-
PDF
- Split View
-
Views
-
Cite
Cite
Dong-Sheng Zhao, Yi Shen, Qing Zhang, Gang Lin, Yi-Hua Lu, Bang-Tao Chen, Lin-Sheng Shi, Jian-Fei Huang, Hui-He Lu, Outcomes of catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a systematic review and meta-analysis, EP Europace, Volume 18, Issue 4, April 2016, Pages 508–520, https://doi.org/10.1093/europace/euv339
Close - Share Icon Share
Abstract
Over the past decade, catheter ablation (CA) has become an established therapy for symptomatic atrial fibrillation (AF). Atrial fibrillation is common in hypertrophic cardiomyopathy (HCM) patients, and restoring sinus rhythm is of great clinical benefit to them. Therefore, we conducted a systematic review and meta-analysis of the available data to evaluate the effectiveness and safety of CA for AF in patients with HCM.
Six databases were searched to identify studies describing outcomes of CA of AF in HCM patients with a mean follow-up of ≥12 months after the index procedure. The following data were extracted: (i) single-procedure success, (ii) multiple-procedure success, and (iii) drug-free success. Fifteen studies involving 531 patients were included. Single-procedure freedom from atrial arrhythmia at the latest follow-up was 45.5% [95% confidence interval (CI): 34.8–56.2%]. With multiple procedures, the final success rate was 66.1% (95% CI: 55.3–76.9%) overall, 71.8% (95% CI: 61.6–82.0%) in paroxysmal AF, and 47.5% (95% CI: 36.0–59.0%) in non-paroxysmal AF. Without anti-arrhythmic drugs (AADs), single-procedure success rate at latest follow-up was 32.9% (95% CI: 21.7–41.1%); after multiple procedures, this raised to 50.4% (95% CI: 39.2–61.6%). The incidence of serious periprocedural complications was acceptable [5.1% (95% CI: 2.8–9.6%)]. Substantial heterogeneity (I2> 50%) was noted in the above groups.
Catheter ablation of AF in patients with HCM is feasible, although more repeat procedures and AAD are needed to prevent AF recurrence.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy. Hypertrophic cardiomyopathy is characterized by the presence of an asymmetric hypertrophied, non-dilated left ventricle (LV) (usually based on two-dimensional echocardiography), with or without relevant outflow tract obstruction, in the absence of local or systemic aetiology that might result in myocardial hypertrophy. Approximately 20–25% of HCM patients will eventually develop atrial fibrillation (AF) during the clinical course of the disease, a percentage that is significantly higher than that of the general population.1,2 It has been demonstrated that cardioversion of AF to sinus rhythm is beneficial to reduce the risk of HCM-related death in obstructed ones,3 mainly because they may rely on left atrium (LA) contraction for LV filling to relieve obstruction.4 Even in the non-obstructive ones, rate control and stroke prevention are not enough because of heart failure-related mortality, stroke, and severe functional disability.3
Recent AF treatment guidelines favour maintaining sinus rhythm in patients with HCM.5,6 Catheter ablation (CA) has been shown to be superior to drug therapy in the aspect of rhythm control of AF.7,8 However, CA in HCM patients may be associated with a higher risk of AF recurrence or complications due to differences in atrial substrates that are essential and/or secondary to ventricular hypertrophy. In the current study, we systematically reviewed the medical literature to evaluate the efficacy and safety of AF ablation in HCM patients.
Methods
We conducted a systematic review using a predefined protocol, in accordance with the Meta-Analysis of Observational Studies in Epidemiology statement,9 and based on a similar system review.10 Both English-based literature databases (PubMed, Embase, and Web of Science) and Chinese-based literature databases (Wanfang, CBM, and Chinese HowNet) were searched for published articles that described the outcome of AF ablation in HCM patients. The search design was established with the assistance of a research librarian and the detailed search methodology employed is presented in Supplementary material online, Appendix S1. This search was supplemented by hand-searching bibliographies of the selected studies and relevant review articles. Citations were included if they involved an evaluation of percutaneous CA outcomes at ≥12 months after the index ablation procedure. Randomized controlled trials, case–control studies, cohort studies, and case series (≥10 cases) were included. Individual case reports, editorials, review articles, and meeting abstracts were excluded. Studies involving surgical AF ablation and atrioventricular nodal ablation, or exclusive right atrial ablation, were also excluded.
The literature search and study selection were conducted by three independent reviewers (D.-S.Z., Q.Z., and G.L.). The differences were resolved by consensus. Selected publications were analysed for the following outcomes: (i) single-procedure success, defined as cumulative survival free of recurrent atrial arrhythmia after the first ablation procedure; (ii) multiple-procedure success, defined as cumulative survival free of recurrent atrial arrhythmia after the last ablation procedure; and (iii) drug-free success, defined as cumulative survival free of recurrent atrial arrhythmia without anti-arrhythmic drug (AAD). We included data presented as Kaplan–Meier analyses or actuarial recurrence rates. Latest follow-up was defined as the latest follow-up time point with ≥10 patients at risk. The definitions of the post-procedure blanking period and the use of AAD were left to individual study design. Study quality was assessed using a modified version of quality assessment criteria for case series.11
The cumulative rates of freedom from atrial arrhythmia were obtained from each study, and these rates at the 12-month follow-up and the latest follow-up were pooled using STATA version 12.0. Arrhythmia-free survival rates were extracted from Kaplan–Meier data using graphic digitization software (Digitizelt). In the absence of standard errors for each Kaplan–Meier curve, the number of patients at risk at the time point of interest was used to conservatively estimate the standard error. A pooled estimate of survival at the same post-operative follow-up point was calculated, using random-effects models based on logit transformed proportions.12 The time point of latest follow-up in a study was defined as the time point reporting a minimum of 10 subjects at risk. At least three studies were needed to perform a meta-analysis. Heterogeneity was assessed using the I2 index, with 50% defined as the threshold for significant heterogeneity. Subgroup analyses were performed according to AF type and AAD usage to explore possible reasons for heterogeneity of study outcomes. Evidence for publication bias was assessed graphically using funnel plots. For the number of procedures per patient, exact Poisson confidence intervals (CIs) were calculated for each study estimate. Study estimates and CIs were then pooled using random-effects models.
