-
PDF
- Split View
-
Views
-
Cite
Cite
David F Briceño, Jorge Romero, Pedro A Villablanca, Alejandra Londoño, Juan C Diaz, Ilir Maraj, Syeda Atiqa Batul, Nidhi Madan, Jignesh Patel, Anand Jagannath, Sanghamitra Mohanty, Prasant Mohanty, Carola Gianni, Domenico Della Rocca, Ahlam Sabri, Soo G Kim, Andrea Natale, Luigi Di Biase, Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis, EP Europace, Volume 20, Issue 1, January 2018, Pages 104–115, https://doi.org/10.1093/europace/eux109
Close - Share Icon Share
Abstract
To compare the long-term outcomes of standard ablation of stable ventricular tachycardia (VT) vs. substrate modification, and of complete vs. incomplete substrate modification in patients with structural heart disease (SHD) presenting with VT.
An electronic search was performed using major databases. The main outcomes were a composite of long-term ventricular arrhythmia (VA) recurrence and all-cause mortality of standard ablation of stable VT vs. substrate modification, and long-term VA recurrence in complete vs. incomplete substrate modification. Six studies were included for the comparison of standard ablation of stable VT vs. substrate modification, with a total of 396 patients (mean age 63 ± 10 years, 87% males), and seven studies were included to assess the impact of extensive substrate modification, with a total of 391 patients (mean age 64 ± years, 90% males). More than 70% of all the patients included had ischaemic cardiomyopathy. Substrate modification was associated with decreased composite VA recurrence/all-cause mortality compared to standard ablation of stable VTs [risk ratio (RR) 0.57, 95% confidence interval (CI) 0.40–0.81]. Complete substrate modification was associated with decreased VA recurrence as compared to incomplete substrate modification (RR 0.39, 95% CI 0.27–0.58).
In patients with SHD who had VT related mainly to ischaemic substrates, there was a significantly lower risk of the composite primary outcome of long-term VA recurrence and all-cause mortality among those undergoing substrate modification compared to standard ablation. Long-term success is improved when performing complete substrate modification.
What’s new?
This is the first meta-analysis evaluating standard ablation of stable ventricular tachycardia (VT) vs. substrate-based ablation, and the long-term outcomes of complete vs. incomplete substrate modification in patients with structural heart disease (SHD) presenting with VT.
The combined risk of ventricular arrhythmia recurrence and all-cause mortality is lower (43% risk reduction) when using a substrate-based approach compared to standard ablation of stable VT during long-term follow-up (mean of 24 months).
Complete substrate modification is associated with lower ventricular arrhythmia recurrence (risk reduction of 61%) as compared with incomplete substrate modification during long-term follow-up (mean of 21 months).
Our results suggest that in patients with SHD presenting with VT, especially with an ischaemic substrate (in our study >70% had ischaemic cardiomyopathy), substrate-based ablation should be selected with the goal of achieving complete substrate modification.
Introduction
Patients with structural heart disease (SHD) are at increased risk for ventricular arrhythmias (VA) and mortality. Even though implantable cardiac defibrillator (ICD) therapy has been efficacious in improving survival, recurrent ventricular tachycardia (VT) with subsequent ICD shocks reduce quality of life and is associated with increased mortality.1 The use of antiarrhythmic drugs (AADs) is limited due to significant side effects and suboptimal effectiveness without any survival benefit.1 Conversely, catheter ablation has evolved as a useful tool to control VA and improve quality of life. Recently, the VANISH trial showed that ablation reduced the composite primary outcome of death, VT storm, or appropriate ICD shock compared to patients receiving an escalation in AADs.2 Nevertheless, although ablation for VT is routinely performed in specialized centres, it is currently uncertain which technique should be the standard of practice to obtain the best results.
VT ablation has been traditionally based on two mapping strategies: standard mapping, where activation and entrainment mapping during an episode of VT is performed; or substrate mapping, in which abnormal electrograms are identified during sinus rhythm or ventricular pacing. Additionally, several techniques for substrate modification have been proposed, with different end points and outcomes. However, the most effective substrate modification strategy remains unknown. Consequently, in the current meta-analysis we analysed the long-term outcomes of standard ablation of stable VTs vs. substrate modification, and of complete vs. incomplete substrate modification in patients with SHD presenting with VT.
Methods
Search strategy
We searched PubMed, Embase, and Cochrane Central Register of Clinical Trials (Cochrane Library, Issue 09, 2016). Our search was limited to human studies in peer-reviewed journals. This was assessed up to September 2016. No language restriction was applied. The reference lists of identified articles were also reviewed. This search was conducted using the terms (VT OR ventricular tachyarrhythmia OR VT ablation) AND (substrate ablation OR standard ablation OR activation mapping OR entrainment mapping OR activation and entrainment mapping OR VT recurrence OR mortality OR SHD OR late potentials OR scar dechanneling OR local abnormal ventricular activities OR core isolation OR homogenization of the scar).
Selection criteria
The PRISMA statement for reporting systematic reviews and meta-analyses was applied to the methods for this study. The studies had to fulfil the following criteria to be included in the analysis of standard ablation of stable VT vs. substrate modification: (i) patients with SHD presenting for VT ablation, (ii) studies that strictly compared standard ablation of stable VT with substrate modification without the use of hybrid strategies (i.e. combining substrate with standard ablation), (iii) studies including VT or VA recurrence and/or total mortality as their endpoints, (iv) studies that were not designed to utilize haemodynamic support for standard VT ablation as a primary strategy.
The studies had to fulfil the following criteria to be included in the analysis of complete vs. incomplete substrate modification: (i) patients with SHD presenting for VT ablation, (ii) studies that reported complete and incomplete substrate modification, (iii) studies including VT or VA recurrence and/or total mortality as their endpoints.
Study outcomes
Primary end points
A composite of long-term VA recurrence and all-cause mortality of standard ablation of stable VT vs. substrate modification.
Long-term VA recurrence of standard ablation of stable VT vs. substrate modification.
Long-term all-cause mortality of standard ablation of stable VT vs. substrate modification.
Long-term VA recurrence in complete vs. incomplete substrate modification.
Secondary end points
Major and minor complications of standard ablation of stable VT vs. substrate modification.
Procedure, radiofrequency, and fluoroscopy times of standard ablation of stable VT vs. substrate modification.
Procedure, radiofrequency, and fluoroscopy times of complete vs. incomplete substrate modification.
Data extraction and quality assessment
Two authors (D.F.B. and P.V.) searched for studies and extracted the data independently and in duplicate. Data were extracted using standardized protocol and reporting forms. Disagreements were resolved by consensus. Two reviewers (D.F.B. and P.V.) independently assessed the quality items and discrepancies were resolved by consensus. The quality and reporting of the non-randomized studies was assessed by the Newcastle-Ottawa scale (NOS). Studies were classified into one of the three categories: (i) high quality 6–9 points (ii) satisfactory quality 3–5 points (iii) unsatisfactory quality 0–2 points.
Statistical analysis
In order to answer both objectives, we performed two separate analyses. The 1st analysis was aimed to compare standard ablation of stable VTs vs. substrate modification. The 2nd analysis compared outcomes of patients undergoing complete vs. incomplete substrate modification.
Overall, the Mantel–Haenszel risk ratio (RR) model was used to summarize data across treatment arms. Summary estimates and 95% confidence interval (CI) were reported for continuous variables as difference in means (DM). We evaluated heterogeneity of effects using the Higgins I-squared (I2) statistic; in cases of heterogeneity (defined as I2 > 25%), random effects models of DerSimonian and Laird were used, otherwise (I2 ≤ 25%) fixed effects models were used. To address publication bias, we used the funnel plots. If any bias was observed, further bias quantification was measured using the Begg–Mazumdar test, Egger test, and the Duval and Tweediés trim and fill test.
Descriptive statistics are presented as means and standard deviations (SD) for continuous variables or number of cases (n) and percentages (%) for dichotomous and categorical variables. The statistical analysis was performed by the comprehensive meta-analysis version 2.0 software (Biostat, Inc., New Jersey, USA).
