10] | 6 [1, 8] | 8 [3, 10] |
| EP procedures before PTA | 1 [1, 2] | 2 [1, 2] | 1 [1, 2] |
| LA diameter | 41 [40, 42] | 41 [39, 43] | 40 [40, 40] |
Variables are mean and standard deviation, median [first, third quartile] or n (%).
AF, atrial fibrillation; CHA2DS2-VASc, stroke risk score based on congestive heart failure, age ≥75 years, diabetes mellitus, prior stroke/transient ischaemic attack/thromboembolism, vascular disease, age 65–74 years, sex; CMP, cardiomyopathy; EP, electrophysiology; HLP, hyperlipoproteinaemia; ICD, implantable cardioverter–defibrillator; LA, left atrium; PTA, percutaneous transluminal angioplasty.
| . | All . | DES only . | LD-BMS only . |
|---|---|---|---|
| Patients | 19 | 6 | 11 |
| Age (years) | 51 ± 14 | 55 ± 12 | 52 ± 13 |
| <50 years | 7 (37) | 2 (33) | 3 (27) |
| Male gender | 13 (68) | 5 (83) | 7 (64) |
| Persistent AF | 3 (16) | 1 (17) | 1 (9) |
| CHA2DS2-VASc score | 1 [0, 1] | 1 [0, 1] | 1 [0, 1] |
| Hypertension | 6 (32) | 4 (67) | 2 (18) |
| Diabetes mellitus | 1 (5) | 0 (0) | 1 (9) |
| HASBLED score | 0 [0, 1] | 1 [0, 1] | 0 [0, 0] |
| HLP | 2 (11) | 1 (17) | 1 (9) |
| Valvular heart disease | 2 (11) | 1 (17) | 0 (0) |
| CMP | 1 (5) | 0 (0) | 1 (9) |
| Pacemaker or ICD | 2 (11) | 1 (17) | 1 (9) |
| Months from ablation to PTA | 7 [3, 10] | 6 [1, 8] | 8 [3, 10] |
| EP procedures before PTA | 1 [1, 2] | 2 [1, 2] | 1 [1, 2] |
| LA diameter | 41 [40, 42] | 41 [39, 43] | 40 [40, 40] |
| . | All . | DES only . | LD-BMS only . |
|---|---|---|---|
| Patients | 19 | 6 | 11 |
| Age (years) | 51 ± 14 | 55 ± 12 | 52 ± 13 |
| <50 years | 7 (37) | 2 (33) | 3 (27) |
| Male gender | 13 (68) | 5 (83) | 7 (64) |
| Persistent AF | 3 (16) | 1 (17) | 1 (9) |
| CHA2DS2-VASc score | 1 [0, 1] | 1 [0, 1] | 1 [0, 1] |
| Hypertension | 6 (32) | 4 (67) | 2 (18) |
| Diabetes mellitus | 1 (5) | 0 (0) | 1 (9) |
| HASBLED score | 0 [0, 1] | 1 [0, 1] | 0 [0, 0] |
| HLP | 2 (11) | 1 (17) | 1 (9) |
| Valvular heart disease | 2 (11) | 1 (17) | 0 (0) |
| CMP | 1 (5) | 0 (0) | 1 (9) |
| Pacemaker or ICD | 2 (11) | 1 (17) | 1 (9) |
| Months from ablation to PTA | 7 [3, 10] | 6 [1, 8] | 8 [3, 10] |
| EP procedures before PTA | 1 [1, 2] | 2 [1, 2] | 1 [1, 2] |
| LA diameter | 41 [40, 42] | 41 [39, 43] | 40 [40, 40] |
Variables are mean and standard deviation, median [first, third quartile] or n (%).
AF, atrial fibrillation; CHA2DS2-VASc, stroke risk score based on congestive heart failure, age ≥75 years, diabetes mellitus, prior stroke/transient ischaemic attack/thromboembolism, vascular disease, age 65–74 years, sex; CMP, cardiomyopathy; EP, electrophysiology; HLP, hyperlipoproteinaemia; ICD, implantable cardioverter–defibrillator; LA, left atrium; PTA, percutaneous transluminal angioplasty.
Characteristics of pulmonary vein stenoses and index procedures
A total of 25 PVS/Os were treated in the 19 patients, with 16 PVS/Os receiving LD-BMS and 9 PVS/Os receiving DES. Fourteen (74%) patients suffered from a single high-degree PVS/O, 4 (21%) patients had 2 PVS/Os, and 1 (5%) patient had 3 PVS/Os (Figure 1A). PVS/Os were located in the left superior PV (LSPV) in 10 (53%) patients, the left inferior PV (LIPV) in another 10 (53%) patients, the right superior PV (RSPV) in 2 (11%) patients, and the right inferior PV (RIPV) in 3 (16%) patients (Figure 1B).