Results
Search and synthesis of literature
A total of 283 unique citations were identified through an electronic retrieval process to be related to AF, HCM, and CA (see ‘Methods’ section). Supplemental hand searches of their bibliographies and relevant review articles were also performed. Of these studies, 221 were excluded after a screening of the abstracts and titles, and another 46 records were excluded after further evaluation of the full text and/or abstract. Of the remaining 16 publications that met the inclusion criteria for this study, one was a Ph.D. thesis that was excluded as the data were published later. The remaining 15 articles were examined in the present review (Figure 1) and the baseline characteristics are presented in Table 1.13–27 Two20,25 of these studies published by the same research centre shared some data, and we had avoided double counting.
| First author, year . | Study design . | Inclusion criteria . | n . | Age (year) . | Male (%) . | PAF (%) . | AF duration (m) . | LAD (mm) . | IVS (mm) . | LVEF (%) . | HOCM (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | Retrospective multicentre | HOCM patients complicated with AF undergoing PVAI | 27 | 55 | 70 | 52 | 64.8 | 50 | 17 | – | 100 |
| Gaita, 200714 | Retrospective multicentre | HC patients with symptomatic drug-refractory AF dating back ≥12 months | 26 | 58 | 69.2 | 50 | 87.6 | 52 | – | 57 | 23 |
| Bunch, 200815 | Prospective single centre | HC patients who underwent an ablation for drug-refractory AF | 33 | 51 | 76 | 64 | 74.4 | – | – | 63 | 24 |
| Lu, 200916 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent catheter ablation | 11 | 52 | 82 | 54.5 | 36 | 45.1 | – | 60 | 9 |
| Di Donna, 201017 | Prospective multicentre | HC patients with symptomatic drug-refractory AF often in the context of disease progression and heart failure | 61 | 54 | 72 | 57 | 68.4 | 51 | – | 59 | 20 |
| Derejko, 201318 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 30 | 48.7 | 67 | 47 | 72 | – | 20.5 | – | 20 |
| Santangeli, 201319 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 43 | 59 | 67 | 28 | 36 | 50.8 | – | 59 | – |
| Yan, 201320 | Prospective single-centre non-randomized case–control study | HC patients undergoing catheter ablation for drug-refractory AF | 24 | 53.4 | 75 | 62.5 | 64.2 | 47.6 | 18.2 | 65 | 33.3 |
| Zhou, 201321 | Retrospective single centre | HC patients complicated with AF undergoing catheter ablation | 57 | 58.6 | 71.9 | 54.4 | 43.5 | 47 | – | 60.4 | 19.3 |
| Hayashi, 201422 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic drug-refractory AF who underwent an initial catheter ablation | 17 | 63 | 71 | 47 | 42 | 46 | 19 | 71 | 24 |
| Mussigbrodt, 201423 | Prospective single-centre non-randomized case–control study | HC patients with highly symptomatic AF, having undergone AF ablation procedures | 22 | 57 | 55 | – | – | 46 | 19 | 60 | 36 |
| Okamatsu, 201424 | Retrospective single centre | HC patients undergoing catheter ablation of paroxysmal or persistent AF | 22 | 65 | 54.5 | 22.7 | 80 | 48 | 13 | 57 | 13.6 |
| Liu, 201425 | Retrospective single centre | HC patients with AF who underwent the first time catheter ablation | 39 | 54 | 74 | 71.8 | 69.8 | 45.8 | – | 65 | – |
| Bassiouny, 201526 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent the first time catheter ablation | 79 | 55.3 | 72 | 43 | 36 | 50.1 | 19 | 56.1 | 28 |
| Contreras-Valdes, 201527 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic AF | 40 | 54.3 | 70 | 32.5 | – | – | – | – | 37.5 |
| First author, year . | Study design . | Inclusion criteria . | n . | Age (year) . | Male (%) . | PAF (%) . | AF duration (m) . | LAD (mm) . | IVS (mm) . | LVEF (%) . | HOCM (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | Retrospective multicentre | HOCM patients complicated with AF undergoing PVAI | 27 | 55 | 70 | 52 | 64.8 | 50 | 17 | – | 100 |
| Gaita, 200714 | Retrospective multicentre | HC patients with symptomatic drug-refractory AF dating back ≥12 months | 26 | 58 | 69.2 | 50 | 87.6 | 52 | – | 57 | 23 |
| Bunch, 200815 | Prospective single centre | HC patients who underwent an ablation for drug-refractory AF | 33 | 51 | 76 | 64 | 74.4 | – | – | 63 | 24 |
| Lu, 200916 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent catheter ablation | 11 | 52 | 82 | 54.5 | 36 | 45.1 | – | 60 | 9 |
| Di Donna, 201017 | Prospective multicentre | HC patients with symptomatic drug-refractory AF often in the context of disease progression and heart failure | 61 | 54 | 72 | 57 | 68.4 | 51 | – | 59 | 20 |
| Derejko, 201318 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 30 | 48.7 | 67 | 47 | 72 | – | 20.5 | – | 20 |
| Santangeli, 201319 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 43 | 59 | 67 | 28 | 36 | 50.8 | – | 59 | – |
| Yan, 201320 | Prospective single-centre non-randomized case–control study | HC patients undergoing catheter ablation for drug-refractory AF | 24 | 53.4 | 75 | 62.5 | 64.2 | 47.6 | 18.2 | 65 | 33.3 |
| Zhou, 201321 | Retrospective single centre | HC patients complicated with AF undergoing catheter ablation | 57 | 58.6 | 71.9 | 54.4 | 43.5 | 47 | – | 60.4 | 19.3 |
| Hayashi, 201422 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic drug-refractory AF who underwent an initial catheter ablation | 17 | 63 | 71 | 47 | 42 | 46 | 19 | 71 | 24 |
| Mussigbrodt, 201423 | Prospective single-centre non-randomized case–control study | HC patients with highly symptomatic AF, having undergone AF ablation procedures | 22 | 57 | 55 | – | – | 46 | 19 | 60 | 36 |
| Okamatsu, 201424 | Retrospective single centre | HC patients undergoing catheter ablation of paroxysmal or persistent AF | 22 | 65 | 54.5 | 22.7 | 80 | 48 | 13 | 57 | 13.6 |
| Liu, 201425 | Retrospective single centre | HC patients with AF who underwent the first time catheter ablation | 39 | 54 | 74 | 71.8 | 69.8 | 45.8 | – | 65 | – |
| Bassiouny, 201526 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent the first time catheter ablation | 79 | 55.3 | 72 | 43 | 36 | 50.1 | 19 | 56.1 | 28 |
| Contreras-Valdes, 201527 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic AF | 40 | 54.3 | 70 | 32.5 | – | – | – | – | 37.5 |
AF, atrial fibrillation; PAF, paroxysmal AF; LAD, left atrial diameter; IVS, interventricular septum thickness; LVEF, left ventricular ejection fraction; HC, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; PVAI, pulmonary vein antral isolation; –, signifies the data that the study did not report.
