-
PDF
- Split View
-
Views
-
Cite
Cite
Jin Iwasawa, Shinsuke Miyazaki, Takamitsu Takagi, Hiroshi Taniguchi, Hiroaki Nakamura, Hitoshi Hachiya, Yoshito Iesaka, Cavotricuspid isthmus ablation using a catheter equipped with mini electrodes on the 8 mm tip: a prospective comparison with an 8 mm dumbbell-shaped tip catheter and 8 mm tip cryothermal catheter, EP Europace, Volume 18, Issue 6, June 2016, Pages 868–872, https://doi.org/10.1093/europace/euv253
Close - Share Icon Share
Abstract
The mini electrodes (ME) placed on the tip of the ablation electrode provide more precise local signal. We evaluated whether ME catheter was effective for the ablation of cavotricuspid isthmus (CTI)-dependent atrial flutter.
Eighty-five consecutive patients (68 men; 62 ± 10 years) underwent CTI ablation either using a catheter equipped with ME on the 8 mm tip (ME catheter) in 25 patients (Group A), 8 mm dumbbell-shaped (DS) tip catheter (DS catheter) in 30 patients (Group B), or 8 mm tip cryothermal catheter (Cryo catheter) in 30 patients (Group C). In cases of failed isthmus block, the catheter was changed to the other catheter, but patients remained in the original group following intention-to-treat analysis. The endpoint was achieved in all patients after 13 ± 7 applications in Group A, 9 ± 4 applications in Group B, and 5 ± 2 applications in Group C (P < 0.001). The fluoroscopic and procedure times were significantly longer in Group A (9 ± 7 and 28 ± 17 min, P = 0.001, and P = 0.002, respectively) when compared with Groups B (6 ± 4 and 13 ± 6 min) and C (4 ± 3 and 14 ± 7 min). A crossover was performed in 14 (56%) Group A patients, and 3 (10%) Group C patients. The mean power delivered in Group A was significantly lower than in Group B (31.3 ± 9.1 vs. 38.6 ± 7.6 W, P = 0.015).
The ME catheter was found to be less effective than the Cryo catheter and a DS catheter for the CTI ablation.
Our data indicate for the first time that ME catheter was less effective than Cryo catheter and dumbbell-shaped catheter for the ablation of CTI-dependent atrial flutter.
Delivered power under the temperature control mode was limited in the ME catheter, which may cause the ineffectiveness of CTI ablation.
Introduction
Various catheter technologies have evolved to improve the procedural success of cavotricuspid isthmus (CTI) block. Irrigated or large-tip catheters have a theoretical advantage of creating wider and deeper lesions than the conventional catheters.1,2 Previous studies investigating the acute and long-term effects of cryoablation for CTI-dependent atrial flutter reported similar efficacy and safety rates as those reported for radiofrequency (RF) ablation.3,4
A new catheter equipped with ME on the 8 mm tip (ME catheter) (IntellaTip MiFi XP, Boston Scientific, Boston, MA, USA) compatible with conventional ablation catheters is now commercially available but no data about its performance in clinical use have been reported. We hypothesized that the addition of small pin electrodes in close proximity to the electrode tip on an 8 mm tip catheter would improve the operator's ability to define effective lesion formation. In addition, pin electrodes would also likely improve the ability to define conduction gaps created during the formation of the CTI linear lesions.
This prospective study evaluated the efficiency and the feasibility of the ME catheter for CTI ablation by comparing it with an 8 mm dumbbell-shaped tip catheter (DS catheter) (Ablaze, Japan Lifeline, Tokyo, Japan) and 8 mm tip cryothermal catheter (Cryo catheter) (Freezor Max, Medtronic CryoCath LP, Quebec, Canada).
Methods
Study population
The study population consisted of 85 consecutive patients (68 men; 62 ± 10 years) with atrial fibrillation (AF) who underwent CTI ablation following an AF ablation. All patients provided their written informed consent before the procedure. The study was approved by our institutional review board. The patients were prospectively divided into three groups utilizing one of the three following ablation catheters: (i) catheter equipped with ME on the 8 mm tip (IntellaTip MiFi XP) (Group A, n = 25). The pin electrodes were 1 mm in diameter, radially distributed around the tip of the ablation catheter at 120° orientations. The electrodes were located 2 mm from the distal 8 mm ablation tip and 2.5 mm from one another in a circumferential orientation (Figure 1A, (i)). (ii) An 8 mm DS tip catheter (Ablaze) (Group B, n = 30). The DS tip was designed to improve the delivered power by increasing the cooling effect (Figure 1B, (ii)). (iii) An 8 mm tip cryothermal catheter (Freezor Max) (Group C, n = 30) (Figure 1C, (iii)).