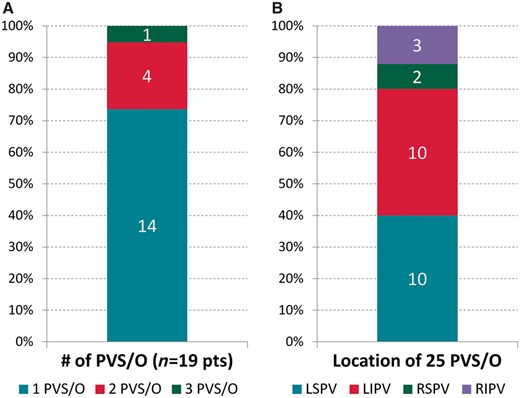
Characteristics of PVS/Os. (A) Distribution of PVS/Os among patient cohorts. (B) Location of PVS/Os.
PVS/Os were treated exclusively with PTA and DES implantation in 6 patients and with PTA and LD-BMS implantation in 11 patients; two patients (Patients 6 and 8) were treated with PTA followed by implantation of both DES and LD-BMS. In these two patients, anatomical circumstances did not allow for LD-BMS implantation in all stenotic lesions, because the side-branch diameter was too small or the long LD-BMS would have protruded into the LA. One of these patients (Patient 8) had two, the other (Patient 6) had three high-grade PVS/Os.
A total of 9 PVS/Os were categorized as occlusions (six LSPV, one LIPV, one RSPV, and one RIPV); of these, two were treated with DES implantation in the index procedure, the remaining seven were treated with LD-BMS implantation. Four of the cases in the DES group and one case in the LD-BMS group were PV bifurcation stenoses with involvement of smaller PV side branches separate from the PV ostium. During the index procedures, a total of 13 DES and 19 LD-BMS were implanted, with DES diameters of 3.0–5.0 mm and LD-BMS diameters of 7.0–10.0 mm (Table 2; Figure 2). The mean ostial PV diameter was 10.2 ± 2.5 mm and 11.1 ± 1.9 mm in patients treated with DES and LD-BMS implantation, respectively (P = 0.34). The mean stent/PV diameter ratio was 0.43 ± 0.13 in the DES group and 0.82 ± 0.13 in the LD-BMS group (P < 0.0001).
| Pt . | PV . | PV stenosis (%) . | PV diameter (mm)a . | Stent type . | Stent diameter (mm) . | Stent/PV ratio . | APT/ anticoa- gulation . | F/U (months) . | Restenosis (Y/N) . | Reinter- vention (Y/N) . | Type of reintervention . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LSPV | 100/95 (bifurcation) | 14.7 | DES | 4.5/3.5 | 0.3/0.24 | Triple | 114.5 | Y | Y | DEB (2×), LD-BMS |
| 2 | LIPV | 95/95 (bifurcation) | 12.7 | DES | 4.0/5.0 | 0.31/0.39 | Triple | 101.3 | N | N | |
| 3 | LSPV | 75 | 7.6 | DES | 5.0 | 0.66 | Triple | 3.3 | Y | Y | |
| 4 | RIPV | 80 | 6.3 | DES | 4.0 | 0.64 | Triple | 2.5 | N | N | |
| 5 | LSPV | 90 | 11.3 | DES | 4.0 | 0.35 | Triple | 11.8 | N | N | |
| 6 | LSPV | 100/100 (bifurcation) | 9.5 | DES | 5.0/3.0 | 0.53/0.32 | DAPT | 21.1 | Y | N | |
| LIPV | 75 | 14.2 | LD-BMS | 9.0 | 0.63 | DAPT | 17.7 | Y | Y | DEB | |
| RIPV | 75 | 9.8 | DES | 4.5 | 0.46 | DAPT | 17.7 | Y | N | ||
| 7 | LIPV | 80/80 (bifurcation) | 10.1 | DES | 5.0/3.5 | 0.50/0.35 | DAPT | 10.2 | Y | Y | DEB, LD-BMS |
| 8 | LSPV | 90 | 11.2 | LD-BMS | 9.0 | 0.80 | DAPT | 19.2 | N | N | |
| LIPV | 80 | 10.1 | DES | 5.0 | 0.50 | DAPT | 19.2 | Y | Y | DES | |
| 9 | RSPV | 100 | 12.5 | LD-BMS | 8.0 | 0.64 | Triple | 45.6 | N | N | |
| 10 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | 23.8 | N | N | |
| 11 | LIPV | 75 | 10.4 | LD-BMS | 10.0 | 0.96 | Triple | 25.4 | N | N | |
| 12 | LSPV | 100 | 9.1 | LD-BMS | 9.0 | 0.99 | Triple | 41.9 | N | N | |
| LIPV | 75 | 8.9 | LD-BMS | 7.0 | 0.79 | Triple | 41.0 | Y | Y | LD-BMS, DEB, DES | |
| 13 | LIPV | 80/80 (bifurcation) | 10.4 | LD-BMS | 9.0/7.0 | 0.87/0.67 | Triple | 39.4 | N | N | |
