-
PDF
- Split View
-
Views
-
Cite
Cite
Sanghamitra Mohanty, Prasant Mohanty, Luigi Di Biase, Chintan Trivedi, Eli Hamilton Morris, Carola Gianni, Pasquale Santangeli, Rong Bai, Javier E Sanchez, Patrick Hranitzky, G Joseph Gallinghouse, Amin Al-Ahmad, Rodney P Horton, Richard Hongo, Salwa Beheiry, Claude S Elayi, Dhanunjaya Lakkireddy, Yaruva Madhu Reddy, Juan F Viles Gonzalez, J David Burkhardt, Andrea Natale, Long-term follow-up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation, EP Europace, Volume 19, Issue 11, November 2017, Pages 1790–1797, https://doi.org/10.1093/europace/euw338
Close - Share Icon Share
Abstract
Left atrial (LA) scarring, a consequence of cardiac fibrosis is a powerful predictor of procedure-outcome in atrial fibrillation (AF) patients undergoing catheter ablation. We sought to compare the long-term outcome in patients with paroxysmal AF (PAF) and severe LA scarring identified by 3D mapping, undergoing pulmonary vein isolation (PVAI) only or PVAI and the entire scar areas (scar homogenization) or PVAI+ ablation of the non-PV triggers.
Totally, 177 consecutive patients with PAF and severe LA scarring were included. Patients underwent PVAI only (n = 45, Group 1), PVAI+ scar homogenization (n = 66, Group 2) or PVAI+ ablation of non-PV triggers (n = 66, Group 3) based on operator’s choice. Baseline characteristics were similar across the groups. After first procedure, all patients were followed-up for a minimum of 2 years. The success rate at the end of the follow-up was 18% (8 pts), 21% (14 pts), and 61% (40 pts) in Groups 1, 2, and 3, respectively. Cumulative probability of AF-free survival was significantly higher in Group 3 (overall log-rank P <0.01, pairwise comparison 1 vs. 3 and 2 vs. 3 P < 0.01). During repeat procedures, non-PV triggers were ablated in all. After average 1.5 procedures, the success rates were 28 (62%), 41 (62%), and 56 (85%) in Groups 1, 2, and 3, respectively (log-rank P< 0.001).
In patients with PAF and severe LA scarring, PVAI+ ablation of non-PV triggers is associated with significantly better long-term outcome than PVAI alone or PVAI+ scar homogenization.
What’s new?
Pulmonary vein isolation plus ablation of non-PV triggers was not only safe without any major complications but also provided significantly higher success rate than PVAI alone or PVAI+ scar homogenization.
Both PVAI alone and PVAI+ scar homogenization approaches had very low success rate after single procedure.
Recurrence was associated with high prevalence of arrhythmogenic triggers from non-PV foci; mostly mapped to left atrial appendage and coronary sinus.
Ablation strategy was an independent predictor of long-term recurrence; compared to PVAI+ non-PV trigger ablation, patients undergoing ablation of PVAI only or homogenization of scar were about 3 times more likely to fail.
Ablation of non-PV triggers during repeat procedures resulted in significantly better outcome in Groups 1 (PVAI alone) and 2 (PVAI+ scar homogenization).
Introduction
Left atrial (LF) tissue fibrosis is a hallmark of arrhythmogenic structural remodelling of the cardiac substrate in atrial fibrillation (AF).1 By providing the substrate needed for the persistence of AF, it is believed to impact the success of catheter ablation, one of the most effective rhythm control strategies in AF.1,2 Atrial fibrosis can be a pathological endpoint in several settings such as advanced age, genetic predisposition, myocardial ischemia, mitral valve disease, and mechanical overload of the heart.3,4 Interestingly, studies have shown similar degree of fibrosis in patients with and without history of AF.5 Therefore, the causal association between LA scar and AF occurrence and persistence still remains to be defined.
After the seminal study by Haissaguerre et al. demonstrating pulmonary vein (PV) triggers initiating AF, PV isolation (PVI), and later PV antrum isolation (PVAI) have been widely performed in paroxysmal AF (PAF) patients with high success rate.6 However, based on results from multiple studies it is clear that PV triggers are not the only mechanism responsible for AF; PAF can be initiated by non-PV ectopic beats.7–9 Therefore, the clinical relevance of these non-PV drivers in influencing ablation-outcome cannot be undermined.