| First author, year . | Study design . | Inclusion criteria . | n . | Age (year) . | Male (%) . | PAF (%) . | AF duration (m) . | LAD (mm) . | IVS (mm) . | LVEF (%) . | HOCM (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | Retrospective multicentre | HOCM patients complicated with AF undergoing PVAI | 27 | 55 | 70 | 52 | 64.8 | 50 | 17 | – | 100 |
| Gaita, 200714 | Retrospective multicentre | HC patients with symptomatic drug-refractory AF dating back ≥12 months | 26 | 58 | 69.2 | 50 | 87.6 | 52 | – | 57 | 23 |
| Bunch, 200815 | Prospective single centre | HC patients who underwent an ablation for drug-refractory AF | 33 | 51 | 76 | 64 | 74.4 | – | – | 63 | 24 |
| Lu, 200916 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent catheter ablation | 11 | 52 | 82 | 54.5 | 36 | 45.1 | – | 60 | 9 |
| Di Donna, 201017 | Prospective multicentre | HC patients with symptomatic drug-refractory AF often in the context of disease progression and heart failure | 61 | 54 | 72 | 57 | 68.4 | 51 | – | 59 | 20 |
| Derejko, 201318 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 30 | 48.7 | 67 | 47 | 72 | – | 20.5 | – | 20 |
| Santangeli, 201319 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 43 | 59 | 67 | 28 | 36 | 50.8 | – | 59 | – |
| Yan, 201320 | Prospective single-centre non-randomized case–control study | HC patients undergoing catheter ablation for drug-refractory AF | 24 | 53.4 | 75 | 62.5 | 64.2 | 47.6 | 18.2 | 65 | 33.3 |
| Zhou, 201321 | Retrospective single centre | HC patients complicated with AF undergoing catheter ablation | 57 | 58.6 | 71.9 | 54.4 | 43.5 | 47 | – | 60.4 | 19.3 |
| Hayashi, 201422 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic drug-refractory AF who underwent an initial catheter ablation | 17 | 63 | 71 | 47 | 42 | 46 | 19 | 71 | 24 |
| Mussigbrodt, 201423 | Prospective single-centre non-randomized case–control study | HC patients with highly symptomatic AF, having undergone AF ablation procedures | 22 | 57 | 55 | – | – | 46 | 19 | 60 | 36 |
| Okamatsu, 201424 | Retrospective single centre | HC patients undergoing catheter ablation of paroxysmal or persistent AF | 22 | 65 | 54.5 | 22.7 | 80 | 48 | 13 | 57 | 13.6 |
| Liu, 201425 | Retrospective single centre | HC patients with AF who underwent the first time catheter ablation | 39 | 54 | 74 | 71.8 | 69.8 | 45.8 | – | 65 | – |
| Bassiouny, 201526 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent the first time catheter ablation | 79 | 55.3 | 72 | 43 | 36 | 50.1 | 19 | 56.1 | 28 |
| Contreras-Valdes, 201527 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic AF | 40 | 54.3 | 70 | 32.5 | – | – | – | – | 37.5 |
| First author, year . | Study design . | Inclusion criteria . | n . | Age (year) . | Male (%) . | PAF (%) . | AF duration (m) . | LAD (mm) . | IVS (mm) . | LVEF (%) . | HOCM (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | Retrospective multicentre | HOCM patients complicated with AF undergoing PVAI | 27 | 55 | 70 | 52 | 64.8 | 50 | 17 | – | 100 |
| Gaita, 200714 | Retrospective multicentre | HC patients with symptomatic drug-refractory AF dating back ≥12 months | 26 | 58 | 69.2 | 50 | 87.6 | 52 | – | 57 | 23 |
| Bunch, 200815 | Prospective single centre | HC patients who underwent an ablation for drug-refractory AF | 33 | 51 | 76 | 64 | 74.4 | – | – | 63 | 24 |
| Lu, 200916 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent catheter ablation | 11 | 52 | 82 | 54.5 | 36 | 45.1 | – | 60 | 9 |
| Di Donna, 201017 | Prospective multicentre | HC patients with symptomatic drug-refractory AF often in the context of disease progression and heart failure | 61 | 54 | 72 | 57 | 68.4 | 51 | – | 59 | 20 |
| Derejko, 201318 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 30 | 48.7 | 67 | 47 | 72 | – | 20.5 | – | 20 |
| Santangeli, 201319 | Prospective single centre | HC patients with symptomatic drug-refractory AF | 43 | 59 | 67 | 28 | 36 | 50.8 | – | 59 | – |
| Yan, 201320 | Prospective single-centre non-randomized case–control study | HC patients undergoing catheter ablation for drug-refractory AF | 24 | 53.4 | 75 | 62.5 | 64.2 | 47.6 | 18.2 | 65 | 33.3 |
| Zhou, 201321 | Retrospective single centre | HC patients complicated with AF undergoing catheter ablation | 57 | 58.6 | 71.9 | 54.4 | 43.5 | 47 | – | 60.4 | 19.3 |
| Hayashi, 201422 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic drug-refractory AF who underwent an initial catheter ablation | 17 | 63 | 71 | 47 | 42 | 46 | 19 | 71 | 24 |