Results
Study selection
A total of 11,280 articles were identified, out of which 10,756 did not meet inclusion criteria based on article and abstract evaluation. After evaluation of the 524 remaining abstracts, 13 studies fulfilled the inclusion criteria and were included in the present meta-analysis. Although the study by Bunch and colleagues fulfilled initial inclusion criteria, it was excluded from the analysis due to patient characteristics (ablation of VT in heamodynamically unstable patients using mechanical support) to avoid bias: none of the other studies included unstable patients and haemodynamic instability during VT limits traditional entrainment and activation mapping.

Baseline characteristics
The baseline characteristics of the patients included are reported in Table 1. The six studies included for the comparison of standard ablation of stable VT vs. substrate modification, had a total of 396 patients (mean age 63 ± 10 years, 87% males), most of which are observational cohort studies with exception of the randomized prospective multicenter study published by our group in 2015.10 The mean follow-up was 24 ± 15 months. The mean left ventricular ejection fraction was 34±10%, and 71% of patients had an ICD implanted at baseline. Most included studies have a preponderance of patients with ischaemic cardiomyopathy as compared to those with non-ischaemic cardiomyopathy (85% vs. 15%), with exception of the study by Makimoto et al.8 (19% vs. 81%). Table 2 depicts the methodological and procedural characteristics of both ablation strategies in each of these studies.
| First Author, Year . | Patients . | Type of study . | Follow-up (months) . | Male (%) . | Age, years (mean ± SD) . | LVEF, % (mean ± SD) . | HTN (%) . | DM (%) . | ICM (%) . | NICM (%) . | Type of NICM . | Mechanical support . | ICD prior to ablation (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Ablation of stable VT vs. substrate modification | |||||||||||||
| Arenal, 200311 | 24 | Prospective cohort | 9 ± 4 | 91.7 | 66 ± 9 | 30 ± 9 | NA | NA | 91.7 | 8.3 | DCM, TOF | None | 50 |
| Volkmer, 20063 | 47 | Prospective cohort | 25 ± 13 | 91.4 | 65 ± 8 | 30 ± 7.5 | NA | NA | 100 | 0 | NA | None | 47 |
| Ventura, 20084 | 30 | Prospective cohort | 14 ± 6 | 93.3 | 65 ± 7 | 32 ± 6 | NA | NA | 100 | 0 | NA | None | 80 |
| Di Biase, 20126 | 92 | Multicenter prospective cohort | 25 ± 10 | 80 | 61.5 ± 9 | 25.5 ±7.5 | 60 | 14.5 | 100 | 0 | NA | None | 100 |
| Makimoto, 20158 | 85 | Prospective cohort | 61 ± 40 | 73 | 53.1 ± 16.2 | 51.7 ± 16.4 | NA | NA | 18.8 | 81.2 | ARVC, DCM, HCM, Sarcoidosis, CHD | None | 51 |
| Di Biase, 201510 | 118 | Prospective Randomized Multicenter | 12 | 93.2 | 66 ± 10.5 | 32.3 ±12 | 73.8 | 36.7 | 100 | 0 | NA | Eight patients during standard ablation | 100 |
| Complete vs. Incomplete Substrate Modification | |||||||||||||
| Jais, 20125 | 70 | Multicentre prospective cohort | 22 | 90 | 67 ± 11 | 35 ±10 | 44 | 26 | 80 | 20 | NICM (excluding ARCD, idiopathic VT, CHD, VHD, HCM and CPVT) | None | 76 |
| Vergara, 20127 | 50 | Prospective cohort | 13 ± 4 | 93.8 | 63.6 ± 13.6 | 32.2 ± 9.4 | NA | NA | 64.1 | 35.9 | DCM | None | NA |
| Tilz, 201413 | 12 | Prospective cohort | 16 (9.9–26)a | 100 | 65 ± 8 | 32 ± 13 | 66.7 | NA | 100 | 0 | NA | None | 92 |
| Berruezo, 20159 | 101 | Prospective cohort | 21 | 91.1 | 65 ± 12 | 36 ± 13 | 74.3 | 22.8 | 74.3 | 25.7 | NA | None | 53 |
| Tzou, 201512 | 44 | Prospective cohort | 17.5 ± 9.0 | 95 | 63 ± 14 | 31 ± 13 | NA | NA | 73 | 27 | DCM, ARVC, surgically repaired TOF, sarcoidosis, HCM, mixed CMP | ECMO in one patient, IABP in two patients | NA |
| Jamil-Copley, 201514 | 21 | Prospective cohort | 16 (14.6–28)a | 95 | 69 ± 7 | 28 ± 6 | NA | NA | 100 | 0 | NA | None | 100 |
| Gokoglan, 201615 | 93 | Multicenter prospective cohort | 14 ± 2 | 67.7 | 55.6 ± 9.4 | 31 ± 6 | 56 | 17 | 0 | 100 | DCM | Seven patients | 100 |
| First Author, Year . | Patients . | Type of study . | Follow-up (months) . | Male (%) . | Age, years (mean ± SD) . | LVEF, % (mean ± SD) . | HTN (%) . | DM (%) . | ICM (%) . | NICM (%) . | Type of NICM . | Mechanical support . | ICD prior to ablation (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Ablation of stable VT vs. substrate modification | |||||||||||||
| Arenal, 200311 | 24 | Prospective cohort | 9 ± 4 | 91.7 | 66 ± 9 | 30 ± 9 | NA | NA | 91.7 | 8.3 | DCM, TOF | None | 50 |
| Volkmer, 20063 | 47 | Prospective cohort | 25 ± 13 | 91.4 | 65 ± 8 | 30 ± 7.5 | NA | NA | 100 | 0 | NA | None | 47 |
| Ventura, 20084 | 30 | Prospective cohort | 14 ± 6 | 93.3 | 65 ± 7 | 32 ± 6 | NA | NA | 100 | 0 | NA | None | 80 |
| Di Biase, 20126 | 92 | Multicenter prospective cohort | 25 ± 10 | 80 | 61.5 ± 9 | 25.5 ±7.5 | 60 | 14.5 | 100 | 0 | NA | None | 100 |
| Makimoto, 20158 | 85 | Prospective cohort | 61 ± 40 | 73 | 53.1 ± 16.2 | 51.7 ± 16.4 | NA | NA | 18.8 | 81.2 | ARVC, DCM, HCM, Sarcoidosis, CHD | None | 51 |
| Di Biase, 201510 | 118 | Prospective Randomized Multicenter | 12 | 93.2 | 66 ± 10.5 | 32.3 ±12 | 73.8 | 36.7 | 100 | 0 | NA | Eight patients during standard ablation | 100 |
| Complete vs. Incomplete Substrate Modification | |||||||||||||
| Jais, 20125 | 70 | Multicentre prospective cohort | 22 | 90 | 67 ± 11 | 35 ±10 | 44 | 26 | 80 | 20 | NICM (excluding ARCD, idiopathic VT, CHD, VHD, HCM and CPVT) | None | 76 |
| Vergara, 20127 | 50 | Prospective cohort | 13 ± 4 | 93.8 | 63.6 ± 13.6 | 32.2 ± 9.4 | NA | NA | 64.1 | 35.9 | DCM | None | NA |
| Tilz, 201413 | 12 | Prospective cohort | 16 (9.9–26)a | 100 | 65 ± 8 | 32 ± 13 | 66.7 | NA | 100 | 0 | NA | None | 92 |
| Berruezo, 20159 | 101 | Prospective cohort | 21 | 91.1 | 65 ± 12 | 36 ± 13 | 74.3 | 22.8 | 74.3 | 25.7 | NA | None | 53 |
| Tzou, 201512 | 44 | Prospective cohort | 17.5 ± 9.0 | 95 | 63 ± 14 | 31 ± 13 | NA | NA | 73 | 27 | DCM, ARVC, surgically repaired TOF, sarcoidosis, HCM, mixed CMP | ECMO in one patient, IABP in two patients | NA |
| Jamil-Copley, 201514 | 21 | Prospective cohort | 16 (14.6–28)a | 95 | 69 ± 7 | 28 ± 6 | NA | NA | 100 | 0 | NA | None | 100 |
| Gokoglan, 201615 | 93 | Multicenter prospective cohort | 14 ± 2 | 67.7 | 55.6 ± 9.4 | 31 ± 6 | 56 | 17 | 0 | 100 | DCM | Seven patients | 100 |
Median (IQR) interquartile range
ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; CMP, cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; DM, diabetes mellitus; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; HCM, hypertrophic cardiomyopathy; HTN, hypertension; LVEF, left ventricular ejection fraction; ICM, ischemic cardiomyopathy; IABP, intra-aortic balloon pump; ICD, implantable cardioverter defibrillator; NA, not available; NICM, non-ischemic cardiomyopathy; TOF, tetralogy of Fallot; VHD, valvular heart disease VT, ventricular tachycardia;
| First Author, Year . | Patients . | Type of study . | Follow-up (months) . | Male (%) . | Age, years (mean ± SD) . | LVEF, % (mean ± SD) . | HTN (%) . | DM (%) . | ICM (%) . | NICM (%) . | Type of NICM . | Mechanical support . | ICD prior to ablation (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Ablation of stable VT vs. substrate modification | |||||||||||||
| Arenal, 200311 | 24 | Prospective cohort | 9 ± 4 | 91.7 | 66 ± 9 | 30 ± 9 | NA | NA | 91.7 | 8.3 | DCM, TOF | None | 50 |
| Volkmer, 20063 | 47 | Prospective cohort | 25 ± 13 | 91.4 | 65 ± 8 | 30 ± 7.5 | NA | NA | 100 | 0 | NA | None | 47 |
| Ventura, 20084 | 30 | Prospective cohort | 14 ± 6 | 93.3 | 65 ± 7 | 32 ± 6 | NA | NA | 100 | 0 | NA | None | 80 |
| Di Biase, 20126 | 92 | Multicenter prospective cohort | 25 ± 10 | 80 | 61.5 ± 9 | 25.5 ±7.5 | 60 | 14.5 | 100 | 0 | NA | None | 100 |
