-
PDF
- Split View
-
Views
-
Cite
Cite
Pietro Palmisano, Michele Accogli, Ennio Carmine Luigi Pisanò, Maria Zaccaria, Sergio De Blasi, Maria Antonietta Ponzetta, Sergio Valsecchi, Giovanni Milanese, Maurelio Lauretti, Francesco Magliari, Reduced long-term overall mortality in heart failure patients with prolonged QRS treated with CRT combined with ICD vs. heart failure patients with narrow QRS treated with ICD only, EP Europace, Volume 18, Issue 9, September 2016, Pages 1374–1382, https://doi.org/10.1093/europace/euv347
Close - Share Icon Share
It is not known whether heart failure (HF) patients with prolonged QRS who undergo cardiac resynchronization therapy combined with a defibrillator (CRT-D) have a prognostic advantage over HF patients with narrow QRS (therefore without indication for CRT) treated with an implantable cardioverter defibrillator (ICD) only. The aim of this study was to compare the long-term mortality of a group of HF patients with prolonged QRS receiving CRT-D with that of a similar group of patients with narrow QRS receiving ICD only.
A total of 312 patients (mean age 66 ± 13 years; 84% male, mean left ventricular ejection fraction 25 ± 4%, mean New York Heart Association class 2.6 ± 0.5) were included in the analysis. Of these, 138 with a QRS complex duration ≥120 ms received a CRT-D. During follow-up, the time and cause of death were assessed. During a median follow-up of 46 months, CRT-D patients showed significantly lower overall mortality (P = 0.038). Compared with patients receiving ICD only, CRT-D patients showed lower HF mortality (P = 0.003). Coronary mortality, non-cardiac mortality, and sudden mortality were similar in both groups (all P > 0.05). A positive response to CRT was an independent predictor of reduced mortality on multivariate analysis (hazard ratio: 0.27; P = 0.047).
In HF patients treated with ICD, the subgroup of patients with prolonged QRS who receive CRT-D displays better long-term survival than narrow QRS ICD recipients, owing to their reduced HF mortality.
In patients undergoing ICD treatment for heart failure and severe left ventricular dysfunction, the subgroup of patients with intraventricular conduction delay who receive cardiac resynchronization therapy combined with ICD displays better long-term survival than those on ICD treatment alone.
The better long-term survival of heart failure patients treated with cardiac resynchronization therapy combined with ICD is the result of reduced heart failure mortality in the subgroup of patients who respond positively to cardiac resynchronization therapy.
Introduction
Patients with dilated cardiomyopathy (DCM) and severe left ventricular (LV) systolic dysfunction have a poor long-term prognosis as a result of their higher mortality due to heart failure (HF) and sudden death (SD). The implantable cardioverter defibrillator (ICD), by effectively preventing sudden arrhythmic death, has significantly improved the survival of these patients.1,2 In patients with intraventricular conduction delay (prolonged QRS complex duration), combining cardiac resynchronization therapy (CRT) with ICD (CRT-D) improves functional class and quality of life and reduces both HF hospitalizations and overall mortality3–7 in comparison with ICD only.
Although it is known that DCM patients with prolonged QRS who are treated with CRT-D have a prognostic advantage over DCM patients with prolonged QRS treated with ICD only, it is not known whether they also have a better prognosis than DCM patients with normal QRS (therefore without indication for CRT) treated with ICD only. Accordingly, the primary aim of this observational, two-centre study was to compare long-term overall mortality in a group of patients with DCM, severe LV systolic dysfunction, and prolonged QRS, who were treated with CRT-D, with that of a group of patients with similar characteristics, but without intraventricular conduction delay, who were treated with ICD only. The secondary aim was to compare the causes of death in both patient groups.
Methods
Study population
We evaluated consecutive patients who had undergone ICD implantation at the two participating centres. Only patients who met the following inclusion criteria were enrolled in the study: (i) DCM (irrespectively of the aetiology) with severe LV systolic dysfunction [LV ejection fraction (LVEF) ≤30%]; (ii) moderate-to-severe functional limitation [New York Heart Association (NYHA) class ≥II]; and (iii) in sinus rhythm. Dilated cardiomyopathy was defined as a reduced LVEF in the presence of increased LV dimension (LV end-diastolic size >115% of that calculated for age and body surface area).8
In accordance with current guidelines,9 patients with a QRS complex duration ≥120 ms received a biventricular ICD.
Device implantation was performed in accordance with ICD and CRT guidelines9,10 by four expert electrophysiologists. The implantation technique used was based on the clinical practice of the two participating centres. For CRT-D implantation, the target position for the coronary sinus lead tip was preferably the lateral or posterolateral wall, midway between base and apex.
The initial device programming was performed by the implanting physician immediately after ICD implantation. For single-chamber device, the programmed pacing mode was VVI with lower rate of 40 b.p.m. Dual and biventricular chamber units were programmed in DDD or DDDR pacing mode in the presence of sinus node disease and/or chronotropic incompetence, with a lower rate limit between 40 and 70 b.p.m. and a maximum, sensor-driven pacing rate between 100 and 130 b.p.m. In ICD-only group, sensed and paced atrioventricular (AV) delays were programmed to promote intrinsic AV conduction, and automatic algorithms designed to minimize unnecessary right ventricular pacing were turned on. In the CRT-D group, the AV delay was programmed empirically to ensure biventricular pacing with a range of 80–120 ms for sensed AV delay, with an additional offset of 30–50 ms for paced AV delay. Ventriculo-ventricular delay was programmed to achieve the narrowest QRS on the surface ECG. In both study groups, tachycardia detection and therapy parameters were programmed at the discretion of the implanting physician taking into consideration the characteristics of each patient.
Data regarding ICD implantation and baseline characteristics of patients were collected prospectively at the two centres for an observational ICD registry and retrospectively analysed for the purpose of this study.
Follow-up
During follow-up, all patients were evaluated regularly at 6-month intervals, or more frequently when clinically indicated. Follow-up evaluations consisted of outpatient clinic visits including echocardiographic assessment of LV volumes, in-hospital or remote device interrogation, and telephone contacts.
In the subgroup of patients with a biventricular ICD, the response to CRT was evaluated 6 months after implantation. Cardiac resynchronization therapy responders were defined as patients who showed an NYHA class improvement of ≥1, who have not been hospitalized for decompensated chronic HF, and who showed an absolute increase ≥10% in LVEF and/or a decrease ≥15% in LV end-systolic volume (LVESV).11,12
In those ICD-only patients who developed an intraventricular conduction delay or bradycardia requiring permanent or frequent (>40%) right ventricular pacing during follow-up, the pacing system was upgraded to biventricular on elective replacement of the device or earlier if there was worsening of HF.9
Assessment of survival status and causes of death
The primary endpoint of the study was death from any cause. Data on patient survival and the causes of death were assessed and collected retrospectively for all patients.
If patients missed a scheduled in-hospital follow-up visit, they or their relatives were contacted by phone. If this contact revealed that the patient had died, the time and cause of death were carefully assessed. If death occurred in one of the two participating hospitals, this information was retrieved from local hospital databases, which are updated regularly. If death occurred in a hospital setting other than the two participating centres, the cause of death was determined by consulting the medical records made during the hospitalization period when death occurred and the results of autopsy (when performed). When death did not occur in a hospital setting, information on the circumstances was provided by the medical reports from the physicians who provided first aid and certified the death, and by family members or witnesses, when available.