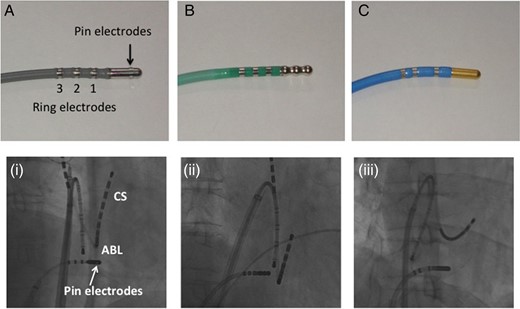
(A) An 8 mm tip catheter equipped with three pin electrodes (IntellaTip MiFi XP, Boston Scientific Corporation, Natick, MA, USA). (B) An 8 mm DS tip catheter (Ablaze). (C) An 8 mm tip cryothermal catheter (Freezor Max, Medtronic CryoCath LP). The radiography image demonstrates the ablation catheter placed on the CTI with the (i) ME catheter, (ii) DS catheter, and (iii) Cryo catheter.
Electrophysiological study and catheter ablation
All anti-arrhythmic drugs were discontinued more than five half-lives prior to the procedure. A 7 Fr 20-pole three-site mapping catheter (BeeAT, Japan Lifeline) was inserted through the right jugular vein, and the eight distal electrodes were positioned in the coronary sinus (CS). In patients with paroxysmal AF, a sole PV isolation was performed using an irrigated-tip catheter or cryoballoon catheter. In patients with persistent AF, additional substrate modification was performed if the AF did not terminate during the PV isolation.5,6 Following the left atrial ablation, a 10-pole mapping catheter was positioned in the lateral right atrium (RA). The surface electrocardiogram and bipolar endocardial electrograms were continuously monitored and recorded (Bard Electrophysiology, Lowell, MA, USA). The intracardiac electrograms were filtered from 30 to 500 Hz and measured at a sweep speed of 100 mm/s. The system was configured to monitor and provide the mean power, temperature, and impedance during each RF application.
Subsequently, the ablation of CTI was performed under pacing from the proximal CS or low lateral RA after the restoration of sinus rhythm. In Groups A and B, the ablation was performed by point-by-point RF applications for a maximum duration of 60 s each. A temperature controlled RF delivery was performed with a maximum power output of 50 W and temperature limit of 58°C. In Group A, the bipolar signals recorded between the microelectrodes were obtained throughout the procedure. In Group C, the individual lesions were placed in a point-by-point fashion for duration of 120 s with a steerable sheath (FlexCath, Medtronic). A temperature controlled ablation was performed at a maximum temperature of −80°C. The procedural endpoint was the creation of a bidirectional isthmus block along the CTI as described elsewhere.7–9 In the case of failed isthmus block at the physician's discretion or within 10 applications, the catheter was exchanged for another type catheter, but the patients remained in the original group following an intention-to-treat analysis.
Statistical analysis
Continuous variables are expressed as the means ± standard deviation, and categorical variables as the frequency (percentage). A χ2 test was used to compare the categorical variables. A two-sample t-test was used to compare the normally distributed continuous variables. A non-parametric test, Mann–Whitney, and Kruskal–Wallis test were used to compare the non-normal continuous variables. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
The mean age was 61.8 ± 9.7 years and 68 (80%) patients were male. The mean left atrial dimension (LAD) was 40.3 ± 5.2 mm and the mean left ventricular ejection fraction (LVEF) was 63.0 ± 8.9%. Hypertension and diabetes mellitus were observed in 37 (43.5%) and 10 (11.8%) patients, respectively, and 6 (7.1%) patients experienced stroke. Six (7.1%) patients also had experienced congestive heart failure.
Table 1 shows the demographic characteristics of the patients in each group. There was no significant difference between the groups in terms of the clinical characteristics except for the LVEF and LAD.