| Makimoto, 20158 | 85 | Prospective cohort | 61 ± 40 | 73 | 53.1 ± 16.2 | 51.7 ± 16.4 | NA | NA | 18.8 | 81.2 | ARVC, DCM, HCM, Sarcoidosis, CHD | None | 51 |
| Di Biase, 201510 | 118 | Prospective Randomized Multicenter | 12 | 93.2 | 66 ± 10.5 | 32.3 ±12 | 73.8 | 36.7 | 100 | 0 | NA | Eight patients during standard ablation | 100 |
| Complete vs. Incomplete Substrate Modification | |||||||||||||
| Jais, 20125 | 70 | Multicentre prospective cohort | 22 | 90 | 67 ± 11 | 35 ±10 | 44 | 26 | 80 | 20 | NICM (excluding ARCD, idiopathic VT, CHD, VHD, HCM and CPVT) | None | 76 |
| Vergara, 20127 | 50 | Prospective cohort | 13 ± 4 | 93.8 | 63.6 ± 13.6 | 32.2 ± 9.4 | NA | NA | 64.1 | 35.9 | DCM | None | NA |
| Tilz, 201413 | 12 | Prospective cohort | 16 (9.9–26)a | 100 | 65 ± 8 | 32 ± 13 | 66.7 | NA | 100 | 0 | NA | None | 92 |
| Berruezo, 20159 | 101 | Prospective cohort | 21 | 91.1 | 65 ± 12 | 36 ± 13 | 74.3 | 22.8 | 74.3 | 25.7 | NA | None | 53 |
| Tzou, 201512 | 44 | Prospective cohort | 17.5 ± 9.0 | 95 | 63 ± 14 | 31 ± 13 | NA | NA | 73 | 27 | DCM, ARVC, surgically repaired TOF, sarcoidosis, HCM, mixed CMP | ECMO in one patient, IABP in two patients | NA |
| Jamil-Copley, 201514 | 21 | Prospective cohort | 16 (14.6–28)a | 95 | 69 ± 7 | 28 ± 6 | NA | NA | 100 | 0 | NA | None | 100 |
| Gokoglan, 201615 | 93 | Multicenter prospective cohort | 14 ± 2 | 67.7 | 55.6 ± 9.4 | 31 ± 6 | 56 | 17 | 0 | 100 | DCM | Seven patients | 100 |
| First Author, Year . | Patients . | Type of study . | Follow-up (months) . | Male (%) . | Age, years (mean ± SD) . | LVEF, % (mean ± SD) . | HTN (%) . | DM (%) . | ICM (%) . | NICM (%) . | Type of NICM . | Mechanical support . | ICD prior to ablation (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Ablation of stable VT vs. substrate modification | |||||||||||||
| Arenal, 200311 | 24 | Prospective cohort | 9 ± 4 | 91.7 | 66 ± 9 | 30 ± 9 | NA | NA | 91.7 | 8.3 | DCM, TOF | None | 50 |
| Volkmer, 20063 | 47 | Prospective cohort | 25 ± 13 | 91.4 | 65 ± 8 | 30 ± 7.5 | NA | NA | 100 | 0 | NA | None | 47 |
| Ventura, 20084 | 30 | Prospective cohort | 14 ± 6 | 93.3 | 65 ± 7 | 32 ± 6 | NA | NA | 100 | 0 | NA | None | 80 |
| Di Biase, 20126 | 92 | Multicenter prospective cohort | 25 ± 10 | 80 | 61.5 ± 9 | 25.5 ±7.5 | 60 | 14.5 | 100 | 0 | NA | None | 100 |
| Makimoto, 20158 | 85 | Prospective cohort | 61 ± 40 | 73 | 53.1 ± 16.2 | 51.7 ± 16.4 | NA | NA | 18.8 | 81.2 | ARVC, DCM, HCM, Sarcoidosis, CHD | None | 51 |
| Di Biase, 201510 | 118 | Prospective Randomized Multicenter | 12 | 93.2 | 66 ± 10.5 | 32.3 ±12 | 73.8 | 36.7 | 100 | 0 | NA | Eight patients during standard ablation | 100 |
| Complete vs. Incomplete Substrate Modification | |||||||||||||
| Jais, 20125 | 70 | Multicentre prospective cohort | 22 | 90 | 67 ± 11 | 35 ±10 | 44 | 26 | 80 | 20 | NICM (excluding ARCD, idiopathic VT, CHD, VHD, HCM and CPVT) | None | 76 |
| Vergara, 20127 | 50 | Prospective cohort | 13 ± 4 | 93.8 | 63.6 ± 13.6 | 32.2 ± 9.4 | NA | NA | 64.1 | 35.9 | DCM | None | NA |
| Tilz, 201413 | 12 | Prospective cohort | 16 (9.9–26)a | 100 | 65 ± 8 | 32 ± 13 | 66.7 | NA | 100 | 0 | NA | None | 92 |
| Berruezo, 20159 | 101 | Prospective cohort | 21 | 91.1 | 65 ± 12 | 36 ± 13 | 74.3 | 22.8 | 74.3 | 25.7 | NA | None | 53 |
| Tzou, 201512 | 44 | Prospective cohort | 17.5 ± 9.0 | 95 | 63 ± 14 | 31 ± 13 | NA | NA | 73 | 27 | DCM, ARVC, surgically repaired TOF, sarcoidosis, HCM, mixed CMP | ECMO in one patient, IABP in two patients | NA |
| Jamil-Copley, 201514 | 21 | Prospective cohort | 16 (14.6–28)a | 95 | 69 ± 7 | 28 ± 6 | NA | NA | 100 | 0 | NA | None | 100 |
| Gokoglan, 201615 | 93 | Multicenter prospective cohort | 14 ± 2 | 67.7 | 55.6 ± 9.4 | 31 ± 6 | 56 | 17 | 0 | 100 | DCM | Seven patients | 100 |
Median (IQR) interquartile range
ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; CMP, cardiomyopathy; CPVT, catecholaminergic polymorphic ventricular tachycardia; DM, diabetes mellitus; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; HCM, hypertrophic cardiomyopathy; HTN, hypertension; LVEF, left ventricular ejection fraction; ICM, ischemic cardiomyopathy; IABP, intra-aortic balloon pump; ICD, implantable cardioverter defibrillator; NA, not available; NICM, non-ischemic cardiomyopathy; TOF, tetralogy of Fallot; VHD, valvular heart disease VT, ventricular tachycardia;
Methodological and procedural characteristics of studies evaluating standard ablation of stable VT vs. substrate modification
| First author, year . | Study design . | Inclusion criteria . | Study population, n . | Study groups . | Ablation strategy . | Procedural endpoints . | Follow-up outcomes . |
|---|---|---|---|---|---|---|---|
| Arenal et al., 200311 | Non-randomized prospective cohort | Consecutive patients with SHD and sustained monomorphic VT documented on EKG and referred for RFA | 24 | Patients with stable tolerated VT, n = 18; Patients with non-tolerated VT or non-inducible VT, n = 6 | Stable tolerated VT: RFA guided by entrainment mapping areas; Non-tolerated VT/non-inducible VT: RFA of E-IDC area (E-IDC, electrogram displaying isolated, delayed component) | (i) Disappearance of all IDCs of a clinical VT-related area in those patients with unmappable VT; and (ii) inducibility suppression of clinical VT | Recurrence of ablated VT and two of a different VT |
| Volkmer et al., 20063 | Non-randomized prospective cohort | Consecutive patients with clinically sustained monomorphic VT with MI > 3 months | 47 | VT mapping group (patients with inducible or spontaneous VT), n = 22; Substrate mapping group (patients in whom a complete VT map was not possible because of catheter-induced mechanical block or because the induced VT was unmappable due to haemodynamic deterioration during VT), n = 25 | VT mapping group: complete activation and propagation map during the clinical and/or any other slower VT combined with conventional entrainment pacing; Substrate mapping group: mapping during sinus rhythm, RVor LV pacing. Sites where pace mapping matched the spontaneous VT morphology and where the delay between the stimulus and the onset of the QRS-complex was at least 50 ms were classified as potential target sites within a protected isthmus | Non-inducibility of the clinical VT and any VT slower than the clinical VT, either at the end of the ablation procedure (VT-mapping group) or in a repeat EP stimulation study before hospital discharge (substrate-mapping group) | Recurrence of clinical VT or any VT slower than the clinical VT and freedom from VT/VF |
| Ventura et al., 20084 | Non-randomized prospective cohort | Consecutive patients referred for RFA ablation of monomorphic VTs in a setting of prior MI | 30 | Patient with haemodynamically tolerated target VT, n = 16; Patients with unstable target VT, n = 14 | If the patient haemodynamically tolerated the target VT, mapping was continued during tachycardia. Concealed entrainment, post-pacing interval and S-QRS interval were evaluated to localize the critical isthmus. In cases of unstable VT, only pace mapping and S-QRS interval were evaluated for isthmus identification | The procedural end-point was considered achieved if no target VTs were inducible | Recurrence of clinical VT |