Deaths were classified according to a modified Hinkle–Thaler classification and categorized into three predefined groups: non-sudden cardiac death, non-cardiac death, and SD.13 The non-sudden cardiac death group was further categorized into HF death, coronary death, and cardiac death but unable to classify further. HF death was diagnosed when patients died of terminal HF, progressive failure of cardiac pump function, or cardiac asthma under maximum inotropic drug support.14 Coronary death was diagnosed when patients died of an acute coronary event with diagnostic changes on serial ECGs or with serum cardiac enzyme concentrations at least twice the normal limit or with coronary artery findings (on autopsy) of acute coronary occlusion.15 The non-cardiac death group was further divided into cerebrovascular death, cancer death, death from pulmonary disease, and non-cardiac death but unable to classify further. Sudden death was defined as death occurring within 1 h of symptoms, during sleep or unwitnessed, in a previously medically stable patient.16 Patients who died suddenly, but with a documented alternative mode of death, were categorized as non-sudden cases (e.g. the mode of death of someone who died suddenly of documented acute myocardial infarction was categorized as ‘cardiac, coronary death’ and not as SD).
Statistical analysis
Descriptive statistics were reported as means ± standard deviations for normally distributed continuous variables and compared by means of Student's t-test and analysis of variance. Continuous variables with skewed distribution were reported as medians with 25–75th percentiles. Categorical data were expressed as percentages, reported in contingency tables, and compared by means of χ2 test or Fisher's exact test, as appropriate. Event and event-free curves were based on Kaplan–Meier analyses, stratified by study group, and compared by means of the log-rank test. The cumulative probability of an event was estimated with its standard error. An individual was censored in the following cases: when death occurred; when the pacing system was removed; when heart transplantation had been performed; in patients receiving CRT-D, when the coronary sinus lead was inactivated for any reason; and finally, in patients receiving ICD only, when the pacing system was upgraded to biventricular. The effect of individual variables on survival was investigated by using univariate Cox proportional hazards models. Variables that showed an effect on survival with a significance level less than 0.2 on univariate analyses were entered into multivariate Cox proportional hazards models. Variables identified as independent predictors of mortality in previous studies17 (specifically: advanced age, higher NYHA class, lower LVEF, prolonged QRS duration, ischaemic cardiomyopathy, chronic kidney disease, and chronic obstructive pulmonary disease) were also included in the multivariate analysis model. Cox model findings are presented as hazard ratios (HRs), tests of significance, and 95% confidence intervals (CIs). Interactions between the covariates were tested for significance in the model. P-values of less than 0.05 were considered statistically significant. The data were analysed by means of the statistical software package Statistica version 6.1 (StatSoft Inc., Tulsa, OK, USA).
Results
Patient characteristics
The initial cohort evaluated for the aim of this study consisted of 541 consecutive patients undergoing ICD implantation in the two participating centres. According to the inclusion criteria listed earlier, the following patients were excluded from the study: 58 patients with arrhythmogenic cardiomyopathies or channelopathies, without significant LV dysfunction; 127 patients with non-severe LV dysfunction (LVEF > 30%); and 44 patients with permanent atrial fibrillation. The remaining 312 patients represented the study population. Of these, 138 received CRT-D. Table 1 shows the baseline patient characteristics of the overall study population and the comparison between patients receiving CRT-D and those receiving ICD only. Patients receiving CRT-D were older, had a longer pre-implantation QRS duration, and a higher LVESV. Moreover, these patients were less likely to have cardiomyopathy of ischaemic aetiology. Other characteristics were similar in the two groups. Table 2 provides details on QRS morphology in patients receiving CRT-D and the distribution of the LV pacing lead position.
Characteristics of the study population and comparison between patients receiving CRT-D and patients receiving ICD only
| Characteristics . | All patients (n = 312) . | Patients receiving CRT-D (n = 138) . | Patients receiving ICD only (n = 174) . | P-value . |
|---|---|---|---|---|
| Indication of ICD | ||||
| Primary prevention, n (%) | 296 (94.9) | 133 (96.4) | 163 (93.7) | 0.283 |
| Secondary prevention, n (%) | 16 (5.1) | 5 (3.6) | 11 (6.3) | 0.283 |
| Baseline characteristics | ||||
| Male, n (%) | 261 (83.7) | 113 (81.9) | 148 (85.1) | 0.452 |
| Age (years), mean + SD | 66.4 ± 12.6 | 68.2 ± 10.6 | 64.9 ± 13.9 | 0.021 |
| QRS duration (ms), mean + SD | 120.0 ± 36.7 | 154.4 ± 29.4 | 94.5 ± 13.2 | <0.001 |
| LVESV (cc), mean ± SD | 136.7 ± 26.7 | 140.8 ± 28.0 | 133.3 ± 25.2 | 0.014 |
| LVEF (%), mean ± SD | 25.5 ± 4.3 | 25.0 ± 4.5 | 25.9 ± 4.1 | 0.069 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.5 ± 0.5 | 0.078 |
| Aetiology | ||||
| Ischaemic cardiomyopathy, n (%) | 181 (58.0) | 71 (51.4) | 110 (63.2) | 0.036 |
| Non-ischaemic cardiomyopathy, n (%) | 131 (42.0) | 67 (48.6) | 64 (36.8) | 0.036 |
| Associated disorders | ||||
| Hypertension on therapy, n (%) | 222 (71.2) | 102 (73.9) | 120 (69.0) | 0.338 |
| Diabetes, n (%) | 94 (30.1) | 42 (30.4) | 52 (29.9) | 0.916 |
| Hospitalizations for congestive heart failurea, n (%) | 107 (34.3) | 54 (39.1) | 53 (30.5) | 0.109 |