| Mussigbrodt, 201423 | Prospective single-centre non-randomized case–control study | HC patients with highly symptomatic AF, having undergone AF ablation procedures | 22 | 57 | 55 | – | – | 46 | 19 | 60 | 36 |
| Okamatsu, 201424 | Retrospective single centre | HC patients undergoing catheter ablation of paroxysmal or persistent AF | 22 | 65 | 54.5 | 22.7 | 80 | 48 | 13 | 57 | 13.6 |
| Liu, 201425 | Retrospective single centre | HC patients with AF who underwent the first time catheter ablation | 39 | 54 | 74 | 71.8 | 69.8 | 45.8 | – | 65 | – |
| Bassiouny, 201526 | Retrospective single centre | HC patients with symptomatic drug-refractory AF who underwent the first time catheter ablation | 79 | 55.3 | 72 | 43 | 36 | 50.1 | 19 | 56.1 | 28 |
| Contreras-Valdes, 201527 | Retrospective single-centre non-randomized case–control study | HC patients with symptomatic AF | 40 | 54.3 | 70 | 32.5 | – | – | – | – | 37.5 |
AF, atrial fibrillation; PAF, paroxysmal AF; LAD, left atrial diameter; IVS, interventricular septum thickness; LVEF, left ventricular ejection fraction; HC, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; PVAI, pulmonary vein antral isolation; –, signifies the data that the study did not report.
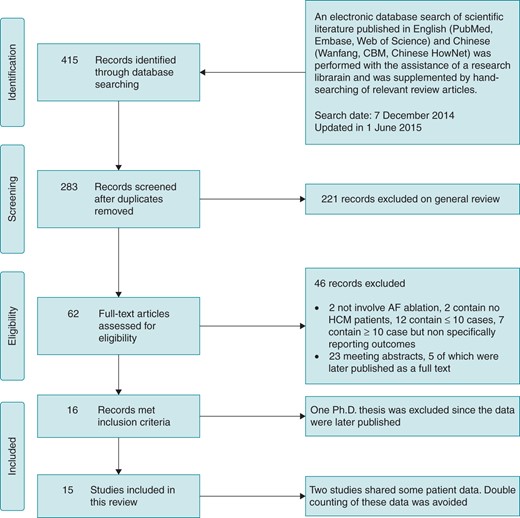
Search criteria and flow diagram for studies included in this systematic review. AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy.
The included studies were published between 2006 and 2015, and the patients involved were enrolled between 1999 and 2013. Three of them13,14,17 were multicentric. Eight studies were based in Europe and/or America and seven studies were based in Asian countries (four from China, two from Japan, and one from India). Prospective recruitment was used in six studies,15,17–20,23 while nine studies recruited retrospectively.13,14,16,21,22,24–27 Study size varied from 11 patients16 to 79 patients.26 Twelve studies13,14,16–19,21–23,25–27 reported a single-procedure success rate. All of the studies except one25 reported multiple-procedure success rates, while this data from one of the studies26 was not extracted because of the short follow-up time (10 months) after the last ablation. Six studies reported outcome data for paroxysmal AF (PAF) patients,14,15,17–19,21 while seven studies reported data for non-paroxysmal AF (NPAF) patients.14,15,17–19,21,24 Eleven studies13–17,19–21,24,26,27 reported success rates in the absence of treatment with AAD, and three studies reported this only.20,21,24
Study quality was assessed using a modified version of previously published quality assessment criteria for case series.11 Study quality was limited and the majority of studies had identifiable limitations in their study design (see Supplementary material online, Appendix S2). Of particular note, one study16 did not clearly report the selection eligibility criteria used, while another study20 did not clarify the method of follow-up. In another six studies,15,22–25,27 loss to follow-up was not explained. Prognostic factors for recurrence or ablation success were also inconsistently reported.
Baseline patient characteristics
A total of 531 patients were included from the 15 studies. The mean age of these patients ranged from 48.7 to 65.0 years and the proportion of male subjects varied from 54.5 to 82.0%. The mean left atrial diameter varied from 45.1 to 52.0 mm and the mean left ventricular ejection fraction varied from 57.0 to 71.0%. The duration of AF varied from 36.0 to 87.6 months and the proportion of PAF cases varied from 22.7 to 71.8% (Table 1).
Catheter ablation approach
Pulmonary vein (PV) isolation using radiofrequency energy was the foundation of the ablations performed in all of the studies examined (Table 2). Most of the studies15–18,20–27 employed a circumferential PV vein isolation strategy with linear ablation15–18,21–23,24–26 and complex fragmented atrial electrogram ablation.16,18,20,21 However, one study14 used segmental PV isolation, while PV antral ablation combined with superior vena cava ablation was used in two other studies.13,19 In addition, the earlier studies tended to use non-irrigated conventional ablation catheters, while the later published studies predominantly used irrigated ablation catheters. More contemporary techniques including contact-force sensing catheters, laser balloon catheters, and multielectrode ablation catheters were not been used.