| Di Biase et al., 20126 | Multicentre non-randomized prospective cohort | Consecutive patients with ischaemic cardiomyopathy and ES (defined as three ICD interventions in 24 h). All patients had ICD placement before ablation | 92 | Group 1, n = 49: activation/entrainment-mapping; Group 2, n = 43: homogenization of the scar | Group 1: conventional mapping techniques including pace mapping, activation mapping, and entrainment mapping; Group 2: based on the substrate map, ablation was empirically extended throughout the entire scar (homogenization of the scar) endocardially. | Non-inducibility of any monomorphic VTs before and after the administration of isoproterenol (up to 5 g/min). | Arrhythmia recurrence defined as arrhythmias receiving device-based treatments (anti tachycardia pacing or shock) |
| Makimoto et al., 20158 | Non-randomized prospective cohort | Consecutive patients with SHD and at least one episode of monomorphic sustained VT who underwent catheter ablation targeting VT between 1999 and 2009 | 85 | Activation/entrainment-mapping, n = 35; Substrate Group, n = 50 | Standard group: activation/entrainment-mapping; Substrate group: substrate-based strategy due to non-inducibility of VT or hemodynamic instability | Standard group: non-inducibility of any VT after the procedure; for patients with no inducible VT before the RF applications and for substrate group: the definitions of a successful ablation were as follows; (i) PVC elimination when PVCs were targeted, (ii) confirmation of a linear lesion blockade when a linear lesion was made, and (iii) elimination of fractionated or isolated delayed potentials when those potentials were targeted | Primary outcome: recurrence of VT defined as sustained VA documented by an ICD or ECG recordings. Secondary outcomes: hospitalizations due to heart failure, sudden cardiac death, and syncopal episodes |
| Di Biase et al., 201510 | Multicentre prospective randomized cohort | Patients who had received an ICD before ablation and suffered from recurrent stable monomorphic VTs that were symptomatic or required ICD therapies despite AADs. | 118 | Clinical VT ablation group, n = 60; extensive substrate-based ablation group, n = 58 | Clinical VT ablation group: conventional mapping techniques (pace-mapping, activation mapping, and entrainment mapping). Linear ablation lesions were placed to transect the VT isthmus and terminate inducible VTs; Substrate based ablation group: voltage and activation mapping, identification of fractionated, delayed, or abnormal electrograms, ablation lesions empirically extended throughout the entire scar based on the substrate map defined by 3D mapping and targeting any abnormal potentials in normal sinus rhythm, VT induction was not required | Clinical VT ablation group: programmed stimulation with and without isoproterenol in all cases to test the inducibility of VAs; Substrate based ablation group: elimination of all abnormal potentials | Primary endpoint: recurrence of any VT during the 12-month post-ablation period, as demonstrated by device interrogation and clinical evaluation. Secondary endpoints: periprocedural complications, 12-month post-procedure mortality, rehospitalization, and combined incidence of rehospitalization and mortality |
| First author, year . | Study design . | Inclusion criteria . | Study population, n . | Study groups . | Ablation strategy . | Procedural endpoints . | Follow-up outcomes . |
|---|---|---|---|---|---|---|---|
| Arenal et al., 200311 | Non-randomized prospective cohort | Consecutive patients with SHD and sustained monomorphic VT documented on EKG and referred for RFA | 24 | Patients with stable tolerated VT, n = 18; Patients with non-tolerated VT or non-inducible VT, n = 6 | Stable tolerated VT: RFA guided by entrainment mapping areas; Non-tolerated VT/non-inducible VT: RFA of E-IDC area (E-IDC, electrogram displaying isolated, delayed component) | (i) Disappearance of all IDCs of a clinical VT-related area in those patients with unmappable VT; and (ii) inducibility suppression of clinical VT | Recurrence of ablated VT and two of a different VT |
| Volkmer et al., 20063 | Non-randomized prospective cohort | Consecutive patients with clinically sustained monomorphic VT with MI > 3 months | 47 | VT mapping group (patients with inducible or spontaneous VT), n = 22; Substrate mapping group (patients in whom a complete VT map was not possible because of catheter-induced mechanical block or because the induced VT was unmappable due to haemodynamic deterioration during VT), n = 25 | VT mapping group: complete activation and propagation map during the clinical and/or any other slower VT combined with conventional entrainment pacing; Substrate mapping group: mapping during sinus rhythm, RVor LV pacing. Sites where pace mapping matched the spontaneous VT morphology and where the delay between the stimulus and the onset of the QRS-complex was at least 50 ms were classified as potential target sites within a protected isthmus | Non-inducibility of the clinical VT and any VT slower than the clinical VT, either at the end of the ablation procedure (VT-mapping group) or in a repeat EP stimulation study before hospital discharge (substrate-mapping group) | Recurrence of clinical VT or any VT slower than the clinical VT and freedom from VT/VF |
| Ventura et al., 20084 | Non-randomized prospective cohort | Consecutive patients referred for RFA ablation of monomorphic VTs in a setting of prior MI | 30 | Patient with haemodynamically tolerated target VT, n = 16; Patients with unstable target VT, n = 14 | If the patient haemodynamically tolerated the target VT, mapping was continued during tachycardia. Concealed entrainment, post-pacing interval and S-QRS interval were evaluated to localize the critical isthmus. In cases of unstable VT, only pace mapping and S-QRS interval were evaluated for isthmus identification | The procedural end-point was considered achieved if no target VTs were inducible | Recurrence of clinical VT |
| Di Biase et al., 20126 | Multicentre non-randomized prospective cohort | Consecutive patients with ischaemic cardiomyopathy and ES (defined as three ICD interventions in 24 h). All patients had ICD placement before ablation | 92 | Group 1, n = 49: activation/entrainment-mapping; Group 2, n = 43: homogenization of the scar | Group 1: conventional mapping techniques including pace mapping, activation mapping, and entrainment mapping; Group 2: based on the substrate map, ablation was empirically extended throughout the entire scar (homogenization of the scar) endocardially. | Non-inducibility of any monomorphic VTs before and after the administration of isoproterenol (up to 5 g/min). | Arrhythmia recurrence defined as arrhythmias receiving device-based treatments (anti tachycardia pacing or shock) |
| Makimoto et al., 20158 | Non-randomized prospective cohort | Consecutive patients with SHD and at least one episode of monomorphic sustained VT who underwent catheter ablation targeting VT between 1999 and 2009 | 85 | Activation/entrainment-mapping, n = 35; Substrate Group, n = 50 | Standard group: activation/entrainment-mapping; Substrate group: substrate-based strategy due to non-inducibility of VT or hemodynamic instability | Standard group: non-inducibility of any VT after the procedure; for patients with no inducible VT before the RF applications and for substrate group: the definitions of a successful ablation were as follows; (i) PVC elimination when PVCs were targeted, (ii) confirmation of a linear lesion blockade when a linear lesion was made, and (iii) elimination of fractionated or isolated delayed potentials when those potentials were targeted | Primary outcome: recurrence of VT defined as sustained VA documented by an ICD or ECG recordings. Secondary outcomes: hospitalizations due to heart failure, sudden cardiac death, and syncopal episodes |