| History of atrial fibrillation, n (%) | 30 (9.6) | 10 (7.2) | 20 (11.5) | 0.206 |
| Chronic kidney disease, n (%) | 68 (21.8) | 34 (24.6) | 34 (19.5) | 0.279 |
| Chronic obstructive pulmonary disease, n (%) | 62 (19.9) | 30 (21.7) | 32 (18.4) | 0.462 |
| Cardiovascular medications | ||||
| Beta-blockers, n (%) | 269 (86.2) | 121 (87.7) | 148 (85.1) | 0.504 |
| ACE inhibitors or AT II antagonist, n (%) | 260 (83.3) | 112 (81.2) | 148 (85.1) | 0.359 |
| Furosemide, n (%) | 251 (80.4) | 116 (84.1) | 135 (77.6) | 0.152 |
| Statins, n (%) | 216 (69.2) | 89 (64.5) | 127 (73.0) | 0.106 |
| Antiplatelet drugs, n (%) | 210 (67.3) | 92 (66.7) | 118 (67.8) | 0.830 |
| Mineralocorticoid receptor antagonists, n (%) | 154 (49.4) | 71 (51.4) | 83 (47.7) | 0.511 |
| Amiodarone, n (%) | 36 (11.5) | 14 (10.1) | 22 (12.6) | 0.493 |
| Oral anticoagulants, n (%) | 42 (13.5) | 17 (12.3) | 25 (14.4) | 0.598 |
| Digoxin, n (%) | 49 (15.7) | 27 (19.6) | 22 (12.6) | 0.095 |
| Other antiarrhythmics, n (%) | 8 (2.6) | 5 (3.6) | 3 (1.7) | 0.292 |
| Characteristics . | All patients (n = 312) . | Patients receiving CRT-D (n = 138) . | Patients receiving ICD only (n = 174) . | P-value . |
|---|---|---|---|---|
| Indication of ICD | ||||
| Primary prevention, n (%) | 296 (94.9) | 133 (96.4) | 163 (93.7) | 0.283 |
| Secondary prevention, n (%) | 16 (5.1) | 5 (3.6) | 11 (6.3) | 0.283 |
| Baseline characteristics | ||||
| Male, n (%) | 261 (83.7) | 113 (81.9) | 148 (85.1) | 0.452 |
| Age (years), mean + SD | 66.4 ± 12.6 | 68.2 ± 10.6 | 64.9 ± 13.9 | 0.021 |
| QRS duration (ms), mean + SD | 120.0 ± 36.7 | 154.4 ± 29.4 | 94.5 ± 13.2 | <0.001 |
| LVESV (cc), mean ± SD | 136.7 ± 26.7 | 140.8 ± 28.0 | 133.3 ± 25.2 | 0.014 |
| LVEF (%), mean ± SD | 25.5 ± 4.3 | 25.0 ± 4.5 | 25.9 ± 4.1 | 0.069 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.5 ± 0.5 | 0.078 |
| Aetiology | ||||
| Ischaemic cardiomyopathy, n (%) | 181 (58.0) | 71 (51.4) | 110 (63.2) | 0.036 |
| Non-ischaemic cardiomyopathy, n (%) | 131 (42.0) | 67 (48.6) | 64 (36.8) | 0.036 |
| Associated disorders | ||||
| Hypertension on therapy, n (%) | 222 (71.2) | 102 (73.9) | 120 (69.0) | 0.338 |
| Diabetes, n (%) | 94 (30.1) | 42 (30.4) | 52 (29.9) | 0.916 |
| Hospitalizations for congestive heart failurea, n (%) | 107 (34.3) | 54 (39.1) | 53 (30.5) | 0.109 |
| History of atrial fibrillation, n (%) | 30 (9.6) | 10 (7.2) | 20 (11.5) | 0.206 |
| Chronic kidney disease, n (%) | 68 (21.8) | 34 (24.6) | 34 (19.5) | 0.279 |
| Chronic obstructive pulmonary disease, n (%) | 62 (19.9) | 30 (21.7) | 32 (18.4) | 0.462 |
| Cardiovascular medications | ||||
| Beta-blockers, n (%) | 269 (86.2) | 121 (87.7) | 148 (85.1) | 0.504 |
| ACE inhibitors or AT II antagonist, n (%) | 260 (83.3) | 112 (81.2) | 148 (85.1) | 0.359 |
| Furosemide, n (%) | 251 (80.4) | 116 (84.1) | 135 (77.6) | 0.152 |
| Statins, n (%) | 216 (69.2) | 89 (64.5) | 127 (73.0) | 0.106 |
| Antiplatelet drugs, n (%) | 210 (67.3) | 92 (66.7) | 118 (67.8) | 0.830 |
| Mineralocorticoid receptor antagonists, n (%) | 154 (49.4) | 71 (51.4) | 83 (47.7) | 0.511 |
| Amiodarone, n (%) | 36 (11.5) | 14 (10.1) | 22 (12.6) | 0.493 |
| Oral anticoagulants, n (%) | 42 (13.5) | 17 (12.3) | 25 (14.4) | 0.598 |
| Digoxin, n (%) | 49 (15.7) | 27 (19.6) | 22 (12.6) | 0.095 |
| Other antiarrhythmics, n (%) | 8 (2.6) | 5 (3.6) | 3 (1.7) | 0.292 |
ACE, angiotensin-converting enzyme; AT, angiotensin; CRT-D, cardiac resynchronization therapy combined with defibrillator; ICD, implantable cardioverter defibrillator; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
Characteristics of the study population and comparison between patients receiving CRT-D and patients receiving ICD only
| Characteristics . | All patients (n = 312) . | Patients receiving CRT-D (n = 138) . | Patients receiving ICD only (n = 174) . | P-value . |
|---|---|---|---|---|
| Indication of ICD | ||||
| Primary prevention, n (%) | 296 (94.9) | 133 (96.4) | 163 (93.7) | 0.283 |
| Secondary prevention, n (%) | 16 (5.1) | 5 (3.6) | 11 (6.3) | 0.283 |
| Baseline characteristics | ||||
| Male, n (%) | 261 (83.7) | 113 (81.9) | 148 (85.1) | 0.452 |
| Age (years), mean + SD | 66.4 ± 12.6 | 68.2 ± 10.6 | 64.9 ± 13.9 | 0.021 |
| QRS duration (ms), mean + SD | 120.0 ± 36.7 | 154.4 ± 29.4 | 94.5 ± 13.2 | <0.001 |
| LVESV (cc), mean ± SD | 136.7 ± 26.7 | 140.8 ± 28.0 | 133.3 ± 25.2 | 0.014 |
| LVEF (%), mean ± SD | 25.5 ± 4.3 | 25.0 ± 4.5 | 25.9 ± 4.1 | 0.069 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.5 ± 0.5 | 0.078 |
| Aetiology | ||||
| Ischaemic cardiomyopathy, n (%) | 181 (58.0) | 71 (51.4) | 110 (63.2) | 0.036 |
| Non-ischaemic cardiomyopathy, n (%) | 131 (42.0) | 67 (48.6) | 64 (36.8) | 0.036 |
| Associated disorders | ||||
| Hypertension on therapy, n (%) | 222 (71.2) | 102 (73.9) | 120 (69.0) | 0.338 |
| Diabetes, n (%) | 94 (30.1) | 42 (30.4) | 52 (29.9) | 0.916 |
| Hospitalizations for congestive heart failurea, n (%) | 107 (34.3) | 54 (39.1) | 53 (30.5) | 0.109 |
| History of atrial fibrillation, n (%) | 30 (9.6) | 10 (7.2) | 20 (11.5) | 0.206 |
| Chronic kidney disease, n (%) | 68 (21.8) | 34 (24.6) | 34 (19.5) | 0.279 |
| Chronic obstructive pulmonary disease, n (%) | 62 (19.9) | 30 (21.7) | 32 (18.4) | 0.462 |
| Cardiovascular medications | ||||
| Beta-blockers, n (%) | 269 (86.2) | 121 (87.7) | 148 (85.1) | 0.504 |
| ACE inhibitors or AT II antagonist, n (%) | 260 (83.3) | 112 (81.2) | 148 (85.1) | 0.359 |
| Furosemide, n (%) | 251 (80.4) | 116 (84.1) | 135 (77.6) | 0.152 |
| Statins, n (%) | 216 (69.2) | 89 (64.5) | 127 (73.0) | 0.106 |
| Antiplatelet drugs, n (%) | 210 (67.3) | 92 (66.7) | 118 (67.8) | 0.830 |
| Mineralocorticoid receptor antagonists, n (%) | 154 (49.4) | 71 (51.4) | 83 (47.7) | 0.511 |
| Amiodarone, n (%) | 36 (11.5) | 14 (10.1) | 22 (12.6) | 0.493 |
| Oral anticoagulants, n (%) | 42 (13.5) | 17 (12.3) | 25 (14.4) | 0.598 |