| 14 | RSPV | 90 | 10.2 | LD-BMS | 10.0 | 0.98 | Triple | 2.7 | N | N | |
| 15 | LIPV | 90 | 10.0 | LD-BMS | 10.0 | 1.00 | Triple | 14.9 | Y | Y | DEB (3×), DES |
| 16 | LSPV | 80 | 12.1 | LD-BMS | 8.0 | 0.66 | Triple | 9.4 | N | N | |
| RIPV | 100 | 8.1 | LD-BMS | 7.0 | 0.86 | Triple | 9.4 | N | N | ||
| 17 | LSPV | 100 | 14.7 | LD-BMS | 10.0 | 0.68 | Triple | 2.5 | N | N | |
| 18 | LIPV | 75 | 11.7 | LD-BMS | 10.0 | 0.85 | Triple | 2.9 | N | N | |
| 19 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | – | n/a | N | |
| LIPV | 100 | 9.5 | LD-BMS | 9.0 | 0.95 | Triple | – | n/a | N |
| Pt . | PV . | PV stenosis (%) . | PV diameter (mm)a . | Stent type . | Stent diameter (mm) . | Stent/PV ratio . | APT/ anticoa- gulation . | F/U (months) . | Restenosis (Y/N) . | Reinter- vention (Y/N) . | Type of reintervention . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LSPV | 100/95 (bifurcation) | 14.7 | DES | 4.5/3.5 | 0.3/0.24 | Triple | 114.5 | Y | Y | DEB (2×), LD-BMS |
| 2 | LIPV | 95/95 (bifurcation) | 12.7 | DES | 4.0/5.0 | 0.31/0.39 | Triple | 101.3 | N | N | |
| 3 | LSPV | 75 | 7.6 | DES | 5.0 | 0.66 | Triple | 3.3 | Y | Y | |
| 4 | RIPV | 80 | 6.3 | DES | 4.0 | 0.64 | Triple | 2.5 | N | N | |
| 5 | LSPV | 90 | 11.3 | DES | 4.0 | 0.35 | Triple | 11.8 | N | N | |
| 6 | LSPV | 100/100 (bifurcation) | 9.5 | DES | 5.0/3.0 | 0.53/0.32 | DAPT | 21.1 | Y | N | |
| LIPV | 75 | 14.2 | LD-BMS | 9.0 | 0.63 | DAPT | 17.7 | Y | Y | DEB | |
| RIPV | 75 | 9.8 | DES | 4.5 | 0.46 | DAPT | 17.7 | Y | N | ||
| 7 | LIPV | 80/80 (bifurcation) | 10.1 | DES | 5.0/3.5 | 0.50/0.35 | DAPT | 10.2 | Y | Y | DEB, LD-BMS |
| 8 | LSPV | 90 | 11.2 | LD-BMS | 9.0 | 0.80 | DAPT | 19.2 | N | N | |
| LIPV | 80 | 10.1 | DES | 5.0 | 0.50 | DAPT | 19.2 | Y | Y | DES | |
| 9 | RSPV | 100 | 12.5 | LD-BMS | 8.0 | 0.64 | Triple | 45.6 | N | N | |
| 10 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | 23.8 | N | N | |
| 11 | LIPV | 75 | 10.4 | LD-BMS | 10.0 | 0.96 | Triple | 25.4 | N | N | |
| 12 | LSPV | 100 | 9.1 | LD-BMS | 9.0 | 0.99 | Triple | 41.9 | N | N | |
| LIPV | 75 | 8.9 | LD-BMS | 7.0 | 0.79 | Triple | 41.0 | Y | Y | LD-BMS, DEB, DES | |
| 13 | LIPV | 80/80 (bifurcation) | 10.4 | LD-BMS | 9.0/7.0 | 0.87/0.67 | Triple | 39.4 | N | N | |
| 14 | RSPV | 90 | 10.2 | LD-BMS | 10.0 | 0.98 | Triple | 2.7 | N | N | |
| 15 | LIPV | 90 | 10.0 | LD-BMS | 10.0 | 1.00 | Triple | 14.9 | Y | Y | DEB (3×), DES |
| 16 | LSPV | 80 | 12.1 | LD-BMS | 8.0 | 0.66 | Triple | 9.4 | N | N | |
| RIPV | 100 | 8.1 | LD-BMS | 7.0 | 0.86 | Triple | 9.4 | N | N | ||
| 17 | LSPV | 100 | 14.7 | LD-BMS | 10.0 | 0.68 | Triple | 2.5 | N | N | |
| 18 | LIPV | 75 | 11.7 | LD-BMS | 10.0 | 0.85 | Triple | 2.9 | N | N | |
| 19 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | – | n/a | N | |
| LIPV | 100 | 9.5 | LD-BMS | 9.0 | 0.95 | Triple | – | n/a | N |
APT, antiplatelet therapy; DAPT, dual antiplatelet therapy; DEB, drug-eluting balloon; DES, drug-eluting stent; F/U, follow-up; LD-BMS, large-diameter bare metal stent; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Measured at PV ostium.
