-
PDF
- Split View
-
Views
-
Cite
Cite
Gian-Battista Chierchia, Giacomo Mugnai, Erwin Ströker, Vedran Velagic, Burak Hünük, Darragh Moran, Ebru Hacioglu, Jan Poelaert, Christian Verborgh, Vincent Umbrain, Stefan Beckers, Diego Ruggiero, Pedro Brugada, Carlo de Asmundis, Incidence of real-time recordings of pulmonary vein potentials using the third-generation short-tip cryoballoon, EP Europace, Volume 18, Issue 8, August 2016, Pages 1158–1163, https://doi.org/10.1093/europace/euv452
Close - Share Icon Share
Abstract
The third-generation Cryoballoon Advance Short-tip (CB-ST) has been designed with a 40% shortened tip length compared with the former second-generation CB Advance device. Ideally, a shorter tip should permit an improved visualization of real-time (RT) recordings in the pulmonary vein (PV) due to a more proximal positioning of the inner lumen mapping catheter. In the present study, we sought to analyse the rate of visualization of RT recordings in our first series of patients with the CB-ST device.
All consecutive patients having undergone CB ablation using CB-ST technology were analysed. Exclusion criteria were the presence of an intracavitary thrombus, uncontrolled heart failure, moderate or severe valvular disease, and contraindications to general anaesthesia. A total of 60 consecutive patients (60.5 ± 11.2 years, 62% males) were evaluated. Real-time recordings were detected in 209 of 240 PVs (87.1%). Specifically, RT recordings could be visualized in 55 left superior PVs (91.7%), 51 left inferior PVs (85.0%), 53 right superior PVs (88.3%), and 50 right inferior PVs (83.3).
The rate of visualization of RT recordings is significantly high during third-generation CB-ST ablation. Real-time recordings can be visualized in ∼87.1% of veins with this novel cryoballoon.
The third-generation cryoballoon short-tip (CB-ST) has been designed with a 40% shortened tip length compared with the former second-generation CB Advance.
The rate of visualization of real-time recordings was significantly high during third-generation CB-ST ablation.
Real-time recordings could be frequently visualized in the CB-ST group in all types of veins.
Real-time recordings can be visualized in ∼87.1% of veins with this novel cryoballoon.
Introduction
Real-time (RT) recordings during cryoballoon (CB) ablation offer valuable information regarding time to isolation (TTI) of the pulmonary veins (PVs) during cryoenergy application. These can be visualized by a dedicated inner lumen mapping catheter (ILMC) (Achieve®, Medtronic©, Minneapolis, MN, USA) that is used in conjunction with the CB. However, due to anatomical variations of the PVs, the ILMC is often positioned more distally to the PV sleeve extension in order to offer stability to the balloon catheter in the PV ostium. In addition, the catheters shaft extends 1 cm beyond the balloon tip creating a distance between the ILMC and the site of ablation.
The third-generation Cryoballoon Advance Short-tip (CB-ST) (Medtronic) has very recently been released on the market. With respect to the former second-generation CB Advance device (CB-A), the CB-ST has been designed with a 40% shortened tip length (Figure 1). Ideally, a shorter tip should permit an improved visualization of the electrical activity in the PV due to a more proximal positioning of the ILMC (Achieve, Medtronic) (Figure 2). The other technical features having led to excellent clinical outcome have not been modified between both devices.1–4
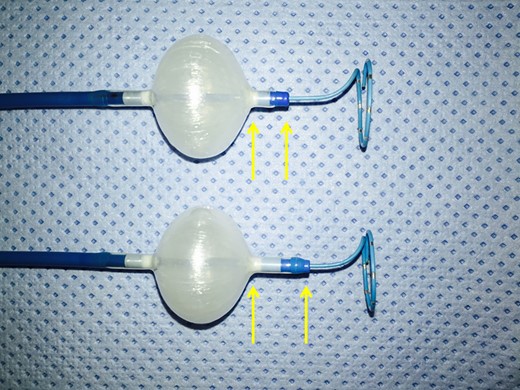
The figure shows the difference between second- and third-generation CBs: with respect to the former CB-A (below), the CB-ST (above) has been designed with a 40% shortened tip length (yellow arrows).