| Digoxin, n (%) | 49 (15.7) | 27 (19.6) | 22 (12.6) | 0.095 |
| Other antiarrhythmics, n (%) | 8 (2.6) | 5 (3.6) | 3 (1.7) | 0.292 |
| Characteristics . | All patients (n = 312) . | Patients receiving CRT-D (n = 138) . | Patients receiving ICD only (n = 174) . | P-value . |
|---|---|---|---|---|
| Indication of ICD | ||||
| Primary prevention, n (%) | 296 (94.9) | 133 (96.4) | 163 (93.7) | 0.283 |
| Secondary prevention, n (%) | 16 (5.1) | 5 (3.6) | 11 (6.3) | 0.283 |
| Baseline characteristics | ||||
| Male, n (%) | 261 (83.7) | 113 (81.9) | 148 (85.1) | 0.452 |
| Age (years), mean + SD | 66.4 ± 12.6 | 68.2 ± 10.6 | 64.9 ± 13.9 | 0.021 |
| QRS duration (ms), mean + SD | 120.0 ± 36.7 | 154.4 ± 29.4 | 94.5 ± 13.2 | <0.001 |
| LVESV (cc), mean ± SD | 136.7 ± 26.7 | 140.8 ± 28.0 | 133.3 ± 25.2 | 0.014 |
| LVEF (%), mean ± SD | 25.5 ± 4.3 | 25.0 ± 4.5 | 25.9 ± 4.1 | 0.069 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.6 ± 0.5 | 2.5 ± 0.5 | 0.078 |
| Aetiology | ||||
| Ischaemic cardiomyopathy, n (%) | 181 (58.0) | 71 (51.4) | 110 (63.2) | 0.036 |
| Non-ischaemic cardiomyopathy, n (%) | 131 (42.0) | 67 (48.6) | 64 (36.8) | 0.036 |
| Associated disorders | ||||
| Hypertension on therapy, n (%) | 222 (71.2) | 102 (73.9) | 120 (69.0) | 0.338 |
| Diabetes, n (%) | 94 (30.1) | 42 (30.4) | 52 (29.9) | 0.916 |
| Hospitalizations for congestive heart failurea, n (%) | 107 (34.3) | 54 (39.1) | 53 (30.5) | 0.109 |
| History of atrial fibrillation, n (%) | 30 (9.6) | 10 (7.2) | 20 (11.5) | 0.206 |
| Chronic kidney disease, n (%) | 68 (21.8) | 34 (24.6) | 34 (19.5) | 0.279 |
| Chronic obstructive pulmonary disease, n (%) | 62 (19.9) | 30 (21.7) | 32 (18.4) | 0.462 |
| Cardiovascular medications | ||||
| Beta-blockers, n (%) | 269 (86.2) | 121 (87.7) | 148 (85.1) | 0.504 |
| ACE inhibitors or AT II antagonist, n (%) | 260 (83.3) | 112 (81.2) | 148 (85.1) | 0.359 |
| Furosemide, n (%) | 251 (80.4) | 116 (84.1) | 135 (77.6) | 0.152 |
| Statins, n (%) | 216 (69.2) | 89 (64.5) | 127 (73.0) | 0.106 |
| Antiplatelet drugs, n (%) | 210 (67.3) | 92 (66.7) | 118 (67.8) | 0.830 |
| Mineralocorticoid receptor antagonists, n (%) | 154 (49.4) | 71 (51.4) | 83 (47.7) | 0.511 |
| Amiodarone, n (%) | 36 (11.5) | 14 (10.1) | 22 (12.6) | 0.493 |
| Oral anticoagulants, n (%) | 42 (13.5) | 17 (12.3) | 25 (14.4) | 0.598 |
| Digoxin, n (%) | 49 (15.7) | 27 (19.6) | 22 (12.6) | 0.095 |
| Other antiarrhythmics, n (%) | 8 (2.6) | 5 (3.6) | 3 (1.7) | 0.292 |
ACE, angiotensin-converting enzyme; AT, angiotensin; CRT-D, cardiac resynchronization therapy combined with defibrillator; ICD, implantable cardioverter defibrillator; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
| Characteristics . | Responder (n = 84) . | Non-responder (n = 54) . | P-value . |
|---|---|---|---|
| Baseline characteristics | |||
| Male, n (%) | 68 (81.0) | 45 (83.3) | 0.723 |
| Age (years), mean + SD | 67.6 ± 10.3 | 69.3 ± 11.1 | 0.379 |
| QRS duration (ms), mean + SD | 158.1 ± 33.2 | 149.1 ± 22.7 | 0.090 |
| LVEF (%), mean ± SD | 25.3 ± 3.7 | 24.6 ± 5.6 | 0.383 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.115 |
| Aetiology | |||
| Ischaemic cardiomyopathy, n (%) | 36 (42.9) | 35 (64.8) | 0.012 |
| Non-ischaemic cardiomyopathy, n (%) | 48 (57.1) | 19 (35.2) | 0.012 |
| QRS morphology | |||
| Left bundle branch block, n (%) | 69 (82.1) | 35 (64.8) | 0.021 |
| Non-left bundle branch block, n (%) | 15 (17.9) | 19 (35.2) | 0.021 |
| Non-specific intraventricular conduction disturbance, n (%) | 11 (14.3) | 12 (22.2) | 0.160 |
| Right bundle branch block, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Associated disorders | |||
| Hospitalizations for congestive heart failurea, n (%) | 28 (33.3) | 26 (48.1) | 0.082 |
| History of atrial fibrillation, n (%) | 4 (4.8) | 6 (11.1) | 0.160 |
| Chronic renal failure, n (%) | 25 (29.8) | 9 (16.7) | 0.081 |
| Chronic obstructive pulmonary disease, n (%) | 16 (19.0) | 14 (25.9) | 0.339 |
| Left ventricular lead position | |||
| Non-apical lateral, n (%) | 64 (76.2) | 31 (57.4) | 0.020 |
| Non-apical posterior, n (%) | 14 (16.7) | 5 (9.3) | 0.218 |
| Non-apical anterior, n (%) | 6 (7.1) | 8 (14.8) | 0.145 |
| All apical, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Characteristics . | Responder (n = 84) . | Non-responder (n = 54) . | P-value . |
|---|---|---|---|
| Baseline characteristics | |||
| Male, n (%) | 68 (81.0) | 45 (83.3) | 0.723 |
| Age (years), mean + SD | 67.6 ± 10.3 | 69.3 ± 11.1 | 0.379 |
| QRS duration (ms), mean + SD | 158.1 ± 33.2 | 149.1 ± 22.7 | 0.090 |
| LVEF (%), mean ± SD | 25.3 ± 3.7 | 24.6 ± 5.6 | 0.383 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.115 |
| Aetiology | |||
| Ischaemic cardiomyopathy, n (%) | 36 (42.9) | 35 (64.8) | 0.012 |
| Non-ischaemic cardiomyopathy, n (%) | 48 (57.1) | 19 (35.2) | 0.012 |
| QRS morphology | |||
| Left bundle branch block, n (%) | 69 (82.1) | 35 (64.8) | 0.021 |
| Non-left bundle branch block, n (%) | 15 (17.9) | 19 (35.2) | 0.021 |
| Non-specific intraventricular conduction disturbance, n (%) | 11 (14.3) | 12 (22.2) | 0.160 |
| Right bundle branch block, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Associated disorders | |||
| Hospitalizations for congestive heart failurea, n (%) | 28 (33.3) | 26 (48.1) | 0.082 |
| History of atrial fibrillation, n (%) | 4 (4.8) | 6 (11.1) | 0.160 |
| Chronic renal failure, n (%) | 25 (29.8) | 9 (16.7) | 0.081 |
| Chronic obstructive pulmonary disease, n (%) | 16 (19.0) | 14 (25.9) | 0.339 |
| Left ventricular lead position | |||
| Non-apical lateral, n (%) | 64 (76.2) | 31 (57.4) | 0.020 |
| Non-apical posterior, n (%) | 14 (16.7) | 5 (9.3) | 0.218 |
| Non-apical anterior, n (%) | 6 (7.1) | 8 (14.8) | 0.145 |
| All apical, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