| First author, year . | Enrolment period . | Ablation procedure . | Catheter type . | Mean follow-up (months) . | Blanking period (months) . | Use of AAD . | Follow-up year 1 . | Follow-up after year 1 . |
|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | 2002–04 | PVAI (ICE guided) + SVC ablation | 8 mm, non-irrigated | 11.4 | 2 | ✓ | Clinic visit and 48-h Holter monitor at 3, 6, and 12 months and CER during the first 3 months (if early recurrence, extended by another 3 months) | Every 6 months |
| Gaita, 200714 | 2002–05 | PVI (segmental) + linear ablation | 4 mm, irrigated | 19 | 1 | ✓ | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring at 1, 3, 6, and 12 months | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring every 6 months |
| Bunch, 200815 | 1999–2006 | CPVI (n = 8) or WACA + linear ablation (n = 25) | 4–5, 8 mm non-irrigated or 3.5 mm irrigated | 18 | 3 | ✓ | – | |
| Lu, 200916 | 2006–08 | CPVI (EAM guided) + linear ablation and CFAE in persistent AF | – | 14 | 3 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, and 6 months, and 12-lead ECG when palpitation reported | 12-Lead ECG when palpitation reported |
| Di Donna, 201017 | 2001–08 | CPVI + linear ablation (EAM or fluoroscopic guided) | 3.5 mm, irrigated | 29 | 1 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and/or communication with the patient's primary referring physician every 6 months |
| Derejko, 201318 | 2008–11 | CPVI + CTI + linear ablation and CFAE in NPAF | Irrigated | 22.8 | 3 | ✓ | 12-Lead ECG, and Holter monitoring at 4 weeks and then every 3–6 months and when palpitation reported | |
| Santangeli, 201319 | – | PVAI + SVC ablation and CFAE in NPAF | 3.5 mm, irrigated | 42 | 3 | ✓ | 12-Lead ECG, and 7-day Holter monitoring at 3, 6, 9, and 12 months and CER during the first 5 months | 12-Lead ECG, and 7-day Holter monitoring every 6 months |
| Yan, 201320 | 2006–11 | CPVI + linear ablation and CFAE in persistent AF | – | 36 | 3 | ✗ | Telephone contact at 3, 6, and 12 months | Telephone contact every 6 months |
| Zhou, 201321 | 2005–12 | CPVI (EAM guided) and linear ablation + CFAE in NPAF | 3.5 mm, irrigated | 36 | 1 | ✗ | 24-h Holter monitoring at 1 and 7 days, and 1, 3, 6, 9, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring when palpitation reported |
| Hayashi, 201422 | 2006–12 | CPVI (EAM guided) + linear ablation | 3.5 mm, irrigated | 29 | 3 | ✓ | 12-Lead ECG every month and CER during the first 4 months | 12-Lead ECG every 2 or 3 months and a 24-h Holter monitoring every 12 months |
| Mussigbrodt, 201423 | 2009–12 | CPVI | Irrigated | – | 3 | ✓ | Clinic visit and 7-day Holter monitor at 6, 12, and 24 months and advise the patients to contact hospital themselves or through their family physicians in case of any symptom recurrence | |
| Okamatsu, 201424 | 2009–12 | CPVI (EAM guided) + linear ablation at the operator's discretion | 3.5 mm, irrigated | 21 | 2 | ✗ | 12-Lead ECG, 24-h Holter monitoring, and assessment of the current condition every 1–3 months in the outpatient clinic | |
| Liu, 201425 | 2006–13 | CPVI + linear ablation in persistent AF | – | 14.8 | 3 | ✗ | Telephone contact, clinic visits every week in the first month, every month afterwards, and 12-lead ECG, 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring every month |
| Bassiouny, 201526 | 2004–13 | PVI (fluoroscopic and 3D navigation system guided) + linear ablation at electrophysiologists’ discretion | 8-mm or 3.5-mm irrigated-tip | 35 | 3 | ✓ | Weekly follow-up telephone calls and transtelephonic ECG transmissions were conducted in the first 4–6 months and 24- to 48-h Holter monitoring at 4–6 months and then every 6 months thereafter, with earlier visits if symptoms develop | |
| Contreras-Valdes, 201527 | 2006–12 | PVI (ICE guided) | – | 54 | – | ✓ | – | |
| First author, year . | Enrolment period . | Ablation procedure . | Catheter type . | Mean follow-up (months) . | Blanking period (months) . | Use of AAD . | Follow-up year 1 . | Follow-up after year 1 . |
|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | 2002–04 | PVAI (ICE guided) + SVC ablation | 8 mm, non-irrigated | 11.4 | 2 | ✓ | Clinic visit and 48-h Holter monitor at 3, 6, and 12 months and CER during the first 3 months (if early recurrence, extended by another 3 months) | Every 6 months |
| Gaita, 200714 | 2002–05 | PVI (segmental) + linear ablation | 4 mm, irrigated | 19 | 1 | ✓ | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring at 1, 3, 6, and 12 months | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring every 6 months |
| Bunch, 200815 | 1999–2006 | CPVI (n = 8) or WACA + linear ablation (n = 25) | 4–5, 8 mm non-irrigated or 3.5 mm irrigated | 18 | 3 | ✓ | – | |
| Lu, 200916 | 2006–08 | CPVI (EAM guided) + linear ablation and CFAE in persistent AF | – | 14 | 3 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, and 6 months, and 12-lead ECG when palpitation reported | 12-Lead ECG when palpitation reported |
| Di Donna, 201017 | 2001–08 | CPVI + linear ablation (EAM or fluoroscopic guided) | 3.5 mm, irrigated | 29 | 1 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and/or communication with the patient's primary referring physician every 6 months |
| Derejko, 201318 | 2008–11 | CPVI + CTI + linear ablation and CFAE in NPAF | Irrigated | 22.8 | 3 | ✓ | 12-Lead ECG, and Holter monitoring at 4 weeks and then every 3–6 months and when palpitation reported | |