Although PAF patients present with severe LA scar from time to time, very little data are available to guide in deciding for the best ablation strategy in this unique subset of AF population. Therefore, we aimed to compare the long-term outcome of three ablation approaches, namely PVAI only and PVAI combined with either scar homogenization or non-PV triggers ablation, in PAF patients with extensive LA scar.
Methods
Study population
One-hundred seventy seven consecutive patients with paroxysmal AF and severe LA scarring undergoing their first ablation for AF at the participating centers from 2009 to 2012 were included in this prospective study. Patients presenting with non-paroxysmal AF or history of any previous catheter ablation, at our centre or elsewhere, were excluded from the study.
Patients in the study underwent ablation of the pulmonary vein antrum (PVAI) only (n = 45, Group 1), PVAI extended to the entire scar areas [scar homogenization (n = 66, Group 2)] or PVAI+ ablation of non-PV triggers (n = 66, Group 3). Choice of ablation strategy was determined by the operator. Flow-chart showing the study design in presented in Figure 1.
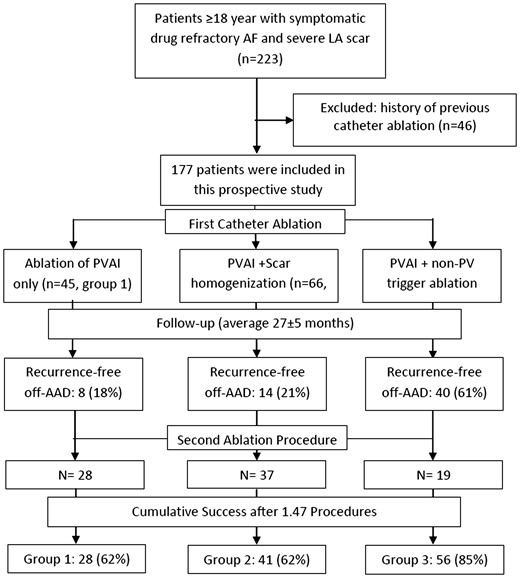
The flow-diagram showing the study design and outcomes after single and multiple ablation procedures.
Definitions
The LA scar was identified by detailed 3D voltage mapping of the LA. Mapping was always performed in sinus rhythm; low-voltage area was defined as a region with bipolar voltage amplitude <0.5 mV.10,–12 The degree of scar was described as severe when > 60% of the LA area was involved (7, 8) (Figure 2).
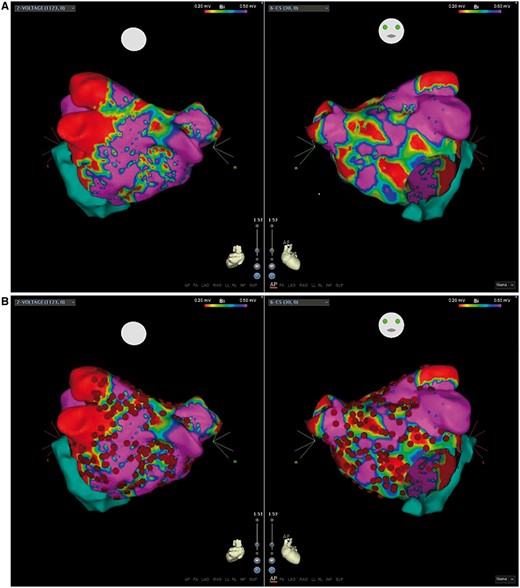
(A) A left atrial bipolar voltage map showing extensive low-voltage areas defined as <0.5 mV; regions in red show scar and other colored regions represent abnormal myocardium, purple regions represent normal myocardium. (B) Ablation points (red dots) illustrating the scar homogenization approach.
Non-PV triggers were defined as ectopic triggers originating from sites other than pulmonary veins such as interatrial septum, superior vena cava, LA appendage (LAA), crista terminalis, and coronary sinus (CS). Both sustained triggers (>30 s) as well as non-sustained drivers including repetitive short-lasting bursts of arrhythmia (<30 s) or premature atrial contractions (≥10 bpm) with earliest activation from non-PV sites were targeted for ablation (Figure 3).