CRT-D, cardiac resynchronization therapy combined with defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
| Characteristics . | Responder (n = 84) . | Non-responder (n = 54) . | P-value . |
|---|---|---|---|
| Baseline characteristics | |||
| Male, n (%) | 68 (81.0) | 45 (83.3) | 0.723 |
| Age (years), mean + SD | 67.6 ± 10.3 | 69.3 ± 11.1 | 0.379 |
| QRS duration (ms), mean + SD | 158.1 ± 33.2 | 149.1 ± 22.7 | 0.090 |
| LVEF (%), mean ± SD | 25.3 ± 3.7 | 24.6 ± 5.6 | 0.383 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.115 |
| Aetiology | |||
| Ischaemic cardiomyopathy, n (%) | 36 (42.9) | 35 (64.8) | 0.012 |
| Non-ischaemic cardiomyopathy, n (%) | 48 (57.1) | 19 (35.2) | 0.012 |
| QRS morphology | |||
| Left bundle branch block, n (%) | 69 (82.1) | 35 (64.8) | 0.021 |
| Non-left bundle branch block, n (%) | 15 (17.9) | 19 (35.2) | 0.021 |
| Non-specific intraventricular conduction disturbance, n (%) | 11 (14.3) | 12 (22.2) | 0.160 |
| Right bundle branch block, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Associated disorders | |||
| Hospitalizations for congestive heart failurea, n (%) | 28 (33.3) | 26 (48.1) | 0.082 |
| History of atrial fibrillation, n (%) | 4 (4.8) | 6 (11.1) | 0.160 |
| Chronic renal failure, n (%) | 25 (29.8) | 9 (16.7) | 0.081 |
| Chronic obstructive pulmonary disease, n (%) | 16 (19.0) | 14 (25.9) | 0.339 |
| Left ventricular lead position | |||
| Non-apical lateral, n (%) | 64 (76.2) | 31 (57.4) | 0.020 |
| Non-apical posterior, n (%) | 14 (16.7) | 5 (9.3) | 0.218 |
| Non-apical anterior, n (%) | 6 (7.1) | 8 (14.8) | 0.145 |
| All apical, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Characteristics . | Responder (n = 84) . | Non-responder (n = 54) . | P-value . |
|---|---|---|---|
| Baseline characteristics | |||
| Male, n (%) | 68 (81.0) | 45 (83.3) | 0.723 |
| Age (years), mean + SD | 67.6 ± 10.3 | 69.3 ± 11.1 | 0.379 |
| QRS duration (ms), mean + SD | 158.1 ± 33.2 | 149.1 ± 22.7 | 0.090 |
| LVEF (%), mean ± SD | 25.3 ± 3.7 | 24.6 ± 5.6 | 0.383 |
| NYHA class, mean ± SD | 2.6 ± 0.5 | 2.7 ± 0.5 | 0.115 |
| Aetiology | |||
| Ischaemic cardiomyopathy, n (%) | 36 (42.9) | 35 (64.8) | 0.012 |
| Non-ischaemic cardiomyopathy, n (%) | 48 (57.1) | 19 (35.2) | 0.012 |
| QRS morphology | |||
| Left bundle branch block, n (%) | 69 (82.1) | 35 (64.8) | 0.021 |
| Non-left bundle branch block, n (%) | 15 (17.9) | 19 (35.2) | 0.021 |
| Non-specific intraventricular conduction disturbance, n (%) | 11 (14.3) | 12 (22.2) | 0.160 |
| Right bundle branch block, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
| Associated disorders | |||
| Hospitalizations for congestive heart failurea, n (%) | 28 (33.3) | 26 (48.1) | 0.082 |
| History of atrial fibrillation, n (%) | 4 (4.8) | 6 (11.1) | 0.160 |
| Chronic renal failure, n (%) | 25 (29.8) | 9 (16.7) | 0.081 |
| Chronic obstructive pulmonary disease, n (%) | 16 (19.0) | 14 (25.9) | 0.339 |
| Left ventricular lead position | |||
| Non-apical lateral, n (%) | 64 (76.2) | 31 (57.4) | 0.020 |
| Non-apical posterior, n (%) | 14 (16.7) | 5 (9.3) | 0.218 |
| Non-apical anterior, n (%) | 6 (7.1) | 8 (14.8) | 0.145 |
| All apical, n (%) | 4 (4.8) | 7 (13.0) | 0.083 |
CRT-D, cardiac resynchronization therapy combined with defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
Follow-up
The cumulative duration of follow-up was 1354 patient-years, with a median follow-up of 46 months per patient (interquartile range: 35–58 months). No patient was lost to follow-up. The duration of follow-up was similar in patients receiving CRT-D and in those only receiving an ICD (43.8 ± 17.0 vs. 44.4 ± 17.6; P = 0.763).
On evaluation 6 months after implantation, 84 of the 138 patients (60.9%) with a CRT-D showed a positive response to CRT, according to the predefined criteria listed earlier. In comparison with the time of implantation, CRT induced significant reverse remodelling (with reduction of LV volumes and improvement of LVEF) and a significant improvement in functional NYHA class in responders, but not in non-responders (data not shown). Compared with non-responders, responders were less likely to have a non-ischaemic cardiomyopathy, a left bundle branch block (LBBB) QRS morphology, and the LV lead placed in a non-apical lateral position (Table 2).
During follow-up, four patients (1.3%) underwent heart transplantation: one (0.7%) in the CRT-D group and three (1.7%) in the ICD-only group (P = 0.436). In three CRT-D patients (2.2%), the coronary sinus lead was inactivated after a mean of 6.8 ± 2.4 months from implantation: in two because of phrenic nerve stimulation irresolvable by device reprogramming and in one because of dislodgement. In seven ICD-only patients (4.0%), the pacing system was upgraded to biventricular after a mean of 46.1 ± 24.3 months from implantation: in four because of the spontaneous development of intraventricular conduction delay and in three because of the development of pacemaker dependency.
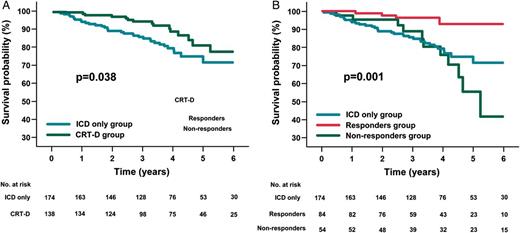
Unadjusted Kaplan–Meier patient survival from death due to any cause in ICD-only and CRT-D patients (A). Comparison between ICD-only patients, responders, and non-responders (B). (A) In an unadjusted Kaplan–Meier analysis, patients receiving ICD only (blue line) displayed lower survival from death due to any cause at 6 years than those receiving CRT-D (green line). (B) Comparison by means of log-rank test of cumulative 6-year survival from death due to any cause shows that: responders to CRT (red line) display better survival than non-responders (green line) (P < 0.001); non-responders to CRT have a survival rate similar to that of ICD-only patients (blue line) (P = 0.450); responders to CRT have a higher survival rate than patients receiving ICD only (P < 0.001). CRT-D, cardiac resynchronization therapy plus defibrillator device; ICD, implantable cardioverter defibrillator.