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Age | 62 ± 10 | 60 ± 10 | 63 ± 10 | 0.408 |
| Male sex | 20 (80%) | 23 (77%) | 25 (83%) | 0.814 |
| LVEF (%) | 64 ± 8 | 59 ± 10 | 66 ± 6 | 0.005 |
| LAD (mm) | 41 ± 4 | 43 ± 5 | 37 ± 5 | <0.001 |
| Hypertension | 11 (44%) | 12 (40%) | 14 (47%) | 0.873 |
| Heart failure | 0 (0%) | 6 (20%) | 0 (0%) | 0.003 |
| Diabetes mellitus | 3 (17%) | 4 (20%) | 3 (10%) | 0.923 |
| Stroke | 2 (15%) | 2 (2%) | 2 (2%) | 0.977 |
| Medication | ||||
| Beta-blockers | 7 (28%) | 9 (30%) | 7 (23%) | 0.84 |
| Calcium channel blockers | 6 (24%) | 8 (27%) | 8 (27%) | 0.968 |
| ACE or ARB | 5 (20%) | 7 (23%) | 9 (36%) | 0.68 |
| Anti-arrhythmic drugs | 5 (20%) | 10 (30%) | 9 (36%) | 0.535 |
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Age | 62 ± 10 | 60 ± 10 | 63 ± 10 | 0.408 |
| Male sex | 20 (80%) | 23 (77%) | 25 (83%) | 0.814 |
| LVEF (%) | 64 ± 8 | 59 ± 10 | 66 ± 6 | 0.005 |
| LAD (mm) | 41 ± 4 | 43 ± 5 | 37 ± 5 | <0.001 |
| Hypertension | 11 (44%) | 12 (40%) | 14 (47%) | 0.873 |
| Heart failure | 0 (0%) | 6 (20%) | 0 (0%) | 0.003 |
| Diabetes mellitus | 3 (17%) | 4 (20%) | 3 (10%) | 0.923 |
| Stroke | 2 (15%) | 2 (2%) | 2 (2%) | 0.977 |
| Medication | ||||
| Beta-blockers | 7 (28%) | 9 (30%) | 7 (23%) | 0.84 |
| Calcium channel blockers | 6 (24%) | 8 (27%) | 8 (27%) | 0.968 |
| ACE or ARB | 5 (20%) | 7 (23%) | 9 (36%) | 0.68 |
| Anti-arrhythmic drugs | 5 (20%) | 10 (30%) | 9 (36%) | 0.535 |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker.
The values are presented as the mean ± SD or as the n (%).
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Age | 62 ± 10 | 60 ± 10 | 63 ± 10 | 0.408 |
| Male sex | 20 (80%) | 23 (77%) | 25 (83%) | 0.814 |
| LVEF (%) | 64 ± 8 | 59 ± 10 | 66 ± 6 | 0.005 |
| LAD (mm) | 41 ± 4 | 43 ± 5 | 37 ± 5 | <0.001 |
| Hypertension | 11 (44%) | 12 (40%) | 14 (47%) | 0.873 |
| Heart failure | 0 (0%) | 6 (20%) | 0 (0%) | 0.003 |
| Diabetes mellitus | 3 (17%) | 4 (20%) | 3 (10%) | 0.923 |
| Stroke | 2 (15%) | 2 (2%) | 2 (2%) | 0.977 |
| Medication | ||||
| Beta-blockers | 7 (28%) | 9 (30%) | 7 (23%) | 0.84 |
| Calcium channel blockers | 6 (24%) | 8 (27%) | 8 (27%) | 0.968 |
| ACE or ARB | 5 (20%) | 7 (23%) | 9 (36%) | 0.68 |
| Anti-arrhythmic drugs | 5 (20%) | 10 (30%) | 9 (36%) | 0.535 |
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Age | 62 ± 10 | 60 ± 10 | 63 ± 10 | 0.408 |
| Male sex | 20 (80%) | 23 (77%) | 25 (83%) | 0.814 |
| LVEF (%) | 64 ± 8 | 59 ± 10 | 66 ± 6 | 0.005 |
| LAD (mm) | 41 ± 4 | 43 ± 5 | 37 ± 5 | <0.001 |
| Hypertension | 11 (44%) | 12 (40%) | 14 (47%) | 0.873 |
| Heart failure | 0 (0%) | 6 (20%) | 0 (0%) | 0.003 |
| Diabetes mellitus | 3 (17%) | 4 (20%) | 3 (10%) | 0.923 |
| Stroke | 2 (15%) | 2 (2%) | 2 (2%) | 0.977 |
| Medication | ||||
| Beta-blockers | 7 (28%) | 9 (30%) | 7 (23%) | 0.84 |
| Calcium channel blockers | 6 (24%) | 8 (27%) | 8 (27%) | 0.968 |
| ACE or ARB | 5 (20%) | 7 (23%) | 9 (36%) | 0.68 |
| Anti-arrhythmic drugs | 5 (20%) | 10 (30%) | 9 (36%) | 0.535 |
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker.