| Pt . | PV . | PV stenosis (%) . | PV diameter (mm)a . | Stent type . | Stent diameter (mm) . | Stent/PV ratio . | APT/ anticoa- gulation . | F/U (months) . | Restenosis (Y/N) . | Reinter- vention (Y/N) . | Type of reintervention . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LSPV | 100/95 (bifurcation) | 14.7 | DES | 4.5/3.5 | 0.3/0.24 | Triple | 114.5 | Y | Y | DEB (2×), LD-BMS |
| 2 | LIPV | 95/95 (bifurcation) | 12.7 | DES | 4.0/5.0 | 0.31/0.39 | Triple | 101.3 | N | N | |
| 3 | LSPV | 75 | 7.6 | DES | 5.0 | 0.66 | Triple | 3.3 | Y | Y | |
| 4 | RIPV | 80 | 6.3 | DES | 4.0 | 0.64 | Triple | 2.5 | N | N | |
| 5 | LSPV | 90 | 11.3 | DES | 4.0 | 0.35 | Triple | 11.8 | N | N | |
| 6 | LSPV | 100/100 (bifurcation) | 9.5 | DES | 5.0/3.0 | 0.53/0.32 | DAPT | 21.1 | Y | N | |
| LIPV | 75 | 14.2 | LD-BMS | 9.0 | 0.63 | DAPT | 17.7 | Y | Y | DEB | |
| RIPV | 75 | 9.8 | DES | 4.5 | 0.46 | DAPT | 17.7 | Y | N | ||
| 7 | LIPV | 80/80 (bifurcation) | 10.1 | DES | 5.0/3.5 | 0.50/0.35 | DAPT | 10.2 | Y | Y | DEB, LD-BMS |
| 8 | LSPV | 90 | 11.2 | LD-BMS | 9.0 | 0.80 | DAPT | 19.2 | N | N | |
| LIPV | 80 | 10.1 | DES | 5.0 | 0.50 | DAPT | 19.2 | Y | Y | DES | |
| 9 | RSPV | 100 | 12.5 | LD-BMS | 8.0 | 0.64 | Triple | 45.6 | N | N | |
| 10 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | 23.8 | N | N | |
| 11 | LIPV | 75 | 10.4 | LD-BMS | 10.0 | 0.96 | Triple | 25.4 | N | N | |
| 12 | LSPV | 100 | 9.1 | LD-BMS | 9.0 | 0.99 | Triple | 41.9 | N | N | |
| LIPV | 75 | 8.9 | LD-BMS | 7.0 | 0.79 | Triple | 41.0 | Y | Y | LD-BMS, DEB, DES | |
| 13 | LIPV | 80/80 (bifurcation) | 10.4 | LD-BMS | 9.0/7.0 | 0.87/0.67 | Triple | 39.4 | N | N | |
| 14 | RSPV | 90 | 10.2 | LD-BMS | 10.0 | 0.98 | Triple | 2.7 | N | N | |
| 15 | LIPV | 90 | 10.0 | LD-BMS | 10.0 | 1.00 | Triple | 14.9 | Y | Y | DEB (3×), DES |
| 16 | LSPV | 80 | 12.1 | LD-BMS | 8.0 | 0.66 | Triple | 9.4 | N | N | |
| RIPV | 100 | 8.1 | LD-BMS | 7.0 | 0.86 | Triple | 9.4 | N | N | ||
| 17 | LSPV | 100 | 14.7 | LD-BMS | 10.0 | 0.68 | Triple | 2.5 | N | N | |
| 18 | LIPV | 75 | 11.7 | LD-BMS | 10.0 | 0.85 | Triple | 2.9 | N | N | |
| 19 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | – | n/a | N | |
| LIPV | 100 | 9.5 | LD-BMS | 9.0 | 0.95 | Triple | – | n/a | N |
| Pt . | PV . | PV stenosis (%) . | PV diameter (mm)a . | Stent type . | Stent diameter (mm) . | Stent/PV ratio . | APT/ anticoa- gulation . | F/U (months) . | Restenosis (Y/N) . | Reinter- vention (Y/N) . | Type of reintervention . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LSPV | 100/95 (bifurcation) | 14.7 | DES | 4.5/3.5 | 0.3/0.24 | Triple | 114.5 | Y | Y | DEB (2×), LD-BMS |
| 2 | LIPV | 95/95 (bifurcation) | 12.7 | DES | 4.0/5.0 | 0.31/0.39 | Triple | 101.3 | N | N | |
| 3 | LSPV | 75 | 7.6 | DES | 5.0 | 0.66 | Triple | 3.3 | Y | Y | |
| 4 | RIPV | 80 | 6.3 | DES | 4.0 | 0.64 | Triple | 2.5 | N | N | |
| 5 | LSPV | 90 | 11.3 | DES | 4.0 | 0.35 | Triple | 11.8 | N | N | |
| 6 | LSPV | 100/100 (bifurcation) | 9.5 | DES | 5.0/3.0 | 0.53/0.32 | DAPT | 21.1 | Y | N | |
| LIPV | 75 | 14.2 | LD-BMS | 9.0 | 0.63 | DAPT | 17.7 | Y | Y | DEB | |
| RIPV | 75 | 9.8 | DES | 4.5 | 0.46 | DAPT | 17.7 | Y | N | ||
| 7 | LIPV | 80/80 (bifurcation) | 10.1 | DES | 5.0/3.5 | 0.50/0.35 | DAPT | 10.2 | Y | Y | DEB, LD-BMS |
| 8 | LSPV | 90 | 11.2 | LD-BMS | 9.0 | 0.80 | DAPT | 19.2 | N | N | |