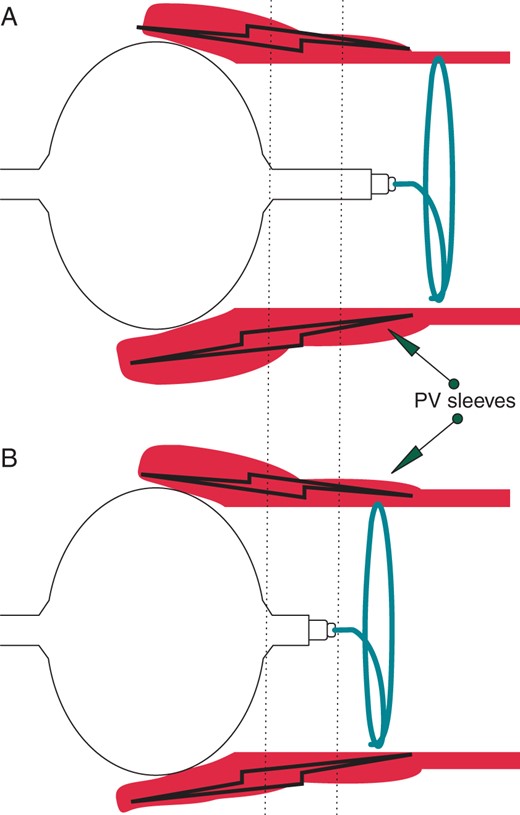
(A) In the CB-A ablation procedures, the ILMC was often positioned more distally to the PV sleeve extension in order to offer stability to the balloon catheter in the PV ostium. In addition, the catheters shaft extended 1 cm beyond the balloon tip, creating a distance between the ILMC and the site of ablation. (B) The shortened tip length has greatly shortened the distance between the ablation site and the ILMC position resulting in a higher probability of the Achieve catheter being in contact with the PV sleeves and therefore providing important electrical information on the effects of ablation in that vein.
Therefore, in the present study, we sought to analyse the rate of visualization of RT recordings in our first series of patients with the CB-ST device.
Methods
Aim of the study
The main aim of the study was to assess the incidence of visualization of RT recordings in patients having undergone atrial fibrillation (AF) ablation with the novel CB-ST technology.
Study population
A total of 60 consecutive patients were included in our retrospective analysis. From the beginning of June 2015, the novel third-generation device (CB-ST) was incepted in our centre. Since then, a total of 60 patients underwent CB ablation with this novel device. The main focus of the study was to assess the incidence of RT recordings visualization in the novel third-generation device. Other acute procedural results were also taken into consideration and compared. Exclusion criteria were the presence of an intracavitary thrombus, uncontrolled heart failure, moderate or severe valvular disease, and contraindications to general anaesthesia. The study was approved by the ethics committee of our institution.
Cryoballoon advance short-tip
The CB-ST has been recently launched on the market with a shorter tip. The CB-ST has an 8 mm long tip that is ∼40% shorter than the previous second-generation CB-A (13 mm). All the other technical features pertaining to the CB-A have been left unmodified.
Pre-procedural management
All patients provided written informed consent to the procedure. Structural heart disease was defined as follows: coronary artery disease, impaired left ventricular ejection fraction (LVEF) <40%, LV hypertrophy >15 mm, valvular insufficiency >2/4, significant valvular stenosis, and prior valve replacements. All antiarrhythmic drugs were discontinued at least 3 days before ablation. For patients under anticoagulant therapy, our standard practice has been recently described.5 A transthoracic echocardiogram was performed within 1 week prior to ablation. To exclude the presence of intracavitary thrombi, all patients underwent transoesophageal echocardiography the day before the procedure. All patients underwent a pre-procedural computed tomography (CT) scan to assess detailed left atrium (LA) and PV anatomy.