The values are presented as the mean ± SD or as the n (%).
Ablation results
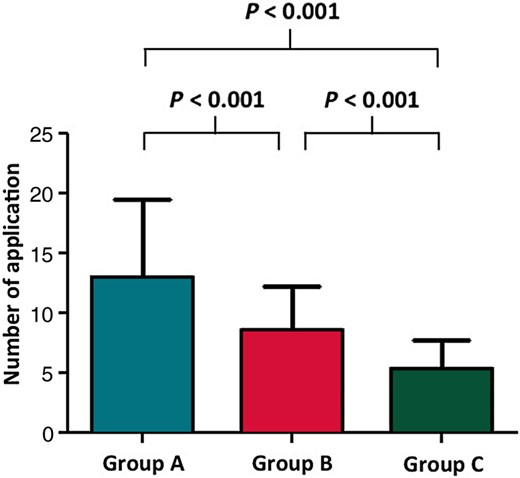
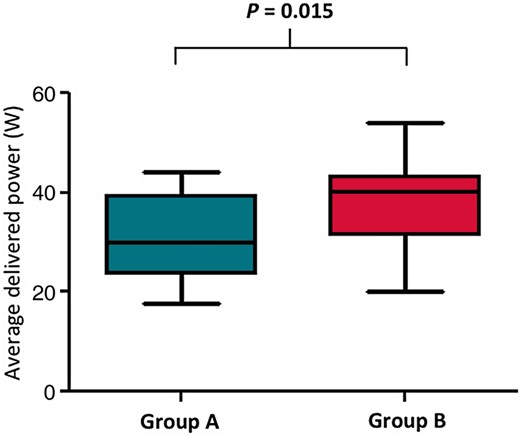
Exchanging the initial catheter to another type of catheter was required in 14 (56%) patients in Group A, and in 3 (10%) patients in Group C (P = <0.001). This resulted in ending up with 25 patients in Group A even though initially it was planned to have 30 in this study for the intention-to-treat analysis. The selected endpoint could be achieved in all patients after 13.0 ± 6.5 applications in Group A, 8.6 ± 3.7 applications in Group B, and 5.3 ± 2.5 applications in Group C (P < 0.001) (Figure 2). The number of RF applications and RF duration to achieve CTI block was significantly longer in Group A than in Groups B and C (P= 0.002). The fluoroscopic time and the total procedure time were also significantly longer in Group A (P = 0.001) than in Groups B and C (Table 2). Other parameters were measured including the temperature, impedance, power, and total energy in Groups A and B in the intention-to-treat analysis. The average power was significantly lower in Group A than in Group B (P = 0.015) (Figure 3), whereas the other parameters were similar between the groups.