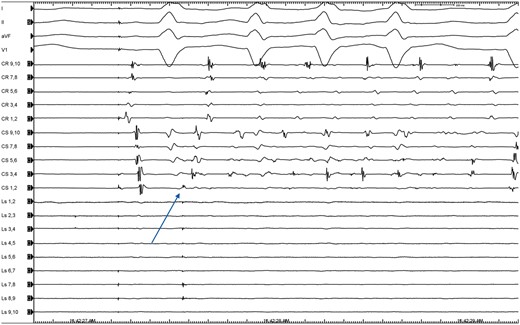
Representative intracardiac electrogram showing a trigger from the coronary sinus in one of the patients included in the analysis; the Lasso is positioned in the LA appendage and shows premature atrial contractions (arrow) during isoproterenol challenge.
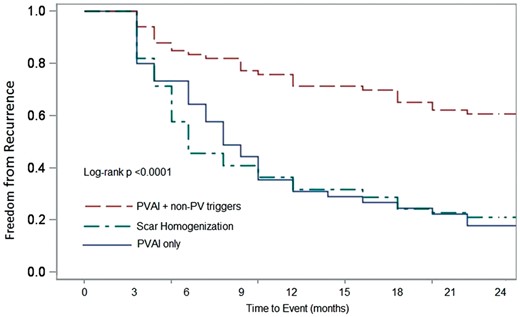
Kaplan–Meier log-rank test indicated the probability of AF-free survival after the first procedure was significantly higher in PVAI+ non-PV trigger ablation group.
Ablation procedure
Pre-ablation electro-anatomic mapping of the scar was conducted in all patients. Scar mapping was always performed in sinus rhythm; if the patient was in AF/atrial tachycardia (AT), cardioversion was carried out prior to mapping. Mapping was performed with the CARTO 3D system (Biosense Webster, Inc., Diamond Bar, CA, USA) and 3.5-mm open irrigated tip ablation catheter (3.5 mm distal electrode separated by 2 mm from a 2-mm proximal electrode) guided by intra-cardiac echocardiogram (ICE) and fluoroscopy. Mean number of points taken to map the extent of the scar was 271.2 ± 96.3.
Standard institutional protocol was followed for the catheter ablation procedures as described in earlier publications from our group.9 Briefly, PVAI and electrical isolation of the posterior wall between the pulmonary veins were performed guided by circular mapping catheter, intra-cardiac echocardiography and a 3D mapping system. Complete abolition of all PV potentials rather than decrease in the amplitudes was the end point and confirmed by entrance and/or exit block. If ablation was unsuccessful in terminating the arrhythmia, cardioversion was performed to restore sinus rhythm. In Group 2, PVAI was extended to the entire scar area (Figure 2) and RF energy was delivered until all abnormal potentials in the low-voltage areas were eliminated. After PVAI, high-dose isoproterenol infusion up to 30 µg/min for 15-20 min was given to detect PV reconnection and non-PV triggers; the later were ablated using additional radiofrequency energy in the Group 3 population only. During repeat procedure, non-PV triggers were ablated in all. For triggers originating from CS, LAA and the superior vena cava, isolation of these structures was the endpoint. For all other non-PV foci, inability to provoke the trigger with repeat isoproterenol infusion was considered as the endpoint.
Follow-up
After overnight observation following ablation, patients were discharged on their previously ineffective antiarrhythmic drugs (AADs) that were continued during the blanking period (12 weeks). After the blanking period AADs were discontinued. In case of recurrence after the blanking period, patients were given either previously ineffective AADs or new anti-arrhythmic agents or were scheduled for repeat-ablation.
Follow-up was performed at 3, 6, 9, and 12 months after the procedure and every 6-9 months thereafter, with cardiology evaluation, 12-lead electrocardiogram (ECG), and 7-day Holter monitoring.
Data collection
Demographic and baseline clinical characteristics, pre- and post-ablation medication use, procedural parameters, and post-ablation follow-up data were collected for all patients. Data were collected using the Institutional Review Board (IRB) approved AF Registry.