Cryoballoon ablation procedure
Our standard ablation procedure has been previously reported in detail.5 Briefly, after obtaining LA access, through a steerable 15 Fr sheath (FlexCath Advance®, Medtronic), an ILMC (Achieve, Medtronic) was advanced in each PV ostium. A 28 mm CB-ST was advanced inflated and positioned in each PV ostium. Optimal vessel occlusion was considered as achieved when selective contrast injection showed total contrast retention with no backflow to the atrium. Once occlusion was documented, cryothermal energy was started. Cryoenergy applications lasted 180 s. Usually, the left superior PV (LSPV) was treated first, followed by the left inferior (LIPV), right inferior (RIPV), and right superior (RSPV). Pulmonary vein activity was recorded with the ILMC at a proximal site in the ostium prior to ablation in each vein. During ablation, if PV potentials (PVPs) were visible during energy delivery, TTI was recorded when PVPs completely disappeared or were dissociated from LA activity. Durable PV isolation (PVI) was assessed at least 20 min after cryoenergy application. In the case of phrenic nerve palsy (PNP), recovery of diaphragmatic contraction was carefully monitored for 30 min.
Further additional cryoenergy applications were not applied when the veins were already isolated. If needed, pacing from the distal coronary sinus (CS) was performed to distinguish eventual far-field atrial signals from PVPs recorded on the mapping catheter, for left-sided PVs. Very seldomly, and not routinely, pacing from the proximal CS or the posterior wall of the right atrium was performed to confirm the presence of PVPs in the right-sided veins. Moreover, after having retrieved the 15 Fr sheath to the right atrium while keeping the ILMC in the LSPV, a bipolar catheter was introduced through the trans-septal access in the left atrial appendage, and pacing was performed in order to distinguish eventual far field of left atrial electrical activity from PVPs. During the whole procedure, activated clotting time was maintained over 250 s by supplementing heparin infusion as required.
Phrenic nerve monitoring
Before ablation of right-sided PVs, a standard decapolar catheter was placed in the superior vena cava cranial to the RSPV or in the subclavian vein in order to pace the right phrenic nerve (20–24 mA at 1.0–2.0 ms pulse width at a cycle length of 1200 ms) during ablation of the right-sided PVs. Phrenic nerve capture was achieved when contraction of the right hemidiaphragm could be observed both under fluoroscopic imaging and with manual palpation of the abdomen. The exact position of optimal phrenic nerve stimulation was then captured in two fluoroscopic projections in order to memorize it. Phrenic nerve pacing started when the temperature reached −20°C in order to avoid balloon dislodgement due to diaphragmatic contraction in the first phase of cryoenergy application. Pacing was continued throughout the whole duration of the cryoenergy delivery. Of note, because the procedure was performed under general anaesthesia, it is important to underline that only short-acting paralytic agents were used to facilitate intubation. These were no longer effective once the right-sided veins were approached. If loss of capture was observed, freeze was immediately aborted and observed for recovery. An immediate deflation technique was performed in the case of PNP as described by Ghosh et al.6 Additional applications were not necessary as all PVs were isolated at the time PNP occurred.
Post-ablation management
Patients were discharged the day following ablation if their clinical status was stable. After the intervention, the patients were continuously monitored with ECG telemetry for at least 18 h. Before hospital discharge, all patients underwent transthoracic echocardiography in order to exclude pericardial effusion and a chest X-ray. Oral anticoagulation was started the same evening of ablation and continued for at least 3 months.
Statistical analysis
Categorical variables are expressed as absolute and relative frequencies. Continuous variables are expressed as mean ± SD or median and range as appropriate. Comparisons of continuous variables were done with a Student's t-test and binomial variables with χ2 or Fisher's test as appropriate. A two-tailed P-value of <0.05 was deemed significant. Statistical analysis was performed using SPSS 20.0.0 (IBM, Inc., Armonk, NY, USA).