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Number of application | 13.0 ± 6.5 | 8.6 ± 3.7 | 5.3 ± 2.5 | <0.001 |
| Ablation time (s) | 21.7 ± 13.6 | 12.5 ± 6.0 | 14.3 ± 7.3 | 0.002 |
| Fluoroscopic time (s) | 9.1 ± 6.6 | 5.7 ± 3.6 | 4.5 ± 3.2 | 0.001 |
| Total procedure time (s) | 28.4 ± 17.3 | 15.0 ± 5.8 | 18.6 ± 9.2 | 0.001 |
| Average temperature (°C) | 49.1 ± 1.6 | 47.4 ± 2.3 | 0.005 | |
| Average impedance (Ω) | 75.3 ± 6.0 | 76.3 ± 6.3 | 0.614 | |
| Average power (W) | 31.3 ± 9.1 | 38.6 ± 7.6 | 0.015 | |
| Total application energy (J) | 17 027 ± 6025 | 18 789 ± 8383 | 0.545 | |
| Crossover | 14 (56%) | 0 (0%) | 3 (10%) | <0.001 |
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Number of application | 13.0 ± 6.5 | 8.6 ± 3.7 | 5.3 ± 2.5 | <0.001 |
| Ablation time (s) | 21.7 ± 13.6 | 12.5 ± 6.0 | 14.3 ± 7.3 | 0.002 |
| Fluoroscopic time (s) | 9.1 ± 6.6 | 5.7 ± 3.6 | 4.5 ± 3.2 | 0.001 |
| Total procedure time (s) | 28.4 ± 17.3 | 15.0 ± 5.8 | 18.6 ± 9.2 | 0.001 |
| Average temperature (°C) | 49.1 ± 1.6 | 47.4 ± 2.3 | 0.005 | |
| Average impedance (Ω) | 75.3 ± 6.0 | 76.3 ± 6.3 | 0.614 | |
| Average power (W) | 31.3 ± 9.1 | 38.6 ± 7.6 | 0.015 | |
| Total application energy (J) | 17 027 ± 6025 | 18 789 ± 8383 | 0.545 | |
| Crossover | 14 (56%) | 0 (0%) | 3 (10%) | <0.001 |
The values are presented as the mean ± SD.
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Number of application | 13.0 ± 6.5 | 8.6 ± 3.7 | 5.3 ± 2.5 | <0.001 |
| Ablation time (s) | 21.7 ± 13.6 | 12.5 ± 6.0 | 14.3 ± 7.3 | 0.002 |
| Fluoroscopic time (s) | 9.1 ± 6.6 | 5.7 ± 3.6 | 4.5 ± 3.2 | 0.001 |
| Total procedure time (s) | 28.4 ± 17.3 | 15.0 ± 5.8 | 18.6 ± 9.2 | 0.001 |
| Average temperature (°C) | 49.1 ± 1.6 | 47.4 ± 2.3 | 0.005 | |
| Average impedance (Ω) | 75.3 ± 6.0 | 76.3 ± 6.3 | 0.614 | |
| Average power (W) | 31.3 ± 9.1 | 38.6 ± 7.6 | 0.015 | |
| Total application energy (J) | 17 027 ± 6025 | 18 789 ± 8383 | 0.545 | |
| Crossover | 14 (56%) | 0 (0%) | 3 (10%) | <0.001 |
| . | Group A (n = 25) . | Group B (n = 30) . | Group C (n = 30) . | P . |
|---|---|---|---|---|
| Number of application | 13.0 ± 6.5 | 8.6 ± 3.7 | 5.3 ± 2.5 | <0.001 |
| Ablation time (s) | 21.7 ± 13.6 | 12.5 ± 6.0 | 14.3 ± 7.3 | 0.002 |
| Fluoroscopic time (s) | 9.1 ± 6.6 | 5.7 ± 3.6 | 4.5 ± 3.2 | 0.001 |
| Total procedure time (s) | 28.4 ± 17.3 | 15.0 ± 5.8 | 18.6 ± 9.2 | 0.001 |
| Average temperature (°C) | 49.1 ± 1.6 | 47.4 ± 2.3 | 0.005 | |
| Average impedance (Ω) | 75.3 ± 6.0 | 76.3 ± 6.3 | 0.614 | |
| Average power (W) | 31.3 ± 9.1 | 38.6 ± 7.6 | 0.015 | |
| Total application energy (J) | 17 027 ± 6025 | 18 789 ± 8383 | 0.545 | |
| Crossover | 14 (56%) | 0 (0%) | 3 (10%) | <0.001 |
The values are presented as the mean ± SD.
Among the 14 patients in Group A, no sharp potentials were identified along the line in 12 patients and a sufficient delivery of the power was not obtained in 7 patients before switching to the ME catheter. Bidirectional CTI block was created by additional 5.2 ± 5.1 applications using the DS catheter and irrigation-tip catheters after the crossover in 3 (21%) and 11 (79%) patients, respectively. In all three patients whom the CTI block failed in Group C using the DS catheter as a second catheter, CTI block was created by an additional 3.0 ± 2.1 applications after the crossover. In those three patients, no transient CTI block was observed during the ablation using the Cryo catheter. In the 14 patients who required a switch of the catheter in Group A, transient CTI block was observed in 6 patients (43%), but persistent block could not be obtained before the crossover. Among those 14 patients, bidirectional CTI block was achieved within one or less than three applications after the crossover in three and nine patients, respectively. In five patients, a higher power obtained by the second catheter compared with the ME catheter resulted in the achievement of persistent CTI block (30 ± 0.3 vs. 22 ± 3.3 W, P = 0.046). In the remaining four patients, an improved catheter–tissue contact after the switching the catheter resulted in the achievement of CTI block with a similar power delivered before and after the crossover.