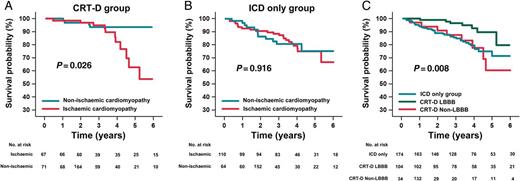
Kaplan–Meier patient survival from death due to any cause: comparison between subgroups. Comparison by means of log-rank test of cumulative 6-year survival from death due to any cause. (A) In the CRT-D group, patients with non-ischaemic cardiomyopathy (blue line) have a higher survival rate than those with ischaemic cardiomyopathy (red line) (P = 0.026). (B) In the ICD-only group, patients with non-ischaemic cardiomyopathy (blue line) have a survival rate similar to patients with ischaemic cardiomyopathy (red line) (P = 0.918). (C) Patients of the CRT-D group with LBBB QRS morphology (green line) showed better survival than patients of the CRT-D group with non-LBBB QRS morphology (red line) (P = 0.015); patients of the CRT-D group with LBBB QRS morphology showed better survival than patients of the ICD-only group (blue line) (P = 0.017); and patients of the CRT-D group with non-LBBB QRS morphology have a survival rate similar to the ICD-only group (P = 0.733). CRT-D, cardiac resynchronization therapy plus defibrillator device; ICD, implantable cardioverter defibrillator; LBBB, left bundle branch block.
Patients of the CRT-D group with LBBB QRS morphology resulted having a better survival compared with those with non-LBBB QRS morphology (P = 0.015) and compared also to patients of the ICD-only group (P = 0.017). The survival of CRT-D patients with non-LBBB QRS morphology was similar to the ICD-only group (P = 0.733) (Figure 2C).
Predictors of mortality and causes of death
In the study population, univariate analysis (see Supplementary material online) showed that CRT was significantly associated with lower all-cause mortality. Patient factors associated with greater all-cause mortality on univariate analysis were: higher age, higher NYHA class, hospitalizations for congestive HF in the year prior to implantation, chronic kidney disease, and chronic obstructive pulmonary disease. Conversely, a positive response to CRT, a longer QRS duration, and a higher LVEF were associated with lower all-cause mortality (Table 3). After multivariate analysis, the predictive factors that were still associated with greater all-cause mortality were higher age and hospitalizations for congestive HF in the year prior to implantation. A positive response to CRT and a higher LVEF remained associated with lower all-cause mortality. Interactions between the covariates were not significant.
Predictors of mortality in the overall study population: univariate and multivariate Cox proportional hazards analysis
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P-value . | Hazard ratio (95% CI) . | P-value . | |
| CRT | 0.466 (0.24–0.89) | 0.022 | 0.402 (0.16–1.01) | 0.053 |
| Response to CRT | 0.192 (0.07–0.55) | 0.002 | 0.274 (0.08–0.98) | 0.047 |
| QRS duration (for increase of +1SD, ms) | 0.990 (0.98–0.99) | 0.039 | 1.007 (0.99–1.02) | 0.403 |
| Age (for increase of +1SD, years) | 1.032 (1.00–1.06) | 0.026 | 1.047 (1.02–1.08) | 0.003 |
| Ejection fraction (for increase of +1SD, %) | 0.925 (0.86–0.99) | 0.025 | 0.904 (0.84–0.98) | 0.011 |
| Ischaemic cardiomyopathy | 1.932 (0.99–3.77) | 0.054 | 0.825 (0.37–1.85) | 0.641 |
| NYHA class (for increase of +1SD) | 1.699 (1.04–2.77) | 0.033 | 1.560 (0.92–2.65) | 0.100 |
| Hospitalizations for congestive heart failurea | 2.207 (1.20–4.07) | 0.011 | 2.549 (1.27–5.10) | 0.008 |
| Chronic renal failure | 2.140 (1.11–4.14) | 0.024 | 1.700 (0.82–3.51) | 0.151 |
| Chronic obstructive pulmonary disease | 2.163 (1.10–4.24) | 0.025 | 1.828 (0.87–3.84) | 0.111 |
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P-value . | Hazard ratio (95% CI) . | P-value . | |
| CRT | 0.466 (0.24–0.89) | 0.022 | 0.402 (0.16–1.01) | 0.053 |
| Response to CRT | 0.192 (0.07–0.55) | 0.002 | 0.274 (0.08–0.98) | 0.047 |
| QRS duration (for increase of +1SD, ms) | 0.990 (0.98–0.99) | 0.039 | 1.007 (0.99–1.02) | 0.403 |
| Age (for increase of +1SD, years) | 1.032 (1.00–1.06) | 0.026 | 1.047 (1.02–1.08) | 0.003 |
| Ejection fraction (for increase of +1SD, %) | 0.925 (0.86–0.99) | 0.025 | 0.904 (0.84–0.98) | 0.011 |
| Ischaemic cardiomyopathy | 1.932 (0.99–3.77) | 0.054 | 0.825 (0.37–1.85) | 0.641 |
| NYHA class (for increase of +1SD) | 1.699 (1.04–2.77) | 0.033 | 1.560 (0.92–2.65) | 0.100 |
| Hospitalizations for congestive heart failurea | 2.207 (1.20–4.07) | 0.011 | 2.549 (1.27–5.10) | 0.008 |
| Chronic renal failure | 2.140 (1.11–4.14) | 0.024 | 1.700 (0.82–3.51) | 0.151 |
| Chronic obstructive pulmonary disease | 2.163 (1.10–4.24) | 0.025 | 1.828 (0.87–3.84) | 0.111 |
CRT, cardiac resynchronization therapy; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
Predictors of mortality in the overall study population: univariate and multivariate Cox proportional hazards analysis
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P-value . | Hazard ratio (95% CI) . | P-value . | |
| CRT | 0.466 (0.24–0.89) | 0.022 | 0.402 (0.16–1.01) | 0.053 |
| Response to CRT | 0.192 (0.07–0.55) | 0.002 | 0.274 (0.08–0.98) | 0.047 |
| QRS duration (for increase of +1SD, ms) | 0.990 (0.98–0.99) | 0.039 | 1.007 (0.99–1.02) | 0.403 |
| Age (for increase of +1SD, years) | 1.032 (1.00–1.06) | 0.026 | 1.047 (1.02–1.08) | 0.003 |
| Ejection fraction (for increase of +1SD, %) | 0.925 (0.86–0.99) | 0.025 | 0.904 (0.84–0.98) | 0.011 |
| Ischaemic cardiomyopathy | 1.932 (0.99–3.77) | 0.054 | 0.825 (0.37–1.85) | 0.641 |
| NYHA class (for increase of +1SD) | 1.699 (1.04–2.77) | 0.033 | 1.560 (0.92–2.65) | 0.100 |
| Hospitalizations for congestive heart failurea | 2.207 (1.20–4.07) | 0.011 | 2.549 (1.27–5.10) | 0.008 |
| Chronic renal failure | 2.140 (1.11–4.14) | 0.024 | 1.700 (0.82–3.51) | 0.151 |
| Chronic obstructive pulmonary disease | 2.163 (1.10–4.24) | 0.025 | 1.828 (0.87–3.84) | 0.111 |
| Variable . | Univariable analysis . | Multivariable analysis . | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) . | P-value . | Hazard ratio (95% CI) . | P-value . | |
| CRT | 0.466 (0.24–0.89) | 0.022 | 0.402 (0.16–1.01) | 0.053 |
| Response to CRT | 0.192 (0.07–0.55) | 0.002 | 0.274 (0.08–0.98) | 0.047 |
| QRS duration (for increase of +1SD, ms) | 0.990 (0.98–0.99) | 0.039 | 1.007 (0.99–1.02) | 0.403 |
| Age (for increase of +1SD, years) | 1.032 (1.00–1.06) | 0.026 | 1.047 (1.02–1.08) | 0.003 |
| Ejection fraction (for increase of +1SD, %) | 0.925 (0.86–0.99) | 0.025 | 0.904 (0.84–0.98) | 0.011 |
| Ischaemic cardiomyopathy | 1.932 (0.99–3.77) | 0.054 | 0.825 (0.37–1.85) | 0.641 |
| NYHA class (for increase of +1SD) | 1.699 (1.04–2.77) | 0.033 | 1.560 (0.92–2.65) | 0.100 |
| Hospitalizations for congestive heart failurea | 2.207 (1.20–4.07) | 0.011 | 2.549 (1.27–5.10) | 0.008 |
| Chronic renal failure | 2.140 (1.11–4.14) | 0.024 | 1.700 (0.82–3.51) | 0.151 |
| Chronic obstructive pulmonary disease | 2.163 (1.10–4.24) | 0.025 | 1.828 (0.87–3.84) | 0.111 |
CRT, cardiac resynchronization therapy; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SD, standard deviation.