Results
Baseline characteristics
A total of 60 patients (60.5 ± 11.2 years, 62% males) with drug-resistant paroxysmal AF having undergone PVI by means of CB technology were taken into consideration for our analysis (Table 1). All had previously failed ≥1 Class I or III antiarrhythmic drugs. Mean time of AF was 22.7 ± 15.6 months. Mean left atrial diameter was 40.8 ± 7.6 mm. No patient was excluded based on pre-procedural CT scan anatomical findings.
| . | CB-ST (n = 60) . |
|---|---|
| Age (years) | 60.5 ± 11.2 |
| Male gender | 37 (62) |
| Duration of symptoms (months) | 22.7 ± 15.6 |
| Persistent AF | 15 (25) |
| Hypertension | 28 (47) |
| Dyslipidaemia | 19 (32) |
| Diabetes mellitus | 7 (12) |
| Coronary artery disease | 4 (7) |
| LVEF (%) | 58.7 ± 7.4 |
| Left atrial diameter (mm) | 40.8 ± 7.6 |
| CHA2DS2-VASc score | 1.61 ± 1.80 |
| Body mass index (kg/m2) | 25.9 ± 3.1 |
| . | CB-ST (n = 60) . |
|---|---|
| Age (years) | 60.5 ± 11.2 |
| Male gender | 37 (62) |
| Duration of symptoms (months) | 22.7 ± 15.6 |
| Persistent AF | 15 (25) |
| Hypertension | 28 (47) |
| Dyslipidaemia | 19 (32) |
| Diabetes mellitus | 7 (12) |
| Coronary artery disease | 4 (7) |
| LVEF (%) | 58.7 ± 7.4 |
| Left atrial diameter (mm) | 40.8 ± 7.6 |
| CHA2DS2-VASc score | 1.61 ± 1.80 |
| Body mass index (kg/m2) | 25.9 ± 3.1 |
Categorical variables are expressed as absolute and percentage (in parentheses). Continuous variables are expressed as mean ± SD. CB-ST, cryoballoon short-tip device; AF, atrial fibrillation.
| . | CB-ST (n = 60) . |
|---|---|
| Age (years) | 60.5 ± 11.2 |
| Male gender | 37 (62) |
| Duration of symptoms (months) | 22.7 ± 15.6 |
| Persistent AF | 15 (25) |
| Hypertension | 28 (47) |
| Dyslipidaemia | 19 (32) |
| Diabetes mellitus | 7 (12) |
| Coronary artery disease | 4 (7) |
| LVEF (%) | 58.7 ± 7.4 |
| Left atrial diameter (mm) | 40.8 ± 7.6 |
| CHA2DS2-VASc score | 1.61 ± 1.80 |
| Body mass index (kg/m2) | 25.9 ± 3.1 |
| . | CB-ST (n = 60) . |
|---|---|
| Age (years) | 60.5 ± 11.2 |
| Male gender | 37 (62) |
| Duration of symptoms (months) | 22.7 ± 15.6 |
| Persistent AF | 15 (25) |
| Hypertension | 28 (47) |
| Dyslipidaemia | 19 (32) |
| Diabetes mellitus | 7 (12) |
| Coronary artery disease | 4 (7) |
| LVEF (%) | 58.7 ± 7.4 |
| Left atrial diameter (mm) | 40.8 ± 7.6 |
| CHA2DS2-VASc score | 1.61 ± 1.80 |
| Body mass index (kg/m2) | 25.9 ± 3.1 |
Categorical variables are expressed as absolute and percentage (in parentheses). Continuous variables are expressed as mean ± SD. CB-ST, cryoballoon short-tip device; AF, atrial fibrillation.