| Di Biase et al., 201510 | Multicentre prospective randomized cohort | Patients who had received an ICD before ablation and suffered from recurrent stable monomorphic VTs that were symptomatic or required ICD therapies despite AADs. | 118 | Clinical VT ablation group, n = 60; extensive substrate-based ablation group, n = 58 | Clinical VT ablation group: conventional mapping techniques (pace-mapping, activation mapping, and entrainment mapping). Linear ablation lesions were placed to transect the VT isthmus and terminate inducible VTs; Substrate based ablation group: voltage and activation mapping, identification of fractionated, delayed, or abnormal electrograms, ablation lesions empirically extended throughout the entire scar based on the substrate map defined by 3D mapping and targeting any abnormal potentials in normal sinus rhythm, VT induction was not required | Clinical VT ablation group: programmed stimulation with and without isoproterenol in all cases to test the inducibility of VAs; Substrate based ablation group: elimination of all abnormal potentials | Primary endpoint: recurrence of any VT during the 12-month post-ablation period, as demonstrated by device interrogation and clinical evaluation. Secondary endpoints: periprocedural complications, 12-month post-procedure mortality, rehospitalization, and combined incidence of rehospitalization and mortality |
AADs, anti-arrhythmic drugs; ICD, implantable-cardioverter defibrillator; E-IDC, electrogram displaying isolated delayed components; EP, electrophysiology; ES, electric storm; ECG, electrocardiogram; MI, myocardial infarction; PVC, premature ventricular contraction; LV, left ventricle; RFA, radiofrequency ablation; RV, right ventricle; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Methodological and procedural characteristics of studies evaluating standard ablation of stable VT vs. substrate modification
| First author, year . | Study design . | Inclusion criteria . | Study population, n . | Study groups . | Ablation strategy . | Procedural endpoints . | Follow-up outcomes . |
|---|---|---|---|---|---|---|---|
| Arenal et al., 200311 | Non-randomized prospective cohort | Consecutive patients with SHD and sustained monomorphic VT documented on EKG and referred for RFA | 24 | Patients with stable tolerated VT, n = 18; Patients with non-tolerated VT or non-inducible VT, n = 6 | Stable tolerated VT: RFA guided by entrainment mapping areas; Non-tolerated VT/non-inducible VT: RFA of E-IDC area (E-IDC, electrogram displaying isolated, delayed component) | (i) Disappearance of all IDCs of a clinical VT-related area in those patients with unmappable VT; and (ii) inducibility suppression of clinical VT | Recurrence of ablated VT and two of a different VT |
| Volkmer et al., 20063 | Non-randomized prospective cohort | Consecutive patients with clinically sustained monomorphic VT with MI > 3 months | 47 | VT mapping group (patients with inducible or spontaneous VT), n = 22; Substrate mapping group (patients in whom a complete VT map was not possible because of catheter-induced mechanical block or because the induced VT was unmappable due to haemodynamic deterioration during VT), n = 25 | VT mapping group: complete activation and propagation map during the clinical and/or any other slower VT combined with conventional entrainment pacing; Substrate mapping group: mapping during sinus rhythm, RVor LV pacing. Sites where pace mapping matched the spontaneous VT morphology and where the delay between the stimulus and the onset of the QRS-complex was at least 50 ms were classified as potential target sites within a protected isthmus | Non-inducibility of the clinical VT and any VT slower than the clinical VT, either at the end of the ablation procedure (VT-mapping group) or in a repeat EP stimulation study before hospital discharge (substrate-mapping group) | Recurrence of clinical VT or any VT slower than the clinical VT and freedom from VT/VF |
| Ventura et al., 20084 | Non-randomized prospective cohort | Consecutive patients referred for RFA ablation of monomorphic VTs in a setting of prior MI | 30 | Patient with haemodynamically tolerated target VT, n = 16; Patients with unstable target VT, n = 14 | If the patient haemodynamically tolerated the target VT, mapping was continued during tachycardia. Concealed entrainment, post-pacing interval and S-QRS interval were evaluated to localize the critical isthmus. In cases of unstable VT, only pace mapping and S-QRS interval were evaluated for isthmus identification | The procedural end-point was considered achieved if no target VTs were inducible | Recurrence of clinical VT |
| Di Biase et al., 20126 | Multicentre non-randomized prospective cohort | Consecutive patients with ischaemic cardiomyopathy and ES (defined as three ICD interventions in 24 h). All patients had ICD placement before ablation | 92 | Group 1, n = 49: activation/entrainment-mapping; Group 2, n = 43: homogenization of the scar | Group 1: conventional mapping techniques including pace mapping, activation mapping, and entrainment mapping; Group 2: based on the substrate map, ablation was empirically extended throughout the entire scar (homogenization of the scar) endocardially. | Non-inducibility of any monomorphic VTs before and after the administration of isoproterenol (up to 5 g/min). | Arrhythmia recurrence defined as arrhythmias receiving device-based treatments (anti tachycardia pacing or shock) |
| Makimoto et al., 20158 | Non-randomized prospective cohort | Consecutive patients with SHD and at least one episode of monomorphic sustained VT who underwent catheter ablation targeting VT between 1999 and 2009 | 85 | Activation/entrainment-mapping, n = 35; Substrate Group, n = 50 | Standard group: activation/entrainment-mapping; Substrate group: substrate-based strategy due to non-inducibility of VT or hemodynamic instability | Standard group: non-inducibility of any VT after the procedure; for patients with no inducible VT before the RF applications and for substrate group: the definitions of a successful ablation were as follows; (i) PVC elimination when PVCs were targeted, (ii) confirmation of a linear lesion blockade when a linear lesion was made, and (iii) elimination of fractionated or isolated delayed potentials when those potentials were targeted | Primary outcome: recurrence of VT defined as sustained VA documented by an ICD or ECG recordings. Secondary outcomes: hospitalizations due to heart failure, sudden cardiac death, and syncopal episodes |
| Di Biase et al., 201510 | Multicentre prospective randomized cohort | Patients who had received an ICD before ablation and suffered from recurrent stable monomorphic VTs that were symptomatic or required ICD therapies despite AADs. | 118 | Clinical VT ablation group, n = 60; extensive substrate-based ablation group, n = 58 | Clinical VT ablation group: conventional mapping techniques (pace-mapping, activation mapping, and entrainment mapping). Linear ablation lesions were placed to transect the VT isthmus and terminate inducible VTs; Substrate based ablation group: voltage and activation mapping, identification of fractionated, delayed, or abnormal electrograms, ablation lesions empirically extended throughout the entire scar based on the substrate map defined by 3D mapping and targeting any abnormal potentials in normal sinus rhythm, VT induction was not required | Clinical VT ablation group: programmed stimulation with and without isoproterenol in all cases to test the inducibility of VAs; Substrate based ablation group: elimination of all abnormal potentials | Primary endpoint: recurrence of any VT during the 12-month post-ablation period, as demonstrated by device interrogation and clinical evaluation. Secondary endpoints: periprocedural complications, 12-month post-procedure mortality, rehospitalization, and combined incidence of rehospitalization and mortality |