Study objective
The primary objective of this study was to assess the benefit of the ablation strategy that involves ‘PVAI+ ablation of non-PV triggers’, over PVAI alone or PVAI extended to the entire scar area, among patients with severe LA scarring.
The secondary objective was to evaluate if PVAI + non-PV ablation strategy was safe when compared with the other two techniques.
Endpoint
Arrhythmia was the primary end point of the study. Recurrence was defined as documentation of AF, atrial flutter (AFL), or atrial tachycardia (AT) of > 30 s duration off antiarrhythmic drugs (AAD) at follow-up. Any episodes that occurred during the first 12-weeks (blanking period) after the procedure were not considered as recurrence.
Peri-procedural complications and major adverse events during follow-up were the considered as secondary endpoint.
Statistical analysis
Patient characteristics
Continuous data are described as mean ± standard deviation [median and inter-quartile range (IQR) for non-normal data] and as counts and percent if categorical. Student’s t-test and χ2 tests were used to compare groups.
Endpoint evaluation
The primary endpoint, arrhythmia recurrence was compared across the groups by unadjusted log-rank test and Kaplan–Meier curves were generated. Patients who were recurrence-free at the end of follow-up were censored and duration from procedure date to data analysis cut-off date was considered as event-free survival time. A censoring variable was created to identify censored observations. Any event during the blanking period was not considered as failure.
A multivariate Cox proportional hazards regression analysis was performed assessing independent predictors of arrhythmia recurrence. Potential confounders were entered into the model based on known or expected clinical relevance, regardless of their statistical significance at univariate analysis. Controlling variables used in the model were age, gender, diabetes mellitus, dyslipidemia, body mass index, and LA diameter. Hazard ratio (HR) and 95% confidence interval (CI) of AF recurrence were computed. Proportional hazards assumption for the covariates was tested by the Schoenfeld residual analysis.
Secondaryendpoint: incidence of peri-procedural complications was compared between the cohorts using Fisher’s exact test.
All tests were two-sided and a P-value of <0.05 was considered statistically significant. Analyses were performed using SAS software version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
Left atrial scar was detected in 1315 of 4112 (31.9%) PAF cases undergoing catheter ablation during that time period, of which 1097 had mild or moderate LA scar and were excluded. Of the 218 cases with severe scar, 41 were excluded because of repeat procedures and 177 patients were included in the study. Baseline clinical characteristics were not different between patients undergoing PVAI only (Group 1: 63 ± 9 years, 71% male, BMI 28 ± 8, LA size 40.7 ± 5.1 mm, failed 2.6 ± 1.2 AADs), PVAI+ homogenization of scar (Group 2: 58 ± 10 years, 71% male, BMI 28 ± 7, LA size 40.3 ± 4.2 mm, failed 2.2 ± 1.8 AADs), and PVAI+ non-PV trigger ablation (Group 3: 60 ± 11 years, 73% male, BMI 29 ± 6, LA size 42.3 ± 7.2 mm, failed 2.7 ± 1.7 AADs). The baseline values are presented in Table 1.