Anatomical characteristics
No patient was excluded due to anatomical reasons based on the pre-procedural CT scan. A left common ostium was observed in 4 patients (6.7%) and a right-sided early branching could be observed in 2 patients (3.3%). No left-sided common trunk was documented. Of note, all left common ostia presented with two distinct branches with a distance from the common ostium to the site of branching of <5 mm. In the case of common ostium, the standard approach was to address, sequentially, the superior and the inferior branches delivering a single cryoenergy application for each one. The mean LSPV diameter was 17.9 ± 1.6 mm, mean LIPV diameter 17.3 ± 1.8 mm, mean RIPV diameter 17.7 ± 2.1 mm, and mean RSPV diameter 20.1 ± 1.6 mm.
Procedural characteristics
In the total population, procedural and fluoroscopy times were, respectively, 66.1 ± 18.9 and 13.6 ± 7.9 min. Procedural characteristics are listed in Table 2. In a total of 240 PVs, a Grade 4 occlusion could be obtained in 58 (97%), and for the remaining PVs (3%), a Grade 3 occlusion was documented. The latter were all RIPV in which a pull-down manoeuvre was performed to obtain isolation. Pulmonary vein isolation was successfully achieved in all veins without the need of additional focal catheter applications.
| . | CB-ST (n = 60) . |
|---|---|
| Procedural time (min) | 66.1 ± 18.9 |
| Fluoroscopy time (min) | 13.6 ± 7.9 |
| Freezes in LSPV | 1.1 ± 0.4 |
| Freezes in LIPV | 1.2 ± 0.5 |
| Freezes in RSPV | 1.2 ± 0.4 |
| Freezes in RIPV | 1.3 ± 0.5 |
| LSPV freeze duration (s) | 235.1 ± 83.8 |
| LIPV freeze duration (s) | 224.1 ± 86.4 |
| RSPV freeze duration (s) | 219.4 ± 67.2 |
| RIPV freeze duration (s) | 238.8 ± 85.3 |
| Min temp in LSPV | −51.2 ± 5.9 |
| Min temp in LIPV | −47.7 ± 6.0 |
| Min temp in RSPV | −50.9 ± 5.3 |
| Min temp in RIPV | −48.5 ± 6.3 |
| TPVI in LSPV (s) | 43.8 ± 20.9 |
| TPVI in LIPV (s) | 29.8 ± 20.0 |
| TPVI in RSPV (s) | 27.7 ± 14.0 |
| TPVI in RIPV (s) | 38.3 ± 15.3 |
| . | CB-ST (n = 60) . |
|---|---|
| Procedural time (min) | 66.1 ± 18.9 |
| Fluoroscopy time (min) | 13.6 ± 7.9 |
| Freezes in LSPV | 1.1 ± 0.4 |
| Freezes in LIPV | 1.2 ± 0.5 |
| Freezes in RSPV | 1.2 ± 0.4 |
| Freezes in RIPV | 1.3 ± 0.5 |
| LSPV freeze duration (s) | 235.1 ± 83.8 |
| LIPV freeze duration (s) | 224.1 ± 86.4 |
| RSPV freeze duration (s) | 219.4 ± 67.2 |
| RIPV freeze duration (s) | 238.8 ± 85.3 |
| Min temp in LSPV | −51.2 ± 5.9 |
| Min temp in LIPV | −47.7 ± 6.0 |
| Min temp in RSPV | −50.9 ± 5.3 |
| Min temp in RIPV | −48.5 ± 6.3 |
| TPVI in LSPV (s) | 43.8 ± 20.9 |
| TPVI in LIPV (s) | 29.8 ± 20.0 |
| TPVI in RSPV (s) | 27.7 ± 14.0 |
| TPVI in RIPV (s) | 38.3 ± 15.3 |
Continuous variables are expressed as mean ± SD. Min temp, minimal temperature; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; CB-A, cryoballoon advance device; CB-ST, cryoballoon short-tip device; TPVI, time from start of freezing to pulmonary vein isolation.