| LIPV | 80 | 10.1 | DES | 5.0 | 0.50 | DAPT | 19.2 | Y | Y | DES | |
| 9 | RSPV | 100 | 12.5 | LD-BMS | 8.0 | 0.64 | Triple | 45.6 | N | N | |
| 10 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | 23.8 | N | N | |
| 11 | LIPV | 75 | 10.4 | LD-BMS | 10.0 | 0.96 | Triple | 25.4 | N | N | |
| 12 | LSPV | 100 | 9.1 | LD-BMS | 9.0 | 0.99 | Triple | 41.9 | N | N | |
| LIPV | 75 | 8.9 | LD-BMS | 7.0 | 0.79 | Triple | 41.0 | Y | Y | LD-BMS, DEB, DES | |
| 13 | LIPV | 80/80 (bifurcation) | 10.4 | LD-BMS | 9.0/7.0 | 0.87/0.67 | Triple | 39.4 | N | N | |
| 14 | RSPV | 90 | 10.2 | LD-BMS | 10.0 | 0.98 | Triple | 2.7 | N | N | |
| 15 | LIPV | 90 | 10.0 | LD-BMS | 10.0 | 1.00 | Triple | 14.9 | Y | Y | DEB (3×), DES |
| 16 | LSPV | 80 | 12.1 | LD-BMS | 8.0 | 0.66 | Triple | 9.4 | N | N | |
| RIPV | 100 | 8.1 | LD-BMS | 7.0 | 0.86 | Triple | 9.4 | N | N | ||
| 17 | LSPV | 100 | 14.7 | LD-BMS | 10.0 | 0.68 | Triple | 2.5 | N | N | |
| 18 | LIPV | 75 | 11.7 | LD-BMS | 10.0 | 0.85 | Triple | 2.9 | N | N | |
| 19 | LSPV | 100 | 12.2 | LD-BMS | 10.0 | 0.82 | Triple | – | n/a | N | |
| LIPV | 100 | 9.5 | LD-BMS | 9.0 | 0.95 | Triple | – | n/a | N |
APT, antiplatelet therapy; DAPT, dual antiplatelet therapy; DEB, drug-eluting balloon; DES, drug-eluting stent; F/U, follow-up; LD-BMS, large-diameter bare metal stent; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; PV, pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Measured at PV ostium.
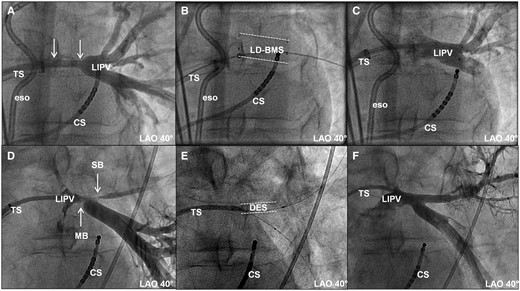
Examples of high-grade PV stenoses in left inferior pulmonary veins. (A–C) Stenosis proximal to PV bifurcation (Patient 15) and (D–F) stenoses in PV bifurcation (Patient 7). (A) Angiographic image after predilatation and before stent implantation. Arrows indicate length of stenosis. (B) Fluoroscopic image after implantation of large-diameter bare metal stent (LD-BMS; dashed lines). (C) Angiographic image post-stenting. (D) Angiographic image after predilatation and before stent implantation in bifurcation lesion. Arrows indicate two stenoses, one in the main branch (MB) and one in the side branch (SB). (E) Fluoroscopic image after implantation of DES into both branches and kissing-balloon dilatation. DES in side branch marked by dashed lines. (F) Final angiographic image. CS, coronary sinus; eso, oesophageal temperature probe; LAO, left anterior oblique view; LIPV, left inferior pulmonary vein; TS, trans-septal sheath/multipurpose catheter.
Clinical follow-up and incidence of restenosis
In all patients but one, at least one angiographic control was conducted after the index procedure at a median of 539 [IQR 99, 774] days. Pertinent follow-up data are given in Table 3. Overall, 9 (39%) of the 23 treated PVs had angiographically proved restenosis during follow-up. Restenosis occurred in 3 of 14 PVs (21%) in the LD-BMS group and in 6 of 9 PVs (67%) in the DES group (P = 0.08, Figure 3). The Kaplan–Meier estimates of 24-month recurrence-free survival after PTA/stenting with LD-BMS and DES were 70% (95% confidence interval 41–99%) and 31% (0–66%), respectively (P = 0.19, Figure 4). While only two of the nine PV occlusions (both in the DES group) had documented restenosis, three of the five cases with bifurcation stenoses developed restenosis (all three in the DES group).