| . | PVAI alone (Group 1, n = 45) . | PVAI+ scar homogenization (Group 2, n = 66) . | PVAI+ non-PV triggers (Group 3, n = 66) . | P-value . |
|---|---|---|---|---|
| Age | 63 ± 9 | 58 ± 10 | 60 ± 11 | 0.23 |
| Male | 32 (71%) | 47 (71%) | 48 (73%) | 0.91 |
| AF duration (year) | 6 ± 4.3 | 6 ± 4.1 | 6 ± 4.2 | 1.00 |
| BMI | 28 ± 8 | 28 ± 7 | 29 ± 6 | 0.65 |
| Hypertension | 29 (64%) | 44 (68%) | 46 (70%) | 0.84 |
| Diabetes | 3 (7%) | 6 (9%) | 5 (8%) | 1.00 |
| CAD | 10 (22%) | 16 (24%) | 17 (26%) | 0.91 |
| LA size (mm) | 40.7 ± 5.1 | 40.3 ± 4.2 | 42.3 ± 7.2 | 0.11 |
| LV EF % | 55 ± 4 | 54 ± 9 | 54 ± 7 | 0.73 |
| Failed AADs | 2.6 ± 1.2 | 2.2 ± 1.8 | 2.7 ± 1.7 | 0.75 |
| Fluoro time (min) | 62 ± 19 | 66 ± 20 | 71 ± 22 | 0.03 |
| RF time (min) | 70 ± 32 | 74 ± 34 | 86 ± 30 | 0.02 |
| Procedure time (hour) | 2.4 ± 1.3 | 2.6 ± 1.1 | 3.2 ± 1.2 | <0.001 |
| . | PVAI alone (Group 1, n = 45) . | PVAI+ scar homogenization (Group 2, n = 66) . | PVAI+ non-PV triggers (Group 3, n = 66) . | P-value . |
|---|---|---|---|---|
| Age | 63 ± 9 | 58 ± 10 | 60 ± 11 | 0.23 |
| Male | 32 (71%) | 47 (71%) | 48 (73%) | 0.91 |
| AF duration (year) | 6 ± 4.3 | 6 ± 4.1 | 6 ± 4.2 | 1.00 |
| BMI | 28 ± 8 | 28 ± 7 | 29 ± 6 | 0.65 |
| Hypertension | 29 (64%) | 44 (68%) | 46 (70%) | 0.84 |
| Diabetes | 3 (7%) | 6 (9%) | 5 (8%) | 1.00 |
| CAD | 10 (22%) | 16 (24%) | 17 (26%) | 0.91 |
| LA size (mm) | 40.7 ± 5.1 | 40.3 ± 4.2 | 42.3 ± 7.2 | 0.11 |
| LV EF % | 55 ± 4 | 54 ± 9 | 54 ± 7 | 0.73 |
| Failed AADs | 2.6 ± 1.2 | 2.2 ± 1.8 | 2.7 ± 1.7 | 0.75 |
| Fluoro time (min) | 62 ± 19 | 66 ± 20 | 71 ± 22 | 0.03 |
| RF time (min) | 70 ± 32 | 74 ± 34 | 86 ± 30 | 0.02 |
| Procedure time (hour) | 2.4 ± 1.3 | 2.6 ± 1.1 | 3.2 ± 1.2 | <0.001 |
| . | PVAI alone (Group 1, n = 45) . | PVAI+ scar homogenization (Group 2, n = 66) . | PVAI+ non-PV triggers (Group 3, n = 66) . | P-value . |
|---|---|---|---|---|
| Age | 63 ± 9 | 58 ± 10 | 60 ± 11 | 0.23 |
| Male | 32 (71%) | 47 (71%) | 48 (73%) | 0.91 |
| AF duration (year) | 6 ± 4.3 | 6 ± 4.1 | 6 ± 4.2 | 1.00 |
| BMI | 28 ± 8 | 28 ± 7 | 29 ± 6 | 0.65 |
| Hypertension | 29 (64%) | 44 (68%) | 46 (70%) | 0.84 |
| Diabetes | 3 (7%) | 6 (9%) | 5 (8%) | 1.00 |
| CAD | 10 (22%) | 16 (24%) | 17 (26%) | 0.91 |
| LA size (mm) | 40.7 ± 5.1 | 40.3 ± 4.2 | 42.3 ± 7.2 | 0.11 |
| LV EF % | 55 ± 4 | 54 ± 9 | 54 ± 7 | 0.73 |
| Failed AADs | 2.6 ± 1.2 | 2.2 ± 1.8 | 2.7 ± 1.7 | 0.75 |
| Fluoro time (min) | 62 ± 19 | 66 ± 20 | 71 ± 22 | 0.03 |
| RF time (min) | 70 ± 32 | 74 ± 34 | 86 ± 30 | 0.02 |
| Procedure time (hour) | 2.4 ± 1.3 | 2.6 ± 1.1 | 3.2 ± 1.2 | <0.001 |
| . | PVAI alone (Group 1, n = 45) . | PVAI+ scar homogenization (Group 2, n = 66) . | PVAI+ non-PV triggers (Group 3, n = 66) . | P-value . |
|---|---|---|---|---|
| Age | 63 ± 9 | 58 ± 10 | 60 ± 11 | 0.23 |
| Male | 32 (71%) | 47 (71%) | 48 (73%) | 0.91 |
| AF duration (year) | 6 ± 4.3 | 6 ± 4.1 | 6 ± 4.2 | 1.00 |