| . | CB-ST (n = 60) . |
|---|---|
| Procedural time (min) | 66.1 ± 18.9 |
| Fluoroscopy time (min) | 13.6 ± 7.9 |
| Freezes in LSPV | 1.1 ± 0.4 |
| Freezes in LIPV | 1.2 ± 0.5 |
| Freezes in RSPV | 1.2 ± 0.4 |
| Freezes in RIPV | 1.3 ± 0.5 |
| LSPV freeze duration (s) | 235.1 ± 83.8 |
| LIPV freeze duration (s) | 224.1 ± 86.4 |
| RSPV freeze duration (s) | 219.4 ± 67.2 |
| RIPV freeze duration (s) | 238.8 ± 85.3 |
| Min temp in LSPV | −51.2 ± 5.9 |
| Min temp in LIPV | −47.7 ± 6.0 |
| Min temp in RSPV | −50.9 ± 5.3 |
| Min temp in RIPV | −48.5 ± 6.3 |
| TPVI in LSPV (s) | 43.8 ± 20.9 |
| TPVI in LIPV (s) | 29.8 ± 20.0 |
| TPVI in RSPV (s) | 27.7 ± 14.0 |
| TPVI in RIPV (s) | 38.3 ± 15.3 |
| . | CB-ST (n = 60) . |
|---|---|
| Procedural time (min) | 66.1 ± 18.9 |
| Fluoroscopy time (min) | 13.6 ± 7.9 |
| Freezes in LSPV | 1.1 ± 0.4 |
| Freezes in LIPV | 1.2 ± 0.5 |
| Freezes in RSPV | 1.2 ± 0.4 |
| Freezes in RIPV | 1.3 ± 0.5 |
| LSPV freeze duration (s) | 235.1 ± 83.8 |
| LIPV freeze duration (s) | 224.1 ± 86.4 |
| RSPV freeze duration (s) | 219.4 ± 67.2 |
| RIPV freeze duration (s) | 238.8 ± 85.3 |
| Min temp in LSPV | −51.2 ± 5.9 |
| Min temp in LIPV | −47.7 ± 6.0 |
| Min temp in RSPV | −50.9 ± 5.3 |
| Min temp in RIPV | −48.5 ± 6.3 |
| TPVI in LSPV (s) | 43.8 ± 20.9 |
| TPVI in LIPV (s) | 29.8 ± 20.0 |
| TPVI in RSPV (s) | 27.7 ± 14.0 |
| TPVI in RIPV (s) | 38.3 ± 15.3 |
Continuous variables are expressed as mean ± SD. Min temp, minimal temperature; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; CB-A, cryoballoon advance device; CB-ST, cryoballoon short-tip device; TPVI, time from start of freezing to pulmonary vein isolation.
Real-time recordings
In the overall study population, RT isolation recording could be observed in 87.1% of all the PVs. Figure 3 shows the distribution of RT recordings for each PV. During LSPV ablation, RT could be visualized in 55 PVs (91.7%), and during LIPV ablation, RT recordings were present in 51 PVs (85%) (Figure 3). In the right-sided PVs cryoablation, the rate of visualization of RT recordings was 83.3% during RIPV ablation (50 out of 60 PVs) and 88.3% during RSPV ablation (53 out of 60 PVs) (Figure 3). Overall, 33 patients (55%) exhibited RT recordings in all PVs and 27 (45%) did not have PVPs in at least one vein. During a mean follow-up period of 3.8 ± 0.7 months (median 4 months), 4 patients of the former group (12%) experienced AF recurrence (4/33) compared with 3 patients of the latter (11%; P = 0.6). Of note, considerable noise was observed in the Achieve catheter during the freeze cycle, limited to 2 dipoles, in 2 patients (3.3%); 1 exhibiting the occurrence of noise 23 s from the beginning of the freeze and the other showing significant noise after 29 s of freezing. In both cases, noise occurred before PVI; however, RT recordings could be clearly assessed in the remaining dipoles for both patients and time to PVI could be evaluated.