| . | All PVs . | PVs treated with DES . | PVs treated with LD-BMS . |
|---|---|---|---|
| Index procedures | 22 | 9 | 13 |
| Duration of FU (months) | 18.7 [10, 40] | 18.7 [10, 40] | 19.5 [10, 41] |
| PVs with stent restenosis | 9 (41) | 6 (67) | 3 (23) |
| Time to stent restenosis (months) | 8 [5, 20] | 13 [6, 22] | 6 [3, 18] |
| Reinterventions | 13 | 4 | 6 |
| Implanted stents (index procedure) | 29 | 13 | 16 |
| Implanted stents (total) | 36 | 18 | 18 |
| Triple therapy during FU | 16 (73) | 5 (56) | 11 (85) |
| DAPT during FU | 6 (27) | 4 (44) | 2 (15) |
| . | All PVs . | PVs treated with DES . | PVs treated with LD-BMS . |
|---|---|---|---|
| Index procedures | 22 | 9 | 13 |
| Duration of FU (months) | 18.7 [10, 40] | 18.7 [10, 40] | 19.5 [10, 41] |
| PVs with stent restenosis | 9 (41) | 6 (67) | 3 (23) |
| Time to stent restenosis (months) | 8 [5, 20] | 13 [6, 22] | 6 [3, 18] |
| Reinterventions | 13 | 4 | 6 |
| Implanted stents (index procedure) | 29 | 13 | 16 |
| Implanted stents (total) | 36 | 18 | 18 |
| Triple therapy during FU | 16 (73) | 5 (56) | 11 (85) |
| DAPT during FU | 6 (27) | 4 (44) | 2 (15) |
Values are median [first, third quartile], n or n (%).
DAPT, dual antiplatelet therapy; DES, drug-eluting stents; FU, follow-up; LD-BMS, large-diameter bare metal stents; PV, pulmonary vein.
| . | All PVs . | PVs treated with DES . | PVs treated with LD-BMS . |
|---|---|---|---|
| Index procedures | 22 | 9 | 13 |
| Duration of FU (months) | 18.7 [10, 40] | 18.7 [10, 40] | 19.5 [10, 41] |
| PVs with stent restenosis | 9 (41) | 6 (67) | 3 (23) |
| Time to stent restenosis (months) | 8 [5, 20] | 13 [6, 22] | 6 [3, 18] |
| Reinterventions | 13 | 4 | 6 |
| Implanted stents (index procedure) | 29 | 13 | 16 |
| Implanted stents (total) | 36 | 18 | 18 |
| Triple therapy during FU | 16 (73) | 5 (56) | 11 (85) |
| DAPT during FU | 6 (27) | 4 (44) | 2 (15) |
| . | All PVs . | PVs treated with DES . | PVs treated with LD-BMS . |
|---|---|---|---|
| Index procedures | 22 | 9 | 13 |
| Duration of FU (months) | 18.7 [10, 40] | 18.7 [10, 40] | 19.5 [10, 41] |
| PVs with stent restenosis | 9 (41) | 6 (67) | 3 (23) |
| Time to stent restenosis (months) | 8 [5, 20] | 13 [6, 22] | 6 [3, 18] |
| Reinterventions | 13 | 4 | 6 |
| Implanted stents (index procedure) | 29 | 13 | 16 |
| Implanted stents (total) | 36 | 18 | 18 |
| Triple therapy during FU | 16 (73) | 5 (56) | 11 (85) |
| DAPT during FU | 6 (27) | 4 (44) | 2 (15) |
Values are median [first, third quartile], n or n (%).
DAPT, dual antiplatelet therapy; DES, drug-eluting stents; FU, follow-up; LD-BMS, large-diameter bare metal stents; PV, pulmonary vein.
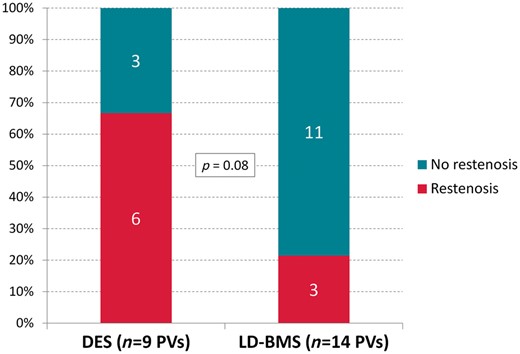
Occurrence of restenosis. (A) DES-treated patients. (B) LD-BMS-treated patients.
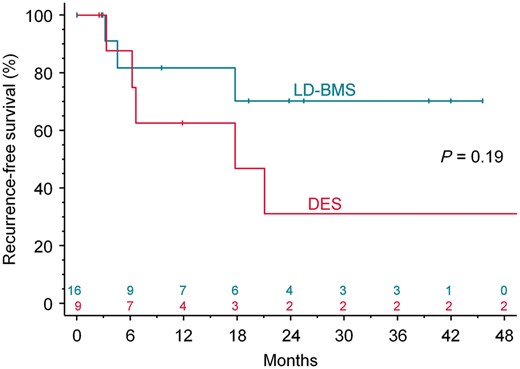
The Kaplan–Meier estimate of recurrence-free survival by stent type.
Repeat interventions
Two of the nine PVs with documented restenosis (75% vessel narrowing in both) were not treated due to lack of symptoms of the affected patients. All other documented restenoses underwent repeat intervention (11 reinterventions in 7 patients/7 PVs; 4 cases in the DES and 3 patients in the LD-BMS group).
In two cases of the DES group, repeat intervention with PTA and repeat DES and LD-BMS implantation was performed 6 and 121 months after the index procedure, respectively. No further restenosis was noted until the end of follow-up for these PVs. The remaining PV with restenosis in the DES group was treated with drug-eluting balloon (DEB) PTA in a first and LD-BMS implantation in a second reintervention 7 and 10 months after the index procedure. This patient did not undergo repeat angiography but was symptom free at the last clinical consultation.