| BMI | 28 ± 8 | 28 ± 7 | 29 ± 6 | 0.65 |
| Hypertension | 29 (64%) | 44 (68%) | 46 (70%) | 0.84 |
| Diabetes | 3 (7%) | 6 (9%) | 5 (8%) | 1.00 |
| CAD | 10 (22%) | 16 (24%) | 17 (26%) | 0.91 |
| LA size (mm) | 40.7 ± 5.1 | 40.3 ± 4.2 | 42.3 ± 7.2 | 0.11 |
| LV EF % | 55 ± 4 | 54 ± 9 | 54 ± 7 | 0.73 |
| Failed AADs | 2.6 ± 1.2 | 2.2 ± 1.8 | 2.7 ± 1.7 | 0.75 |
| Fluoro time (min) | 62 ± 19 | 66 ± 20 | 71 ± 22 | 0.03 |
| RF time (min) | 70 ± 32 | 74 ± 34 | 86 ± 30 | 0.02 |
| Procedure time (hour) | 2.4 ± 1.3 | 2.6 ± 1.1 | 3.2 ± 1.2 | <0.001 |
Intra-procedural parameters
All patients had symptomatic AF refractory to conventional antiarrhythmic therapy. Persistence of AF/AT at the end of the procedure was observed in 24 (21%) patients; sinus rhythm was achieved in these patients by cardioversion. The mean fluoroscopy time, procedure time, and radiofrequency times were relatively higher in Group 3 patients (Table1). In Group 3, the non-PV triggers were mostly clustered in areas such as CS (56%), LAA (62.2%), inter-atrial septum (22.7%), and crista terminalis/superior vena cava (7.5%).
Outcome
Primary outcome—arrhythmia recurrence
All patients in the study were included in the primary analysis. Patients were followed-up for a minimum of 2 years, and the average follow-up duration for the study was 27 ± 5 months. After the first procedure, 8 (18%) in Group 1, 14 (21%) in Group 2, and 40 (61%) in Group 3 were recurrence-free off-AAD (log-rank P < 0.0001). The pairwise log-rank test indicated that the cumulative probability of AF-free survival was significantly higher in Group 3 (Group 1 vs. 3 and Group 2 vs. 3 was significant at P < 0.01). The Kaplan–Meier curve comparing cumulative freedom from recurrence off-AADs across the three groups after the first procedure is presented in Figure4.
Predictor of arrhythmia recurrence
Multivariable analysis for AF recurrence was performed using the Cox model. The covariates in the model are described in the statistical analysis section. After adjusting for the covariates, ablation strategy emerged as an independent predictor of long-term recurrence. Compared with PVAI+ non-PV trigger ablation, patients undergoing ablation of PVAI only (HR 3.2 [95% CI 2.0–5.1], P < 0.001) or homogenization of scar (HR 2.7 [95% CI 1.5–4.3], P < 0.001) were about three times more likely to fail.
Peri-procedural complications
No major procedural complications were observed in any of the study groups. One patient in Group 2 (1.5%) had minor pericardial effusion that was conservatively managed with fresh-frozen plasma and protamine. One groin hematoma in Group 1 (2.2%) and 1 (1.5%) in Group 3 were reported. The distribution of peri-procedural complications did not show any significant association with ablation strategy. No incidence of stroke or any other major adverse events were reported during follow-up of the study population.