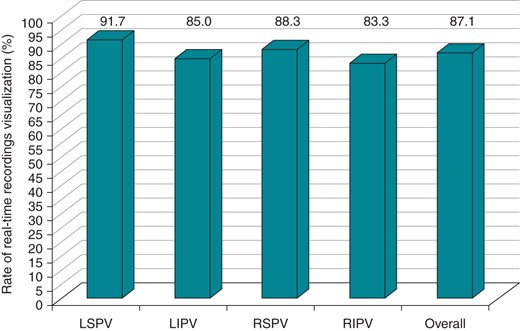
The histogram illustrates the rate of visualization of RT recordings for each vein during CB-ST ablation technology.
Complications
In the overall study, population major complications occurred in 1 of 60 patients (1.7%). The only serious adverse event was constituted by a femoral pseudoaneurysm treated with percutaneous thrombin injection, without significant sequelae. A total amount of 2 transient PNPs (3.3%) were observed; of note, no persistent PNPs occurred.
Discussion
To the best of our knowledge, this is the first article focusing on the novel third-generation CB. The main findings of our study are (i) the rate of visualization of RT recordings of PVPs is considerably high during CB-ST ablation and (ii) the shorter tip does not jeopardize catheter manoeuvrability in the LA.
Real-time visualization of PV electrograms during the application of cryoenergy might provide interesting information on time to PVI and consequently on the conduction properties of the LA-PV junction.7–9 Furthermore, it seems that shorter times to isolation are associated with sustained LA-PV disconnection.7,8 Conversely, longer times to isolation tend to reliably predict early recovery of PV conduction.9 Anatomically, the PV sleeves are often thicker and occupy more of the circumference at the atrial end, but they tend to become thinner as you progressively move more distally into the vein.10
Recent publications reported a 50–60% rate of RT recordings visualization during CB ablation.1,11 Given the above-mentioned considerations, this percentage might appear deceiving. Although the current strategy usually commences by recording baseline electrical activity at the PV ostium level before attempting occlusion and subsequently ablation, TTI might not only be crucial in offering valuable information on LA-PV conduction, but it might also guide the operator in tailoring the dosing strategy of the freeze–thaw cycle. In fact, recent data in the literature seem to indicate that shorter application times seem to offer similar clinical outcome if compared with the recommended ‘2 times 240 s’ strategy.5,12,13 However, although shortening the application duration has proved effective and might lead to shorter procedural times and fluoroscopy times, these findings need to be confirmed in large and randomized trials. As mentioned above, time to PVI has been reported as one of the most important predictor of durable lesion, and RT recordings provide to the operators valuable information about the risk of PV reconnection and freezing efficacy. However, in our standard practice, in case of no RT recordings, the decision to perform an additional freeze was based on the temperature drop and the minimal temperature achieved.
In our study, RT recordings could be visualized in ∼90% of veins when using the ILMC in conjunction with the CB-ST (Figure 3). This high rate of RT recordings observed during CB-ST ablation might be explained by the 40% shorter shaft tip the balloon is mounted on in the third-generation device (Figure 1). This feature has greatly shortened the distance between the ablation site and the ILMC position. This results in a higher probability of the Achieve catheter being in contact with the PV sleeves and therefore providing important electrical information on the effects of ablation in that vein (Figure 2).
Similarly to previous reports, RT recordings were more frequently observed in the superior veins if compared with the lower PVs.1,11 One potential explanation lies in fact that the CB tends to be coaxial to the venous course when being positioned in the antra of both upper veins, while more catheter manipulation is usually required to occlude the inferior veins.14 In fact, when positioning the balloon in the lower veins, the ILMC often needs to be positioned more distally in the vein in order to offer better catheter stability.15 In addition, the PV sleeves are typically shorter in the inferior veins in comparison with the superior ones.16 Consequently, the chances of visualizing RT recordings might diminish drastically.