Discussion
Main findings
To the best of our knowledge, this is the first clinical study to evaluate the efficacy of the ME catheter for CTI ablation. The study results showed that (i) the efficacy of ME catheter was significantly inferior to that of the DS and Cryo catheters and suggested that (ii) a limited power delivery under the temperature control mode was the major reason for the lower efficacy in the ME group.
Efficacy and safety
A crossover to complete the CTI block was required in 56% of the patients in Group A. We hypothesized that the addition of small pin electrodes in close proximity to the electrode tip on an 8 mm tip catheter would improve the operator's ability to define an effective lesion formation. However, in this study, the procedure time and the fluoroscopic time were significantly longer for the CTI ablation with the ME catheter than the Cryo and DS catheters. Transient block in 6 of 14 patients (43%) occurred during the ablation with the ME catheter, significantly more than with the other catheters. This suggested that it was difficult to create a transmural lesion with the ME catheter. The electrograms recorded from the ME along the CTI line were sufficiently attenuated after the ablation, but complete conduction block was not achieved in numerous cases. We ablated the site where large spiky potentials were recorded on the ME, which would result in shallow lesions if the ME recordings were limited to superficial tissues underlying the ablation electrode.10
The RF power delivered to the tissue determines the lesion size. With good contact between the catheter tip and tissue and lower cooling of the catheter tip, the target temperature can be reached with little power resulting in a fairly small lesion even though a high tip temperature is being measured.11–13 In this study, insufficient power was delivered in some patients, while in others with poor tissue contact of the ablation catheter requiring switch of the catheter, caused incomplete CTI block during a temperature controlled RF ablation occurred. The former could be explained by the fact that a higher power obtained by the second catheter than the ME catheter resulted in the achievement of persistent CTI block. The latter was probably due to the successful CTI ablation by changing the catheter although there were no sharp potentials along the CTI line after ablation.
Cryotherapy could be performed without any significant prolongation of the procedure or fluoroscopy times, which is in accordance with the other studies.14,15 Since the Cryo catheter is firmly attached to the tissue during each cryoapplication, in particular, with the deflectable sheath used in the present study, there is no need to check the catheter position repeatedly with fluoroscopy.16
There were no adverse events associated with the use of either catheter. Therefore, under the RF settings used in this study and with close monitoring of the impedance and temperature, all catheters appeared to be safe for the ablation of the CTI.
The mini electrodes catheter vs. the DS catheter
The number of applications, ablation time, fluoroscopic time, and total procedure time significantly differed between the Groups A and B (Table 2). This difference could be partly explained by the temperature sensor position on the catheter tip. The ME catheter had an isolated tip temperature sensor. We needed to deliver more appropriate power levels to maintain the target temperature than when using a catheter with an embedded tip temperature sensor because the isolated tip temperature sensor provided a more accurate measurement of the actual peak tissue temperature. Considering that the addition of the ME around the circumference of the catheter tip focused the recording at only the tissues that came in contact with the ablation electrode, the number of applications should have decreased since it should have taken less time to search for the optimal potentials on the CTI line. However, in fact, the number of applications was significantly greater in Group A than in Group B. Our results showed that this was caused by a lower power delivered during ablation with the ME catheter. The catheter with DS tip was designed to improve the delivered power by increasing the cooling effect due to the intramyocardial blood flow. The potentials obtained by the ME may not be useful because the ME could detect only superficial potentials, and therefore those would not reflect the transmural lesions.
Study limitation
First, the durability of the CTI block was not evaluated in the repeat sessions.
Secondly, the impact of CTI anatomy on the ablation results was not evaluated.
Conclusion
The ME catheter was found to be less effective than the Cryo and DS catheters for CTI ablation. A limited power delivery under the temperature control mode presumably due to the tip design was the major reason for the lower efficacy.
Conflict of interest: none declared.