Repeat procedure
Repeat procedure was offered to all 115 patients who failed the first procedure. A total of 84 patients; 28 of 37 (76%) patients in Group 1, 37 of 52 (71%) in Group 2, and 19 of 26 (73%) in Group 3, underwent repeat ablation. Recurrence of organized AT/AFL was observed in 15/28 (53.5%), 21/37 (56.7%), and 14/19 (73.7%) in Groups 1, 2, and 3, respectively. During the repeat procedure reconnection of the pulmonary veins was detected in in 12 (14.3%) patients, while non-PV triggers were detected in 74 of 84 (88.1%) patients. Mean number of non-PV triggers was 2.36 ± 0.8. The non-PV foci were most commonly mapped to LAA (69%), CS (64%), interatrial septum (22%), and crista terminalis/superior vena cava (9%). Of the 74 patients with non-PV triggers, 60 (81.1%) had more than 1 non-PV focus identified. The non-PV triggers were mostly (90.5%) located in areas outside the scar region (Figure 3). Sixty-three (75%) patients [20 of 28 (71%) in Group 1, 27 of 37 (73%) Groups 2, and 16 of 19 (84%) in Group 3] remained recurrence free after 9 ± 3 months of the second procedure.
After average 1.5 procedures, the cumulative success rates among the groups were 28 (62%) in Group 1, 41 (62%) in Group 2, and 56 (85%) in Group 3 (log-rank P < 0.001).
Discussion
To the best of our knowledge, this prospective analysis is the first study to compare the efficacy and safety of three ablation approaches in paroxysmal AF patients presenting with severe LA scar. Our major findings were the following: (i) PVAI+ ablation of non-PV triggers was not only safe without any major complications but also provided significantly higher success rate than PVAI alone or PVAI+ scar homogenization, (ii) scar homogenization combined with PVAI did not provide any additional advantage compared with PVAI alone; both approaches had very low-success rate after single procedure, (iii) recurrence was associated with high prevalence of arrhythmogenic triggers from non-PV foci, (iv) ablation strategy was an independent predictor of long-term recurrence, and (v) ablation of non-PV triggers during repeat procedures resulted in significantly better outcome in Groups 1 and 2.
Pulmonary vein isolation is the cornerstone of ablation procedures for PAF patients. However, its efficacy is limited by a considerable rate of recurrence of arrhythmia including AF, AT, and AFL. Several factors impacting procedural-outcome have been identified of which the ablation strategy is notable. Attainment of permanent PV isolation, the presence of triggers outside PVs and ablation of these extra-PV triggers at the time of first procedure may significantly influence recurrence rate.13 PVAI has a reported single-procedure off-drug success rate in the range of 80%.14 However, we observed a very high-recurrence rate (82%) after PVAI alone in the current study. Verma et al.10 had reported 57% recurrence rate post-index procedure in AF patients with pre-existent scar. The reason they had a relatively lower recurrence rate can be the inclusion of patients with varying degree of scarring in their population whereas all our patients had severe left atrial scarring (LAS).10 Although there is an ongoing debate on whether voltage mapping and late-gadolinium enhancement magnetic resonance imaging (LGE-MRI) are equally effective or not in assessing scar regions, there is a consensus among electrophysiologists that the presence of severe LAS characterizes a population that has highest risk of recurrence after PVAI.1,15 Oakes et al.11 reported the rate of recurrence at 14% with minimal enhancement, 43.3% with moderate enhancement and 75% with extensive enhancement with DE-MRI. Based on their findings, they suggested that PVAI for AF with extensive scarring should be offered with a reduced expectation of long-term success.11 The recurrence rate of 82% after PVAI alone in our study population with severe scarring was in agreement with their result. By altering the atrial substrate, scarring may increase vulnerability to AF induction by sources apart from the PV antral area.10 In fact, we detected a very high prevalence of triggers originating from extra-PV regions during the repeat procedures.