| First author, year . | Study design . | Inclusion criteria . | Study population, n . | Study groups . | Ablation strategy . | Procedural endpoints . | Follow-up outcomes . |
|---|---|---|---|---|---|---|---|
| Arenal et al., 200311 | Non-randomized prospective cohort | Consecutive patients with SHD and sustained monomorphic VT documented on EKG and referred for RFA | 24 | Patients with stable tolerated VT, n = 18; Patients with non-tolerated VT or non-inducible VT, n = 6 | Stable tolerated VT: RFA guided by entrainment mapping areas; Non-tolerated VT/non-inducible VT: RFA of E-IDC area (E-IDC, electrogram displaying isolated, delayed component) | (i) Disappearance of all IDCs of a clinical VT-related area in those patients with unmappable VT; and (ii) inducibility suppression of clinical VT | Recurrence of ablated VT and two of a different VT |
| Volkmer et al., 20063 | Non-randomized prospective cohort | Consecutive patients with clinically sustained monomorphic VT with MI > 3 months | 47 | VT mapping group (patients with inducible or spontaneous VT), n = 22; Substrate mapping group (patients in whom a complete VT map was not possible because of catheter-induced mechanical block or because the induced VT was unmappable due to haemodynamic deterioration during VT), n = 25 | VT mapping group: complete activation and propagation map during the clinical and/or any other slower VT combined with conventional entrainment pacing; Substrate mapping group: mapping during sinus rhythm, RVor LV pacing. Sites where pace mapping matched the spontaneous VT morphology and where the delay between the stimulus and the onset of the QRS-complex was at least 50 ms were classified as potential target sites within a protected isthmus | Non-inducibility of the clinical VT and any VT slower than the clinical VT, either at the end of the ablation procedure (VT-mapping group) or in a repeat EP stimulation study before hospital discharge (substrate-mapping group) | Recurrence of clinical VT or any VT slower than the clinical VT and freedom from VT/VF |
| Ventura et al., 20084 | Non-randomized prospective cohort | Consecutive patients referred for RFA ablation of monomorphic VTs in a setting of prior MI | 30 | Patient with haemodynamically tolerated target VT, n = 16; Patients with unstable target VT, n = 14 | If the patient haemodynamically tolerated the target VT, mapping was continued during tachycardia. Concealed entrainment, post-pacing interval and S-QRS interval were evaluated to localize the critical isthmus. In cases of unstable VT, only pace mapping and S-QRS interval were evaluated for isthmus identification | The procedural end-point was considered achieved if no target VTs were inducible | Recurrence of clinical VT |
| Di Biase et al., 20126 | Multicentre non-randomized prospective cohort | Consecutive patients with ischaemic cardiomyopathy and ES (defined as three ICD interventions in 24 h). All patients had ICD placement before ablation | 92 | Group 1, n = 49: activation/entrainment-mapping; Group 2, n = 43: homogenization of the scar | Group 1: conventional mapping techniques including pace mapping, activation mapping, and entrainment mapping; Group 2: based on the substrate map, ablation was empirically extended throughout the entire scar (homogenization of the scar) endocardially. | Non-inducibility of any monomorphic VTs before and after the administration of isoproterenol (up to 5 g/min). | Arrhythmia recurrence defined as arrhythmias receiving device-based treatments (anti tachycardia pacing or shock) |
| Makimoto et al., 20158 | Non-randomized prospective cohort | Consecutive patients with SHD and at least one episode of monomorphic sustained VT who underwent catheter ablation targeting VT between 1999 and 2009 | 85 | Activation/entrainment-mapping, n = 35; Substrate Group, n = 50 | Standard group: activation/entrainment-mapping; Substrate group: substrate-based strategy due to non-inducibility of VT or hemodynamic instability | Standard group: non-inducibility of any VT after the procedure; for patients with no inducible VT before the RF applications and for substrate group: the definitions of a successful ablation were as follows; (i) PVC elimination when PVCs were targeted, (ii) confirmation of a linear lesion blockade when a linear lesion was made, and (iii) elimination of fractionated or isolated delayed potentials when those potentials were targeted | Primary outcome: recurrence of VT defined as sustained VA documented by an ICD or ECG recordings. Secondary outcomes: hospitalizations due to heart failure, sudden cardiac death, and syncopal episodes |
| Di Biase et al., 201510 | Multicentre prospective randomized cohort | Patients who had received an ICD before ablation and suffered from recurrent stable monomorphic VTs that were symptomatic or required ICD therapies despite AADs. | 118 | Clinical VT ablation group, n = 60; extensive substrate-based ablation group, n = 58 | Clinical VT ablation group: conventional mapping techniques (pace-mapping, activation mapping, and entrainment mapping). Linear ablation lesions were placed to transect the VT isthmus and terminate inducible VTs; Substrate based ablation group: voltage and activation mapping, identification of fractionated, delayed, or abnormal electrograms, ablation lesions empirically extended throughout the entire scar based on the substrate map defined by 3D mapping and targeting any abnormal potentials in normal sinus rhythm, VT induction was not required | Clinical VT ablation group: programmed stimulation with and without isoproterenol in all cases to test the inducibility of VAs; Substrate based ablation group: elimination of all abnormal potentials | Primary endpoint: recurrence of any VT during the 12-month post-ablation period, as demonstrated by device interrogation and clinical evaluation. Secondary endpoints: periprocedural complications, 12-month post-procedure mortality, rehospitalization, and combined incidence of rehospitalization and mortality |
AADs, anti-arrhythmic drugs; ICD, implantable-cardioverter defibrillator; E-IDC, electrogram displaying isolated delayed components; EP, electrophysiology; ES, electric storm; ECG, electrocardiogram; MI, myocardial infarction; PVC, premature ventricular contraction; LV, left ventricle; RFA, radiofrequency ablation; RV, right ventricle; VA, ventricular arrhythmia; VF, ventricular fibrillation; VT, ventricular tachycardia.
Seven studies were included to assess the impact of complete substrate modification. Complete substrate modification was defined as substrate-based ablation achieving a procedural end point beyond VT inducibility established within each technique such as complete elimination of all sharp high-frequency ventricular potentials (local abnormal ventricular activities), elimination of all identified conducting channels (scar dechanneling), failure to capture the ventricle with pacing from inside the lesion (core isolation), and empirical elimination of all abnormal electrograms (homogenization), among others (Table 3). A total of 391 patients were included (mean age 64 ± 11 years, 90% males). The mean follow-up was 21±5 months. The mean left ventricular ejection fraction was 32 ± 10%, most of the patients had an ICD implanted at baseline (84%), and had ischaemic cardiomyopathy (70%).