| Santangeli, 201319 | – | PVAI + SVC ablation and CFAE in NPAF | 3.5 mm, irrigated | 42 | 3 | ✓ | 12-Lead ECG, and 7-day Holter monitoring at 3, 6, 9, and 12 months and CER during the first 5 months | 12-Lead ECG, and 7-day Holter monitoring every 6 months |
| Yan, 201320 | 2006–11 | CPVI + linear ablation and CFAE in persistent AF | – | 36 | 3 | ✗ | Telephone contact at 3, 6, and 12 months | Telephone contact every 6 months |
| Zhou, 201321 | 2005–12 | CPVI (EAM guided) and linear ablation + CFAE in NPAF | 3.5 mm, irrigated | 36 | 1 | ✗ | 24-h Holter monitoring at 1 and 7 days, and 1, 3, 6, 9, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring when palpitation reported |
| Hayashi, 201422 | 2006–12 | CPVI (EAM guided) + linear ablation | 3.5 mm, irrigated | 29 | 3 | ✓ | 12-Lead ECG every month and CER during the first 4 months | 12-Lead ECG every 2 or 3 months and a 24-h Holter monitoring every 12 months |
| Mussigbrodt, 201423 | 2009–12 | CPVI | Irrigated | – | 3 | ✓ | Clinic visit and 7-day Holter monitor at 6, 12, and 24 months and advise the patients to contact hospital themselves or through their family physicians in case of any symptom recurrence | |
| Okamatsu, 201424 | 2009–12 | CPVI (EAM guided) + linear ablation at the operator's discretion | 3.5 mm, irrigated | 21 | 2 | ✗ | 12-Lead ECG, 24-h Holter monitoring, and assessment of the current condition every 1–3 months in the outpatient clinic | |
| Liu, 201425 | 2006–13 | CPVI + linear ablation in persistent AF | – | 14.8 | 3 | ✗ | Telephone contact, clinic visits every week in the first month, every month afterwards, and 12-lead ECG, 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring every month |
| Bassiouny, 201526 | 2004–13 | PVI (fluoroscopic and 3D navigation system guided) + linear ablation at electrophysiologists’ discretion | 8-mm or 3.5-mm irrigated-tip | 35 | 3 | ✓ | Weekly follow-up telephone calls and transtelephonic ECG transmissions were conducted in the first 4–6 months and 24- to 48-h Holter monitoring at 4–6 months and then every 6 months thereafter, with earlier visits if symptoms develop | |
| Contreras-Valdes, 201527 | 2006–12 | PVI (ICE guided) | – | 54 | – | ✓ | – | |
✗ signifies that this approach not used and ✓ that this approach was used in the study; – signifies the data that the study did not report.
AF, atrial fibrillation; NPAF, non-paroxysmal AF; AAD, anti-arrhythmic drug; PVAI, pulmonary vein antral isolation; ICE, intracardiac echocardiography; SVC, superior vena cava; CER, cardiac event recorder; PVI, pulmonary vein isolation; CPVI, circumferential pulmonary vein isolation; EAM, electroanatomic mapping; WACA, wide area circumferential ablation; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus.
| First author, year . | Enrolment period . | Ablation procedure . | Catheter type . | Mean follow-up (months) . | Blanking period (months) . | Use of AAD . | Follow-up year 1 . | Follow-up after year 1 . |
|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | 2002–04 | PVAI (ICE guided) + SVC ablation | 8 mm, non-irrigated | 11.4 | 2 | ✓ | Clinic visit and 48-h Holter monitor at 3, 6, and 12 months and CER during the first 3 months (if early recurrence, extended by another 3 months) | Every 6 months |
| Gaita, 200714 | 2002–05 | PVI (segmental) + linear ablation | 4 mm, irrigated | 19 | 1 | ✓ | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring at 1, 3, 6, and 12 months | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring every 6 months |
| Bunch, 200815 | 1999–2006 | CPVI (n = 8) or WACA + linear ablation (n = 25) | 4–5, 8 mm non-irrigated or 3.5 mm irrigated | 18 | 3 | ✓ | – | |
| Lu, 200916 | 2006–08 | CPVI (EAM guided) + linear ablation and CFAE in persistent AF | – | 14 | 3 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, and 6 months, and 12-lead ECG when palpitation reported | 12-Lead ECG when palpitation reported |
| Di Donna, 201017 | 2001–08 | CPVI + linear ablation (EAM or fluoroscopic guided) | 3.5 mm, irrigated | 29 | 1 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and/or communication with the patient's primary referring physician every 6 months |
| Derejko, 201318 | 2008–11 | CPVI + CTI + linear ablation and CFAE in NPAF | Irrigated | 22.8 | 3 | ✓ | 12-Lead ECG, and Holter monitoring at 4 weeks and then every 3–6 months and when palpitation reported | |
| Santangeli, 201319 | – | PVAI + SVC ablation and CFAE in NPAF | 3.5 mm, irrigated | 42 | 3 | ✓ | 12-Lead ECG, and 7-day Holter monitoring at 3, 6, 9, and 12 months and CER during the first 5 months | 12-Lead ECG, and 7-day Holter monitoring every 6 months |
| Yan, 201320 | 2006–11 | CPVI + linear ablation and CFAE in persistent AF | – | 36 | 3 | ✗ | Telephone contact at 3, 6, and 12 months | Telephone contact every 6 months |
| Zhou, 201321 | 2005–12 | CPVI (EAM guided) and linear ablation + CFAE in NPAF | 3.5 mm, irrigated | 36 | 1 | ✗ | 24-h Holter monitoring at 1 and 7 days, and 1, 3, 6, 9, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring when palpitation reported |
| Hayashi, 201422 | 2006–12 | CPVI (EAM guided) + linear ablation | 3.5 mm, irrigated | 29 | 3 | ✓ | 12-Lead ECG every month and CER during the first 4 months | 12-Lead ECG every 2 or 3 months and a 24-h Holter monitoring every 12 months |