Ectopic triggers from non-PV sites can be responsible for AF initiation; it has been detected in up to 69% of PAF cases, as reported in recent publications.7,9,16 In a study conducted by our group, the very late recurrences in PAF patients were due to non-PV triggers in the presence of permanent isolation of PVs and ablation of those yielded substantially higher cumulative success (87%) after a decade of follow-up.8 The significance of non-PV triggers have been further illustrated by Hayashi et al.; in their trial the recurrence rate was 68% in patients with AF originating from non-PV foci but without all foci being ablated vs. 8.8% in patients with AF originating from non-PV foci and all the foci successfully ablated (P < 0.0001).22 In an earlier publication, we have also reported similar findings in PAF patients with reduced left ventricular function (75 vs. 32.2% success rate in non-PV triggers ablated vs. not ablated respectively, P < 0.001).9
The presence of pre-existent LAS of varying degrees is observed in PAF patients undergoing their first catheter ablation.18 In the electroanatomic mapping, these scars are primarily seen in the posterior wall and the antero-septal region.11 As our standard PVAI procedure anyway involved electrical isolation of the posterior wall between the PVs, we did not observe any additional benefit when scar homogenization per se was combined with standard PVAI in the Group 2 patients compared with PVAI only in Group 1. Moreover, in the current study the non-PV triggers were mostly found to be located in areas with higher voltage and rarely seen in the scar tissue, which was in accordance with earlier findings from our group.9 Therefore, when these non-PV triggers were not targeted for ablation during the index procedure in PVAI only and PVAI+ scar homogenization group, the success rate was low and improved substantially after the repeat procedure that included ablation of non-PV triggers in all. These results propose that non-PV foci are predominantly associated with diseased atrial substrate and should be addressed when performing AF ablation in PAF patients with severe LA scar.
Pulmonary vein reconnection was not the predominant finding (11.9%) in patients with recurrence in our study population in contrast to earlier published reports with reconnection rate from 58 to 94%.19,20 This differential observation can be due to our standard practice of using higher power (40 W) during ablation and high-dose isoproterenol challenge at the end of the procedure to reveal PV reconnection which upon detection was ablated by additional application of RF energy until all potentials were abolished. These steps presumably minimized the chance of recovery of conduction.
The ablation strategy was detected to be an independent predictor of AF recurrence in the current study. Compared with PVAI+ non-PV trigger ablation, patients undergoing ablation of PVAI only or PVAI+ scar homogenization were found to be three times more likely to fail. Previous evidences corroborate with our results. Earlier studies have shown that the presence of non-PV triggers during the index procedure, even in PAF patients, is associated with higher arrhythmia recurrence at long-term.9,17,21 Based on their study findings, Chen et al. emphasized that the provocation of non-PV triggers should be considered in both the first ablation procedure and the redo ablation procedures, and not only limited to the redo procedures.21 These evidences collectively suggest the necessity to abolish all non-PV foci to improve the outcomes of PAF ablation procedures as well as prevent its progression into persistent AF.17
Lastly, the scar homogenization procedure was technically not more demanding in terms of duration and fluoroscopic time compared to PVAI alone, unlike PVAI + non-PV triggers ablation approach that required significantly longer procedure, radiofrequency, and fluoroscopic time. However, this extensive ablation approach of PVAI+ ablation of non-PV triggers proved to be a safe and more effective treatment strategy in PAF patients with severe left atrial scarring.
Limitations
Certain limitations in our study need to be acknowledged: (i) non-randomized study design with inherent risk of bias, (ii) without continuous atrial-rhythm monitoring device, recurrence rates may have been underestimated although patients were closely followed up by regular office visits, 7-day Holter monitoring every 3 months and phone calls by our research staffs regarding AF symptoms, (iii) small sample size; however, it is similar to the sample size of other more definitive studies in the field of catheter ablation where different ablation approaches have been compared in paroxysmal AF patients, (iv) our definition of scar was based solely on voltage mapping; but it is a validated method and has been used extensively in other published studies.22
Conclusions
In patients with paroxysmal AF and severe left atrial scarring, scar homogenization did not prove to be the most effective strategy; PVAI+ ablation of non-PV triggers was associated with significantly better long-term outcome than PVAI alone or PVAI+ scar homogenization as the first procedure. Moreover, recurrences were majorly associated with non-pulmonary vein triggers that were largely detected outside the scar regions and ablation of those during repeat procedures resulted in substantial improvement in the procedure outcome in this population.
Conflict of interest: A.N. received speaker honorariums from Boston Scientific, Biosense Webster, St Jude Medical, Biotronik and Medtronic, and is a consultant for Biosense Webster St Jude Medical and Janssen. J.D.B. is a consultant for Biosense-Webster and Stereotaxis. L.D.B. is a consultant for Biosense Webster, Boston Scientific Stereotaxis and St Jude Medical, and received speaker honoraria/travel from Medtronic, Atricure, EPiEP and Biotronik. All the remaining authors have no disclosures.