| Study . | Year . | Substrate technique . | Procedural end point . |
|---|---|---|---|
| Jais5 | 2012 | LAVA | Elimination of all sharp high-frequency ventricular potentials, occurring anytime during or after the far-field ventricular electrogram in sinus rhythm or before the far-field ventricular electrogram during VT |
| Vergara7 | 2012 | Late potentials | Complete abolition of all late potentials |
| Tilz13 | 2014 | Substrate isolation | Isolation of the entire substrate and defined as (i) lack of fractionated, double or late potentials inside the encircled area 20 min post-ablation, (ii) non-capture of the LV during pacing with maximal output at multiple sites within the encircled area, and (iii) after a maximum of 40 RF applications |
| Berruezo9 | 2015 | Scar dechanneling | Elimination of all identified CCs at the CC entrance during sinus rhythm |
| Tzou12 | 2015 | Core isolation | Failure to capture the ventricle with pacing from inside the lesion set (exit block) that conforms the isolated core |
| Jamil-Copley14 | 2015 | RMCC | Ablation overlapping all RMCCs |
| Gokoglan15 | 2016 | Scar homogenization | Empirical elimination of all abnormal electrograms throughout the entire scar |
| Study . | Year . | Substrate technique . | Procedural end point . |
|---|---|---|---|
| Jais5 | 2012 | LAVA | Elimination of all sharp high-frequency ventricular potentials, occurring anytime during or after the far-field ventricular electrogram in sinus rhythm or before the far-field ventricular electrogram during VT |
| Vergara7 | 2012 | Late potentials | Complete abolition of all late potentials |
| Tilz13 | 2014 | Substrate isolation | Isolation of the entire substrate and defined as (i) lack of fractionated, double or late potentials inside the encircled area 20 min post-ablation, (ii) non-capture of the LV during pacing with maximal output at multiple sites within the encircled area, and (iii) after a maximum of 40 RF applications |
| Berruezo9 | 2015 | Scar dechanneling | Elimination of all identified CCs at the CC entrance during sinus rhythm |
| Tzou12 | 2015 | Core isolation | Failure to capture the ventricle with pacing from inside the lesion set (exit block) that conforms the isolated core |
| Jamil-Copley14 | 2015 | RMCC | Ablation overlapping all RMCCs |
| Gokoglan15 | 2016 | Scar homogenization | Empirical elimination of all abnormal electrograms throughout the entire scar |
CC, conducting channel; LAVAs, local abnormal ventricular activities; LV, left ventricle; RF, radiofrequency; RMCCs, ripple mapping conduction channels; VT, ventricular tachycardia.
| Study . | Year . | Substrate technique . | Procedural end point . |
|---|---|---|---|
| Jais5 | 2012 | LAVA | Elimination of all sharp high-frequency ventricular potentials, occurring anytime during or after the far-field ventricular electrogram in sinus rhythm or before the far-field ventricular electrogram during VT |
| Vergara7 | 2012 | Late potentials | Complete abolition of all late potentials |
| Tilz13 | 2014 | Substrate isolation | Isolation of the entire substrate and defined as (i) lack of fractionated, double or late potentials inside the encircled area 20 min post-ablation, (ii) non-capture of the LV during pacing with maximal output at multiple sites within the encircled area, and (iii) after a maximum of 40 RF applications |
| Berruezo9 | 2015 | Scar dechanneling | Elimination of all identified CCs at the CC entrance during sinus rhythm |
| Tzou12 | 2015 | Core isolation | Failure to capture the ventricle with pacing from inside the lesion set (exit block) that conforms the isolated core |
| Jamil-Copley14 | 2015 | RMCC | Ablation overlapping all RMCCs |
| Gokoglan15 | 2016 | Scar homogenization | Empirical elimination of all abnormal electrograms throughout the entire scar |
| Study . | Year . | Substrate technique . | Procedural end point . |
|---|---|---|---|
| Jais5 | 2012 | LAVA | Elimination of all sharp high-frequency ventricular potentials, occurring anytime during or after the far-field ventricular electrogram in sinus rhythm or before the far-field ventricular electrogram during VT |
| Vergara7 | 2012 | Late potentials | Complete abolition of all late potentials |
| Tilz13 | 2014 | Substrate isolation | Isolation of the entire substrate and defined as (i) lack of fractionated, double or late potentials inside the encircled area 20 min post-ablation, (ii) non-capture of the LV during pacing with maximal output at multiple sites within the encircled area, and (iii) after a maximum of 40 RF applications |
| Berruezo9 | 2015 | Scar dechanneling | Elimination of all identified CCs at the CC entrance during sinus rhythm |
| Tzou12 | 2015 | Core isolation | Failure to capture the ventricle with pacing from inside the lesion set (exit block) that conforms the isolated core |
| Jamil-Copley14 | 2015 | RMCC | Ablation overlapping all RMCCs |
| Gokoglan15 | 2016 | Scar homogenization | Empirical elimination of all abnormal electrograms throughout the entire scar |
CC, conducting channel; LAVAs, local abnormal ventricular activities; LV, left ventricle; RF, radiofrequency; RMCCs, ripple mapping conduction channels; VT, ventricular tachycardia.
Primary end points
Standard ablation of stable ventricular tachycardias vs. substrate modification
Five studies had data available to assess the composite end point of VA recurrence and all-cause mortality (Figure 2). Substrate modification was associated with decreased VA recurrence/all-cause mortality compared to standard ablation of stable VTs (RR 0.57, 95% CI 0.40–0.81).
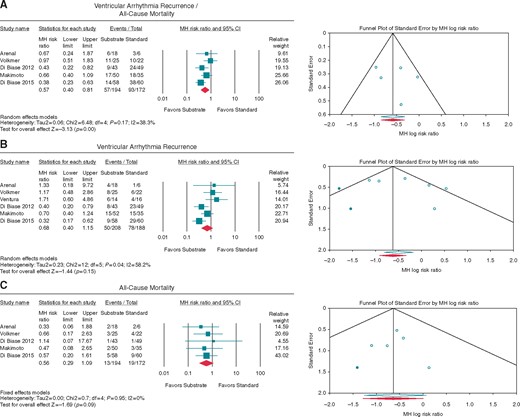
Forest plot reporting the MH RR outcomes in substrate vs. standard ablation. (A) composite VA recurrence/all-cause mortality, (B) VA recurrence, and (C) all-cause mortality, with the respective funnel plot for bias. Diamond indicates overall summary estimate for the analysis (width of the diamond represents the 95% CI); width of the shaded square, size of the population. CI, Confidence interval; MH, Mantel–Haenszel.
Six studies had data available to assess the VA recurrence and five studies for all-cause mortality (Figure 2). Although there was a trend favouring substrate modification, there was no difference in VA recurrence or in all-cause mortality between substrate modification vs. standard ablation of stable VTs when doing separate analyses (RR 0.68, 95% CI 0.40–1.15; RR 0.56, 95% CI 0.29–1.09, respectively).
Complete vs. incomplete substrate modification
Seven studies had data available to assess the impact of complete substrate modification within different substrate based strategies (Figure 3). Complete substrate modification was associated with decreased VA recurrence as compared to incomplete substrate modification (RR 0.39, 95% CI 0.27–0.58) (Figure 4).
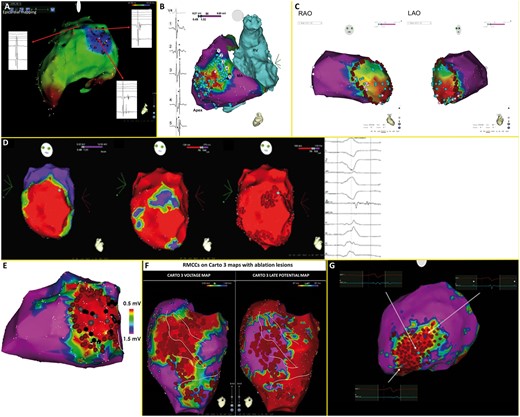
Strategies for substrate modification of studies included (A) local abnormal ventricular activities, (B) scar dechanneling, (C) substrate isolation, (D) late potentials, (E) core isolation, (F) ripple mapping conduction channels, (G) scar homogenization. Adapted from Jais et al.5, Vergara et al.7, Tilz et al.13, Berruezo et al.9, Tzou et al.12, Jamil-Copley et al.14, Gokoglan et al.15
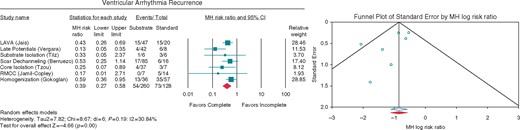
Forest plot reporting the MH RR for (A) ventricular arrhythmia recurrence in complete vs. incomplete substrate modification, with the funnel plot for bias. Diamond indicates overall summary estimate for the analysis (width of the diamond represents the 95% CI); width of the shaded square, size of the population. CI, Confidence interval; MH, Mantel–Haenszel.
Secondary end-points
Standard ablation of stable ventricular tachycardias vs. substrate modification
There was no difference in minor or major complications (RR 1.06, 95% CI 0.27–4.11; RR 1.00, 95% CI 0.14–7.25, respectively). Similarly, no difference was seen for procedure, fluoroscopy or radiofrequency times (DM −2.85, 95% CI −58.48–52.79; DM 1.71, 95% CI −4.69–8.11; DM 21.83, 95% CI −5.18–48.84, respectively) (see Supplementary material online, Figure S1).