One PV in the LD-BMS group with documented restenosis was treated with repeated LD-BMS implantation 3 months after the index procedure, with DEB PTA after another 3 months, and with PTA plus DES implantation 10 months after the index procedure. At the last angiographic control 20 months after the index procedure, the PV was without restenosis. The two remaining PVs in the LD-BMS group with restenosis were treated with repeat DEB PTA. In the first case, TOE control after the repeat procedure revealed elevated peak velocity and velocity time integral values (108 cm/s and 130 cm, respectively). Control angiography was not performed, because the patient showed no symptoms of restenosis. In the second case, PV restenosis was documented 7 months after reintervention and treated with repeat DEB PTA. Angiographic controls 15, 21, and 30 months after the index procedure showed recurrent restenosis, which was subsequently treated with DES implantation and repeat DEB PTA.
Procedural safety
Major complications were noted in 2 (6%) of the 33 procedures. In detail, one PV perforation occurred during reintervention PTA with subsequent cardiac tamponade requiring pericardiocentesis (Patient 7). Bleeding stopped after balloon occlusion of the affected PV for 10 min; subsequently, a LD-BMS was implanted. One case of pulmonary bleeding requiring endotracheal intubation occurred in another patient. Additionally, minor complications occurred in another two interventions (one case of false aneurysm and one case of pericardial effusion, each requiring no interventional or surgical treatment). Of note, minor haemoptysis occurred in all patients after stenting; it resolved spontaneously in all patients but the one mentioned above.
Antiplatelet therapy
Sixteen patients (5 DES-treated patients and 11 LD-BMS-treated patients) received a triple combination of aspirin and clopidogrel as dual antiplatelet therapy (DAPT), combined with oral anticoagulation for at least 6 weeks and up to a maximum of 12 months after stent implantation. The remaining three patients were administered only DAPT for 6–12 months (1 DES-treated patient and two patients with both DES and LD-BMS implantation). With respect to the applied treatment strategy for each PV, a total of 5 DES-treated and 14 LD-BMS-treated PVs received triple therapy and 4 DES-treated and 2 LD-BMS-treated PVs received DAPT after the index procedure (P = 0.14).
Discussion
We report the long-term follow-up after interventional treatment of PVS/O with either the implantation of coronary DES or peripheral LD-BMS after PTA. In this study, although not statistically significant, LD-BMS implantation was associated with a two-third lower incidence of restenosis compared with DES implantation. Procedural safety seems to be acceptable, with only 2 major complications in 33 procedures.
Patient characteristics
The majority of the patients in our cohort were younger males suffering from, and treated for, paroxysmal AF. This important patient characteristic has been reported in earlier studies.4–8 Approximately 75% of the PVS/Os in our patient cohort were located in the lateral PVs, which is in line with previous investigations.4,7–10 Thus, our patient cohort seems to be comparable to the patients investigated in the larger, previously reported cohorts of patients treated for iatrogenic PVS/O.
Previous studies of interventional treatment for iatrogenic pulmonary vein stenosis
Since the first reports of iatrogenic PVS/O after catheter ablation of AF, different groups have reported on different interventional treatment strategies, including the application of stand-alone PTA as well as the implantation of LD-BMS both as a primary treatment and after failed PTA. The numbers of patients included in these studies have generally been low due to the rarity of symptomatic PVS/O after catheter ablation. Early studies documented a high incidence of restenosis after interventional treatment ranging from 33% to 47% after 7–18 months.4,8,9,11 The above-mentioned studies did not show different outcomes after PTA alone and after PTA with stenting.4,8,9,11 Of note, in two studies, stents were implanted only after PV dissection during PTA or after acute failure of stand-alone PTA.8,11
In contrast, the study of Prieto et al.5 demonstrated better outcomes after primary LD-BMS implantation compared with PTA alone in a total of 55 treated PVs (33% vs. 77% restenosis after 25 months). Additionally, Neumann et al.6,12 reported a high incidence of restenosis (77% after 2 months) after PTA and a favourable outcome after reintervention with LD-BMS implantation in another 15 PVs (restenosis in 23% of PVs after >4 years).
In a recently published investigation, Fender et al. reported on the largest patient population with symptomatic PVS.13 They studied a total of 113 patients with 178 stenotic PVs undergoing either stand-alone PTA or PTA with concomitant stenting at the Mayo Clinic between 2000 and 2014. In this patient population, peripheral or bile duct BMS with maximum diameters of 10 mm and maximum lengths of 19 mm were mostly implanted.13 A significantly better outcome was reported in that registry for the patients initially treated with stent implantation as opposed to stand-alone PTA.13 Based on the limited available data, one may conclude that PTA with stenting is superior to stand-alone PTA in PVS treatment.