aIn the year prior to implantation.
Causes of death in the study population and comparison between patients receiving CRT-D and patients receiving ICD only
| Causes of death . | Deaths in overall population (n = 50) . | Deaths in CRT-D patients (n = 15) . | Deaths in ICD-only patients (n = 35) . | P-value . |
|---|---|---|---|---|
| Non-sudden cardiac death, n (%) | 26 (52.0) | 5 (33.3) | 21 (60.0) | 0.084 |
| Heart failure death, n (%) | 23 (46.0) | 3 (20.0) | 20 (57.1) | 0.016 |
| Coronary death, n (%) | 3 (6.0) | 2 (13.3) | 1 (2.9) | 0.153 |
| Non-cardiac death, n (%) | 19 (38.0) | 8 (53.3) | 11 (31.4) | 0.144 |
| Cancer death, n (%) | 8 (16.0) | 3 (20.0) | 5 (14.3) | 0.614 |
| Cerebrovascular death, n (%) | 3 (6.0) | 1 (6.7) | 2 (5.7) | 0.897 |
| Death from pulmonary disease, n (%) | 2 (4.0) | 1 (6.7) | 1 (2.9) | 0.529 |
| Other non-cardiac deaths, n (%) | 6 (12.0) | 3 (20.0) | 3 (8.6) | 0.254 |
| Sudden death, n (%) | 5 (10.0) | 2 (13.3) | 3 (8.6) | 0.607 |
| Causes of death . | Deaths in overall population (n = 50) . | Deaths in CRT-D patients (n = 15) . | Deaths in ICD-only patients (n = 35) . | P-value . |
|---|---|---|---|---|
| Non-sudden cardiac death, n (%) | 26 (52.0) | 5 (33.3) | 21 (60.0) | 0.084 |
| Heart failure death, n (%) | 23 (46.0) | 3 (20.0) | 20 (57.1) | 0.016 |
| Coronary death, n (%) | 3 (6.0) | 2 (13.3) | 1 (2.9) | 0.153 |
| Non-cardiac death, n (%) | 19 (38.0) | 8 (53.3) | 11 (31.4) | 0.144 |
| Cancer death, n (%) | 8 (16.0) | 3 (20.0) | 5 (14.3) | 0.614 |
| Cerebrovascular death, n (%) | 3 (6.0) | 1 (6.7) | 2 (5.7) | 0.897 |
| Death from pulmonary disease, n (%) | 2 (4.0) | 1 (6.7) | 1 (2.9) | 0.529 |
| Other non-cardiac deaths, n (%) | 6 (12.0) | 3 (20.0) | 3 (8.6) | 0.254 |
| Sudden death, n (%) | 5 (10.0) | 2 (13.3) | 3 (8.6) | 0.607 |
CRT-D, cardiac resynchronization therapy plus defibrillator device; ICD, implantable cardioverter defibrillator.
Causes of death in the study population and comparison between patients receiving CRT-D and patients receiving ICD only
| Causes of death . | Deaths in overall population (n = 50) . | Deaths in CRT-D patients (n = 15) . | Deaths in ICD-only patients (n = 35) . | P-value . |
|---|---|---|---|---|
| Non-sudden cardiac death, n (%) | 26 (52.0) | 5 (33.3) | 21 (60.0) | 0.084 |
| Heart failure death, n (%) | 23 (46.0) | 3 (20.0) | 20 (57.1) | 0.016 |
| Coronary death, n (%) | 3 (6.0) | 2 (13.3) | 1 (2.9) | 0.153 |
| Non-cardiac death, n (%) | 19 (38.0) | 8 (53.3) | 11 (31.4) | 0.144 |
| Cancer death, n (%) | 8 (16.0) | 3 (20.0) | 5 (14.3) | 0.614 |
| Cerebrovascular death, n (%) | 3 (6.0) | 1 (6.7) | 2 (5.7) | 0.897 |
| Death from pulmonary disease, n (%) | 2 (4.0) | 1 (6.7) | 1 (2.9) | 0.529 |
| Other non-cardiac deaths, n (%) | 6 (12.0) | 3 (20.0) | 3 (8.6) | 0.254 |
| Sudden death, n (%) | 5 (10.0) | 2 (13.3) | 3 (8.6) | 0.607 |
| Causes of death . | Deaths in overall population (n = 50) . | Deaths in CRT-D patients (n = 15) . | Deaths in ICD-only patients (n = 35) . | P-value . |
|---|---|---|---|---|
| Non-sudden cardiac death, n (%) | 26 (52.0) | 5 (33.3) | 21 (60.0) | 0.084 |
| Heart failure death, n (%) | 23 (46.0) | 3 (20.0) | 20 (57.1) | 0.016 |
| Coronary death, n (%) | 3 (6.0) | 2 (13.3) | 1 (2.9) | 0.153 |
| Non-cardiac death, n (%) | 19 (38.0) | 8 (53.3) | 11 (31.4) | 0.144 |
| Cancer death, n (%) | 8 (16.0) | 3 (20.0) | 5 (14.3) | 0.614 |
| Cerebrovascular death, n (%) | 3 (6.0) | 1 (6.7) | 2 (5.7) | 0.897 |
| Death from pulmonary disease, n (%) | 2 (4.0) | 1 (6.7) | 1 (2.9) | 0.529 |
| Other non-cardiac deaths, n (%) | 6 (12.0) | 3 (20.0) | 3 (8.6) | 0.254 |
| Sudden death, n (%) | 5 (10.0) | 2 (13.3) | 3 (8.6) | 0.607 |
CRT-D, cardiac resynchronization therapy plus defibrillator device; ICD, implantable cardioverter defibrillator.