| Mussigbrodt, 201423 | 2009–12 | CPVI | Irrigated | – | 3 | ✓ | Clinic visit and 7-day Holter monitor at 6, 12, and 24 months and advise the patients to contact hospital themselves or through their family physicians in case of any symptom recurrence | |
| Okamatsu, 201424 | 2009–12 | CPVI (EAM guided) + linear ablation at the operator's discretion | 3.5 mm, irrigated | 21 | 2 | ✗ | 12-Lead ECG, 24-h Holter monitoring, and assessment of the current condition every 1–3 months in the outpatient clinic | |
| Liu, 201425 | 2006–13 | CPVI + linear ablation in persistent AF | – | 14.8 | 3 | ✗ | Telephone contact, clinic visits every week in the first month, every month afterwards, and 12-lead ECG, 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring every month |
| Bassiouny, 201526 | 2004–13 | PVI (fluoroscopic and 3D navigation system guided) + linear ablation at electrophysiologists’ discretion | 8-mm or 3.5-mm irrigated-tip | 35 | 3 | ✓ | Weekly follow-up telephone calls and transtelephonic ECG transmissions were conducted in the first 4–6 months and 24- to 48-h Holter monitoring at 4–6 months and then every 6 months thereafter, with earlier visits if symptoms develop | |
| Contreras-Valdes, 201527 | 2006–12 | PVI (ICE guided) | – | 54 | – | ✓ | – | |
| First author, year . | Enrolment period . | Ablation procedure . | Catheter type . | Mean follow-up (months) . | Blanking period (months) . | Use of AAD . | Follow-up year 1 . | Follow-up after year 1 . |
|---|---|---|---|---|---|---|---|---|
| Kilicaslan, 200613 | 2002–04 | PVAI (ICE guided) + SVC ablation | 8 mm, non-irrigated | 11.4 | 2 | ✓ | Clinic visit and 48-h Holter monitor at 3, 6, and 12 months and CER during the first 3 months (if early recurrence, extended by another 3 months) | Every 6 months |
| Gaita, 200714 | 2002–05 | PVI (segmental) + linear ablation | 4 mm, irrigated | 19 | 1 | ✓ | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring at 1, 3, 6, and 12 months | Clinical evaluation, 12-lead ECG, echocardiography, and 24-h Holter monitoring every 6 months |
| Bunch, 200815 | 1999–2006 | CPVI (n = 8) or WACA + linear ablation (n = 25) | 4–5, 8 mm non-irrigated or 3.5 mm irrigated | 18 | 3 | ✓ | – | |
| Lu, 200916 | 2006–08 | CPVI (EAM guided) + linear ablation and CFAE in persistent AF | – | 14 | 3 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, and 6 months, and 12-lead ECG when palpitation reported | 12-Lead ECG when palpitation reported |
| Di Donna, 201017 | 2001–08 | CPVI + linear ablation (EAM or fluoroscopic guided) | 3.5 mm, irrigated | 29 | 1 | ✓ | 12-Lead ECG and 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and/or communication with the patient's primary referring physician every 6 months |
| Derejko, 201318 | 2008–11 | CPVI + CTI + linear ablation and CFAE in NPAF | Irrigated | 22.8 | 3 | ✓ | 12-Lead ECG, and Holter monitoring at 4 weeks and then every 3–6 months and when palpitation reported | |
| Santangeli, 201319 | – | PVAI + SVC ablation and CFAE in NPAF | 3.5 mm, irrigated | 42 | 3 | ✓ | 12-Lead ECG, and 7-day Holter monitoring at 3, 6, 9, and 12 months and CER during the first 5 months | 12-Lead ECG, and 7-day Holter monitoring every 6 months |
| Yan, 201320 | 2006–11 | CPVI + linear ablation and CFAE in persistent AF | – | 36 | 3 | ✗ | Telephone contact at 3, 6, and 12 months | Telephone contact every 6 months |
| Zhou, 201321 | 2005–12 | CPVI (EAM guided) and linear ablation + CFAE in NPAF | 3.5 mm, irrigated | 36 | 1 | ✗ | 24-h Holter monitoring at 1 and 7 days, and 1, 3, 6, 9, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring when palpitation reported |
| Hayashi, 201422 | 2006–12 | CPVI (EAM guided) + linear ablation | 3.5 mm, irrigated | 29 | 3 | ✓ | 12-Lead ECG every month and CER during the first 4 months | 12-Lead ECG every 2 or 3 months and a 24-h Holter monitoring every 12 months |
| Mussigbrodt, 201423 | 2009–12 | CPVI | Irrigated | – | 3 | ✓ | Clinic visit and 7-day Holter monitor at 6, 12, and 24 months and advise the patients to contact hospital themselves or through their family physicians in case of any symptom recurrence | |
| Okamatsu, 201424 | 2009–12 | CPVI (EAM guided) + linear ablation at the operator's discretion | 3.5 mm, irrigated | 21 | 2 | ✗ | 12-Lead ECG, 24-h Holter monitoring, and assessment of the current condition every 1–3 months in the outpatient clinic | |
| Liu, 201425 | 2006–13 | CPVI + linear ablation in persistent AF | – | 14.8 | 3 | ✗ | Telephone contact, clinic visits every week in the first month, every month afterwards, and 12-lead ECG, 24-h Holter monitoring at 1, 3, 6, and 12 months | Telephone contact, clinic visits, and 12-lead ECG, 24-h Holter monitoring every month |
| Bassiouny, 201526 | 2004–13 | PVI (fluoroscopic and 3D navigation system guided) + linear ablation at electrophysiologists’ discretion | 8-mm or 3.5-mm irrigated-tip | 35 | 3 | ✓ | Weekly follow-up telephone calls and transtelephonic ECG transmissions were conducted in the first 4–6 months and 24- to 48-h Holter monitoring at 4–6 months and then every 6 months thereafter, with earlier visits if symptoms develop | |
| Contreras-Valdes, 201527 | 2006–12 | PVI (ICE guided) | – | 54 | – | ✓ | – | |
✗ signifies that this approach not used and ✓ that this approach was used in the study; – signifies the data that the study did not report.