A recent study meticulously described specific manoeuvres designed to maximize RT recordings during second-generation CB-A ablation.17 The authors concluded that electrical information could be gathered in most PVs. However, the operator had to rely often on exit block verification during CB application. Although unidirectional block is extremely rare during PVI18 and therefore exit block may be an effective alternative to confirm PV, these manoeuvres might be difficult to achieve in some cases on the ILMC. Moreover, direct disappearance or dissociation of PVPs from LA activity might be significantly more simple to interpret. Finally, when pacing to analyse RT documentation of PV exit block in the right-sided veins, an additional external pacing source is needed to pace the phrenic nerve and monitor diaphragmatic contraction. These manoeuvres might therefore render a somewhat originally straightforward ablation procedure more complex and cumbersome.
The shorter tip did not jeopardize catheter manoeuvrability in the LA. In fact, all the other procedural parameters did not differ significantly between both devices. In fact, apart from the modification to the distal portion of the shaft, the CB-ST has been designed with all the previously improved features that characterize the excellent performances of the second-generation device.19,20 Occlusion as a surrogate marker of catheter stability in the PV ostium might hypothetically be more challenging with a shorter device tip mounted on a guidewire. Fortunately, this was not the case, as in most veins a successful Grade 4 occlusion could be achieved with the CB-ST. Interestingly, in 2 patients (3.3%), important noise in 2 dipoles of the Achieve catheter during the freeze cycle was recorded, probably related to the proximity to the balloon. However, in both patients, RT recordings could be clearly assessed in the remaining channels for both patients and time to PVI could be evaluated.
In our centre, the current procedural protocol is to perform a standard 3 min single freeze per vein. Other centres currently performing CB ablation with longer freezing times and with an additional bonus freeze may consider basing their dosing strategy on TTI in those patients with RT recordings. In this context, we may hypothesize that the CB-ST could lead to faster procedural times. Of note, during the follow-up, the rate of AF recurrence did not differ between patients with RT recordings in all PVs and those without (12 vs. 11%; P = 0.6). However, the short follow-up period and the relatively small number of patients in the present study may represent an important limitation. It should be also mentioned that electrical isolation could be obtained in all veins without the need of focal tip catheter touch-up irrespective of the anatomical variations of the PV drainage pattern. This finding underlines once more the technological improvements with respect to the first-generation balloon but might also question the utility of a pre-procedural CT scan prior to CB ablation.
Finally, the rate of serious adverse events in our series is considerably low (1.7%) consistently with previously published studies on second-generation CB technology. Only 2 transient PNPs (3.3%) occurred. Interestingly, in this cohort of patients having undergone CB-ST ablation, we did not observe persistent PNP. This might be due on the one hand to the relatively small number of the study population but also to a growing experience with this ablation procedure and in the systematic performance of an immediate deflation technique, as described by Ghosh et al.6 This technique has proved very effective in pre-empting persistent phrenic nerve damage.
Limitations
Although conducted on a large cohort of patients, this study bares the limitations of being single centre and non-randomized. We did not use a thermal oesophageal probe. Therefore, a certain amount of oesophageal lesions might have gone unnoticed. We did not perform cMAP during right phrenic nerve pacing. The incidence of PNP might have been lower.
Conclusions
The rate of visualization of RT recordings of PVPs could be visualized in ∼90% of veins during third-generation CB-ST ablation. The shorter tip did not jeopardize catheter manoeuvrability in the LA nor the safety of the procedure.
Conflict of interest: G.-B.C. and C.d.A. receive compensation for teaching purposes and proctoring from AF solutions, Medtronic. P.B. receives research grants on behalf of the centre from Biotronik, Medtronic, St Jude Medical, Sorin, Boston Scientific and speakers fees from Biosense-Webster, Biotronik, Medtronic. C.d.A. is consultant for Daiichi Sankyo. G.M. is currently receiving an educational grant from Medtronic for Postgraduate in Cardiac Electrophysiology and Pacing academic course. V.V. is currently receiving an EHRA educational grant sponsored by St Jude Medical.
References
Author notes
Contributed as first author.