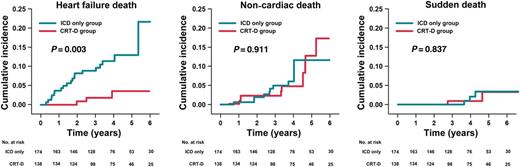
Cumulative incidence of HF death, non-cardiac death, and SD in the ICD-only and CRT-D groups. Cumulative mortality is presented separately for the ICD-only group (blue line) and the CRT-D group (red line). Causes of death other than those described were censored. CRT-D, cardiac resynchronization therapy plus defibrillator device; HF, heart failure; ICD, implantable cardioverter defibrillator; SD, sudden death.
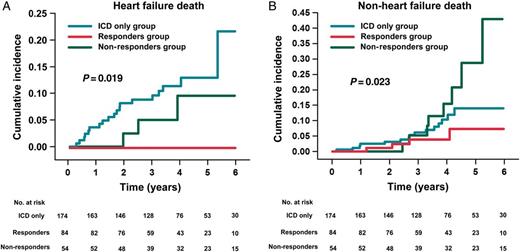
Cumulative incidence of HF death in responders, non-responders, and ICD-only patients (A). Cumulative incidence of non-HF death in responders, non-responders, and ICD-only patients (B). (A) Comparison by means of log-rank test of cumulative 6-year incidence of HF death shows that: responders (red line) display significantly lower cumulative incidence than non-responders (green line) (P = 0.019); non-responders have a cumulative incidence similar to that of ICD-only patients (blue line) (P = 0.340); and responders have a lower cumulative incidence than ICD-only patients (P = 0.012). (B) Comparison by means of log-rank test of cumulative 6-year incidence of non-HF death shows that: responders display significantly lower cumulative incidence than non-responders (P = 0.023); non-responders have a cumulative incidence similar to that of ICD-only patients (P = 0.168); and responders have a cumulative incidence similar to that of ICD-only patients (P = 0.212). CRT-D, cardiac resynchronization therapy plus defibrillator device; HF, heart failure; ICD, implantable cardioverter defibrillator.
Discussion
Main results
This observational, two-centre study provides relevant data regarding survival and causes of death in a selected ICD population from routine clinical practice. The results showed lower long-term overall mortality in patients treated with CRT-D for DCM, severe LV systolic dysfunction, and prolonged QRS than in similar patients with normal QRS duration who were treated with ICD only. With the exceptions of age and the aetiology of cardiomyopathy, the two populations were similar regarding other relevant predictors of mortality,17 specifically LVEF, NYHA class, and comorbidities.
When CRT-D patients were subdivided according to response to CRT, survival analysis showed that the beneficial impact of CRT on patient survival was due to the particularly favourable prognosis shown by patients positively responding to CRT. This finding was confirmed by multivariate analysis, which specifically identified a positive response to CRT as an independent predictor of lower overall mortality.
On analysing the causes of death separately, our results suggested that the lower mortality in CRT-D patients was due to a significant reduction in death due to terminal HF, particularly in responders.
As has been amply demonstrated, the ICD improves the survival of DCM patients by reducing SD mortality.1,2 However, it has no effect on HF mortality.1 In DCM patients with intraventricular conduction delay treated with CRT-D, the defibrillator capability reduces SD mortality, whereas, CRT, by correcting cardiac dyssynchrony, increasing cardiac pump efficiency, and inducing LV reverse remodelling, reduces HF mortality.11 In our study, according to baseline patient characteristics, CRT-D patients had higher expected overall mortality (higher age) and similar expected HF mortality (similar LVEF and NYHA class) in comparison to ICD-only patients. Despite this, they displayed significantly better survival, probably because they benefited from the effect of CRT on HF mortality.
In accordance with the results of previous studies,11,18 in our study, patients with a positive response to CRT displayed significantly better survival than non-responders. This beneficial effect of CRT is probably due to the LV reverse remodelling that it induces in responders. Indeed, several studies11,18 have shown that CRT induced LV reverse remodelling is closely associated with improved long-term survival. This beneficial effect is particularly evident in super-responders,18 in whom CRT can lead to normalization of the LV function, thereby improving survival to levels comparable to those of the general population.
Non-responders, in contrast, displayed a survival rate similar to that of patients treated with ICD only. Moreover, on Kaplan–Meier survival analysis, the survival curves of the two groups diverged from the 4th year of follow-up onwards (Figure 2B); it could therefore be hypothesized that, if follow-up had been extended further, non-responders would have displayed lower survival than ICD-only patients. Our results were not able to explain this finding; however, some hypotheses can be put forward. It is possible that, in a subgroup of non-responders, CRT did not correct, or even worsened, their baseline mechanical dyssynchrony, thus negatively impacting on patient survival.12 Alternatively, it is also possible that a subgroup did not have pre-implantation mechanical dyssynchrony, but that CRT induced a de novo mechanical dyssynchrony, thereby worsening cardiac performance and, consequently, their clinical outcome.19
Comparison with previous studies
To the best of our knowledge, only two previous studies have compared survival in HF patients with prolonged QRS treated with CRT-D vs. that in HF patients with narrow QRS treated with ICD only. In a single-centre, observational study, Desai et al.20 compared long-term survival in a group of HF patients treated with CRT-D vs. a group of HF patients treated with ICD only. Their survival analysis, similar to ours, revealed significantly lower all-cause mortality in the CRT-D group, and multivariate analysis identified CRT as an independent predictor of reduced mortality. The causes of death were not assessed.
In a large, unselected ICD population, Thijssen et al.14 evaluated patient survival and causes of death during long-term follow-up (median 3.4 years). Their study population was divided into three groups: patients receiving ICD for primary prevention, patients receiving ICD for secondary prevention, and patients receiving CRT-D. During follow-up, patients receiving CRT-D showed significantly higher unadjusted all-cause mortality than the other groups. Categorization by causes of death showed that CRT-D patients had a significantly higher cumulative incidence of death owing to HF than the other groups. These findings are in contrast with our results, probably because, in Thijssen's study, there were several significant differences in baseline patient characteristics between the study groups. Specifically, CRT-D patients were older, had a lower LVEF and renal clearance, and had a higher prevalence of atrial fibrillation than patients in the other groups. Finally, a multivariate analysis to assess the impact of CRT on mortality was not performed.
Study limitations
Although data were collected prospectively for an observational registry, the analysis performed was retrospective and is therefore subject to all of the limitations of a retrospective analysis. The results of this study should therefore be interpreted with caution, as confounding factors cannot be entirely excluded. Further prospective, large population analyses are needed to confirm them.
Although we found some significant differences between the two study groups, the study was severely underpowered to detect differences in the mode of death.
The incidence and the risk of sustained ventricular arrhythmias in the two study groups were not evaluated.
Conclusions
In patients undergoing ICD treatment for DCM and severe LV dysfunction, the subgroup of patients with intraventricular conduction delay who receive CRT combined with ICD displays better long-term survival than those on ICD treatment alone. The positive impact of CRT is the result of reduced HF mortality in the subgroup of patients who respond positively to CRT.
In contrast, patients who do not respond positively to CRT, as well as displaying lower survival than responders, also tend to have a worse prognosis even than patients who are not candidates for CRT and who are treated with ICD alone.
These results underline the need to deepen our knowledge of the factors that predict a response to CRT, in order to better identify those patients who will benefit from this therapy and reduce the number of patients in whom it will yield no benefit or even be deleterious.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.