Complete vs. incomplete substrate modification
Although there was a trend favouring incomplete substrate modification for procedure, fluoroscopy, and radiofrequency times, there was no difference seen for these outcomes (DM 7.06, 95% CI −28.84–42.97; DM 4.82, 95% CI −1.24–10.88; DM 4.45, 95% CI −15.91–24.82, respectively) (see Supplementary material online, Figure S1).
Publication bias
Funnel plots suggested minimal publication bias for VA recurrence, all-cause mortality, radiofrequency time of substrate vs. standard VT ablation, and procedure time of complete vs. incomplete substrate modification. However, after quantifying with other methods (Begg–Mazumdar, Egger and Duval, and Tweedie’s trim and fill test), there was no evidence of publication bias (see Supplementary material online, Figure S2).
Quality assessment
Based on NOS, eleven studies were of high quality, while two were of satisfactory quality (see Supplementary material online, Table 1).
Discussion
This is the first meta-analysis assessing different ablation strategies for VT in patients with SHD. Our results are highly representative of a population with VT related to ischaemic substrates as more than 70% of all the patients studied had ischaemic cardiomyopathy. These results have two major implications for this population:
The long-term (mean of 24 months) combined risk of VA recurrence and all-cause mortality is lower (43% risk reduction) when using a substrate-based approach compared to standard ablation of stable VT.
A complete substrate modification is associated with lower VA recurrence (61% risk reduction) as compared with incomplete substrate modification during long-term follow-up (mean of 21 months).
Substrate modification vs. standard ablation
Our results show a benefit of substrate modification over standard ablation for the combined endpoint of VA recurrence and all-cause mortality without a benefit for each individual outcome, probably because the individual studies were not powered to detect differences except for the randomized VISTA trial.10 Therefore, we embedded a composite endpoint into our primary analysis to empower the measurement of small effects of each individual study included in this meta-analysis. This combined endpoint is valuable in patients with VAs, as it includes the two desirable outcomes for the procedure.
Substrate based ablation has evolved since its conception in the 1970s by Josephson and colleagues, and with the advent of improved catheter technology and three-dimensional mapping systems, substrate-based strategies have improved significantly.17 Since then, multiple substrate modification approaches with different ablation goals have been described, most of which have been included in our analysis (Table 3).17 On the other hand, standard ablation (activation and entrainment mapping) has major limitations including the inherent technical difficulties of performing ablation during VT with the subsequent hemodynamic consequences and high recurrence rate.16 The evidence favouring one ablation strategy vs. the other has remained elusive. The only randomized controlled study evaluating these strategies is the VISTA trial, which supports our results and previous non-randomized studies evaluating different substrate based strategies in SHD. A recent meta-analysis also evaluated substrate vs. standard ablation of VT, however, they included patients with both stable and unstable VTs in the standard ablation group (different from our study).18 Even though they showed no difference in VT recurrence when comparing these two strategies (no composite analysis done), their results suggest a trend favouring substrate modification (0.72, 95% CI 0.44–1.18).
The potential advantage of substrate modification over standard ablation seen in our study might be related to multiple isthmuses and exits, or even multiple circuits, especially in complex scars, which are three-dimensional structures with intricate dynamics that would be missed by standard ablation. Additionally, the advantage of substrate modification seen in our study could be related to the persistence of electrical activity surrounding scar channels and isthmuses producing areas with the potential to generate VT substrates when performing standard ablation.17 Thus, the likelihood of eliminating these complex circuits might be higher when aiming to modify the substrate.
Complete vs. incomplete substrate modification
Whether more extensive ablation could be more successful at long-term was largely unknown until 2012 when several authors showed that complete substrate modification was associated with decreased VT recurrence as compared to an incomplete substrate modification.5,7 Similar results were recently published in the setting of non-ICM, showing the superiority of complete substrate modification based on scar homogenization compared to an incomplete substrate ablation strategy.15
A potential explanation for the benefit of complete substrate modification in these studies is that incomplete endocardial ablation does not eliminate all potential channels and re-entrant circuits. Complete substrate modification is also justifiable in view of the substrate complexity in different types of SHD. Thus, complete elimination of scar related abnormal signals is a relevant ablation goal, and is the basis of different substrate-based ablation strategies, which might explain the variable success rates of different substrate modification strategies, when a more extensive (i.e. homogenization) vs. a more conservative approach is used.17 Our meta-analysis is the first study to document this concept across different substrate-based strategies, showing that complete substrate modification is associated with a 61% risk reduction when compared to incomplete substrate modification, regardless of the strategy used, without increasing procedure, fluoroscopy, or radiofrequency times. These data are also essential to highlight the importance that no matter which technique is used, efforts should be made to accomplish the established ablation end point (e.g. in core isolation, creating ‘exit block’ of the core). Unfortunately, in clinical practice, operators don't usually have a clear endpoint when performing substrate modification, which can be worsened by fatigue after extensive ablation limiting the achievement of a specific ablation goal. Stereotaxis can potentially mitigate this limitation and should be further explored, especially when aiming for complete substrate modification.
Finally, our results highlight the paramount importance of selecting the appropriate ablation strategy, which should be made based on the underlying heart disease and potential arrhythmogenic substrate, taking into consideration the possible risks and efficacy of each strategy (i.e. substrate vs. standard ablation). Thus, based on our results, in patients with SHD, especially with an ischaemic substrate (in our study >70% had ICM), substrate-based ablation should be selected as the preferred treatment strategy with the goal of achieving complete substrate modification. However, is there any role combining substrate and standard strategies? Recent evidence illustrates that using both substrate (i.e. scar dechanneling) and standard ablation prolongs the procedure, radiation exposure, and the need for electrical cardioversion without improving acute results and long-term ablation outcomes, as compared to substrate ablation alone, with the caveat that most of the patients evaluated had an ischaemic substrate (37 out of 48 patients).19 Nonetheless, combining both strategies in patients with non-ischaemic substrates, who often have mid-myocardial scar, which is not evident in endocaridal or endocardial voltage maps might still be of value and needs further exploration.
Needless to say, understanding the substrate is the quintessence of VT ablation, and unfortunately it seems we are far from this with the current knowledge and technology. However, as mapping technologies evolve and ablation strategies develop, outcomes seems to improve and overcome the limitations encountered with entrainment mapping.20 Further studies are needed to continue clarifying what is the best ablation approach in specific clinical scenarios and substrates.
Limitations
Our meta-analysis has several potential limitations. First, there is only one randomized study in our analysis comparing both ablation strategies, which likely provides accurate assessment and comparison of both ablation approaches, in contrast to the other studies included. Second, the number of patients included in each of the studies is small in view of the specific population studied, hence the need to do a composite outcome. However, the use of the composite endpoint improves the resolving ability of the meta-analysis, strengthening its capacity to pick out weaker signals of effect from the background noise of sampling error. Third, the substrate modification strategies are all different between studies, hence it is difficult to generalize that all these are better than standard-ablation. Fourth, our results apply mostly to ICM and should not be generalized to non-ischaemic substrates. Fifth, as any meta-analysis including interventions, there is a potential for significant bias related to the operators’ experience from each study centre. Nonetheless, given the high quality of the studies included (as assessed by the NOS) our meta-analysis provides the best evidence currently available regarding the best ablation strategies for the treatment of VT in SHD.
Conclusion
In patients with SHD who had VT related mainly to ischaemic substrates, there was a significantly lower risk of the composite primary outcome of long-term VA recurrence and all-cause mortality among those undergoing substrate modification compared to standard ablation. Long-term success is improved when performing complete substrate modification.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: Dr Di Biase is a consultant for Stereotaxis, Biosense Webster, St Jude Medical and received speaker honorarium/travel reimbursement from Biotronik, Medtronic, Boston Scientific, Janssen, Pfizer and EpiEP. Dr Natale is a consultant and received speaker honorarium/travel from Boston Scientific, Biosense Webster, Medtronic, Biotronik, St. Jude Medical, EpiEP and Janssen. None of the remaining authors have disclosures to report.