Comparison of drug-eluting stent and large-diameter bare metal stent implantation
The DES and LD-BMS commercially available today differ with regard to size and the characteristics of drug elution and stent expansion. Both stent types have their own advantages and disadvantages, which are summarized in Table 4. The use of DES for the treatment of PVS/O has initially been reported by our group.14 These results have been promising, with only one (14%) of the seven PVs requiring reintervention after DES implantation at 3 months.14 The Mayo Clinic Registry reported on four additional PVs treated with DES implantation.13 To our knowledge, there has been no randomized comparison of DES versus LD-BMS implantation as a primary treatment strategy. In our patient cohort, after a median follow-up of 18.7 months, LD-BMS implantation as the primary treatment strategy was associated with a lower incidence of high-grade restenosis than DES implantation. Our data suggest a better long-term outcome after LD-BMS stenting for the treatment of PVS/O, despite the lack of a statistically significant difference, which is likely due to the low number of patients in our study.
| . | DES . | LD-BMS . |
|---|---|---|
| Advantage |
|
|
| Disadvantage |
|
|
| . | DES . | LD-BMS . |
|---|---|---|
| Advantage |
|
|
| Disadvantage |
|
|
DES, drug-eluting stents; FU, follow-up; LD-BMS, large-diameter bare metal stents.
| . | DES . | LD-BMS . |
|---|---|---|
| Advantage |
|
|
| Disadvantage |
|
|
| . | DES . | LD-BMS . |
|---|---|---|
| Advantage |
|
|
| Disadvantage |
|
|
DES, drug-eluting stents; FU, follow-up; LD-BMS, large-diameter bare metal stents.
Mechanisms of restenosis
The potential mechanisms for a better outcome of BMS stenting compared with DES implantation are unknown. In contrast to the development of restenosis after stent implantation in coronary arteries, the underlying pathomechanisms of PV restenosis have yet not been identified. The incidence of PVS/O is higher in young individuals undergoing AF ablation13 and fibrosis and neointimal proliferation are potential key factors in the development of PVS/O and restenosis. Therefore, the use of DES seems to be a viable treatment option, analogous to the treatment of coronary artery lesions. However, the available maximal stent diameter of commercially available DES is limited to 5 mm. Studies have linked inadequate stent dimensions to a higher rate of restenosis in patients treated for coronary artery disease.15 In our study, the maximum available diameter of DES was 5 mm, as opposed to 10 mm for LD-BMS. This large difference in stent sizes was the reason for us to switch from DES to LD-BMS. Generally, the diameter of the PVs close to the ostium ranges between 7 mm and 12 mm. The use of LD-BMS promised an optimization of the vessel diameter after stenting, which is reflected in our study by a significantly better stent/PV diameter match in the LD-BMS group than in the DES group.
One limitation of implanting LD-BMS of greater than 10 mm in diameter is the length of commercially available LD-BMS. If wider stents are needed, the next available length will be 29–30 mm, which bears the risk of the stent protruding into the LA. This was the reason why we implanted DES in two patients after we had decided to implant LD-BMS as a general rule.
Antiplatelet therapy
Our regime of antiplatelet therapy and oral anticoagulation was determined on an individual basis, related to the complexity of the treated lesions with the amount of implanted stents and the underlying CHADS2/CHA2DS2-VASc score. There are no prospective trials that have evaluated different medical treatment strategies after stenting of PVS/Os. The rationale behind our medical treatment strategy was the prevention of acute stent thrombosis using antiplatelet therapy. Since no data are available for the medical therapy of stenting in veins, we recommended an antiplatelet strategy analogous to that of coronary artery stenting to balance the prevention of acute stent thrombosis on the one side and the risk of bleeding on the other side. This analogy takes into account that the risk of acute stent thrombosis after stenting in veins is not known but should potentially be higher than in arteries because of the low pressure in venous systems. Oral anticoagulation was mainly added to antiplatelet therapy in patients with an indication for anticoagulation due to their thromboembolic stroke risk related to the patient’s history of AF.
Limitations
This study was a retrospective study with a limited number of patients and its typical limitations. Furthermore, patients were treated in a non-randomized fashion. We cannot exclude an influence of lesion complexity on the incidence of restenosis. However, our study reflects the decision-making process at a high-volume ablation centre where patients are treated to the best of the physicians’ knowledge, yet based on empirical rather than scientific evidence. This is reflected by the fact that we started PVS/O treatment with PTA only and poor results1 and continued first with small-diameter DES14 and then with large-diameter self-expanding BMS. Still, ours is one of the largest patient series with one of the longest follow-up durations.
Conclusion
PVS/O is a disastrous complication of PV isolation in patients with AF, particularly in young patients. No standardized treatment exists for this complication. In this study, the treatment of PVS/O with LD-BMS implantation after catheter ablation of AF demonstrated a lower incidence of stent restenosis than treatment with DES implantation. Therefore, as long as DES diameters larger than 5 mm are not available, LD-BMS implantation may be the preferred treatment option in these patients—even if a bifurcation lesion is involved. In cases of small, stenotic PV side branches, the implantation of a LD-BMS in the main branch with or without additional DES implantation in the small side branch might be feasible. Finally, the best treatment of PVS/O is definitely the prevention of this severe complication.
Conflict of interest: K.-H.K. reports having received consulting fees/honoraria from Biosense Webster, Medtronic, Boston Scientific, and Abbott. A.M. reports having received speaker’s honoraria from Medtronic. R.R.T. reports having received research grants from St. Jude Medical and speaker’s honoraria from Biosense Webster, Biotronik, Pfizer, Topera, Bristol-Myers Squibb, Bayer, and Sanofi Aventis. The other authors report no conflicts of interest.