AF, atrial fibrillation; NPAF, non-paroxysmal AF; AAD, anti-arrhythmic drug; PVAI, pulmonary vein antral isolation; ICE, intracardiac echocardiography; SVC, superior vena cava; CER, cardiac event recorder; PVI, pulmonary vein isolation; CPVI, circumferential pulmonary vein isolation; EAM, electroanatomic mapping; WACA, wide area circumferential ablation; CFAE, complex fractionated atrial electrogram; CTI, cavotricuspid isthmus.
Follow-up
Mean or median duration of follow-up was reported in all of the included studies except one,23 and this period varied from 11.4 to 54.0 months. Follow-up intensity also differed between the studies. Within the first year of follow-up, 12-lead electrocardiogram (ECG) Holter monitoring was performed (24-h to 7-day) three to five times. In three studies,13,19,22 a cardiac event recorder was used. After the first year, follow-up intensity was generally reduced in most of the studies, yet was not reduced in three of the studies.18,23,24 In two studies,15,27 the method of follow-up was not clarified (Table 2). The detail of AAD usage post-ablation is listed in Table 3.
| First author, year . | Proportion . | Number of AADs used per user . | The kinds of drugs to be used . |
|---|---|---|---|
| Kilicaslan, 200613 | 6/25a | Not reported | Not reported |
| Gaita, 200714 | 6/16a | 1 | Amiodarone in 3 and sotalol in 3 |
| 14/26b | 1 | Amiodarone in 8, sotalol in 5, and botolal in 1 | |
| Bunch, 200815 | Not reported | Not reported | Not reported |
| Lu, 200916 | 3/7a | 1 | Amiodarone in 3 |
| 6/11b | 1 | Amiodarone in 3 and propafenone in 3 | |
| Di Donna, 201017 | 22/41a | 1 | Amiodarone in 15, sotalol in 3, and flecainide in 4 |
| 31/61b | Not reported | Amiodarone in 24, sotalol in 3, and flecainide in 4 | |
| Derejko, 201318 | 11/16a | 1 | Amiodarone in 7, sotalol in 3, and dronedarone in 1 |
| Santangeli, 201319 | 6/39a,c | Not reported | Not reported |
| Hayashi, 201422 | 8/17b | Not reported | Amiodarone or d,l-sotalol |
| Mussigbrodt, 201423 | 6/22b | Not reported | Amiodarone in 4 and sotalol in 2 |
| Bassiouny, 201526 | 4/23a,d | 1 | Not reported |
| Contreras-Valdes, 201527 | 5/24a | Not reported | Not reported |
| 18/40b | Not reported | Not reported |
| First author, year . | Proportion . | Number of AADs used per user . | The kinds of drugs to be used . |
|---|---|---|---|
| Kilicaslan, 200613 | 6/25a | Not reported | Not reported |
| Gaita, 200714 | 6/16a | 1 | Amiodarone in 3 and sotalol in 3 |
| 14/26b | 1 | Amiodarone in 8, sotalol in 5, and botolal in 1 | |
| Bunch, 200815 | Not reported | Not reported | Not reported |
| Lu, 200916 | 3/7a | 1 | Amiodarone in 3 |
| 6/11b | 1 | Amiodarone in 3 and propafenone in 3 | |
| Di Donna, 201017 | 22/41a | 1 | Amiodarone in 15, sotalol in 3, and flecainide in 4 |
| 31/61b | Not reported | Amiodarone in 24, sotalol in 3, and flecainide in 4 | |
| Derejko, 201318 | 11/16a | 1 | Amiodarone in 7, sotalol in 3, and dronedarone in 1 |
| Santangeli, 201319 | 6/39a,c | Not reported | Not reported |
| Hayashi, 201422 | 8/17b | Not reported | Amiodarone or d,l-sotalol |
| Mussigbrodt, 201423 | 6/22b | Not reported | Amiodarone in 4 and sotalol in 2 |
| Bassiouny, 201526 | 4/23a,d | 1 | Not reported |
| Contreras-Valdes, 201527 | 5/24a | Not reported | Not reported |
| 18/40b | Not reported | Not reported |
aThe proportion of patients who were taking drugs in the group maintaining sinus rhythm successfully.
bThe proportion in the overall study population.
cThe time point at 1-year follow-up.
dLast follow-up after the initial ablation.
| First author, year . | Proportion . | Number of AADs used per user . | The kinds of drugs to be used . |
|---|---|---|---|
| Kilicaslan, 200613 | 6/25a | Not reported | Not reported |
| Gaita, 200714 | 6/16a | 1 | Amiodarone in 3 and sotalol in 3 |
| 14/26b | 1 | Amiodarone in 8, sotalol in 5, and botolal in 1 | |
| Bunch, 200815 | Not reported | Not reported | Not reported |
| Lu, 200916 | 3/7a | 1 | Amiodarone in 3 |
| 6/11b | 1 | Amiodarone in 3 and propafenone in 3 | |
| Di Donna, 201017 | 22/41a | 1 | Amiodarone in 15, sotalol in 3, and flecainide in 4 |
| 31/61b | Not reported | Amiodarone in 24, sotalol in 3, and flecainide in 4 | |
| Derejko, 201318 | 11/16a | 1 | Amiodarone in 7, sotalol in 3, and dronedarone in 1 |
| Santangeli, 201319 | 6/39a,c | Not reported | Not reported |
| Hayashi, 201422 | 8/17b | Not reported | Amiodarone or d,l-sotalol |
| Mussigbrodt, 201423 | 6/22b | Not reported | Amiodarone in 4 and sotalol in 2 |
| Bassiouny, 201526 | 4/23a,d | 1 | Not reported |
| Contreras-Valdes, 201527 | 5/24a | Not reported | Not reported |
| 18/40b | Not reported | Not reported |
| First author, year . | Proportion . | Number of AADs used per user . | The kinds of drugs to be used . |
|---|---|---|---|
| Kilicaslan, 200613 | 6/25a | Not reported | Not reported |
| Gaita, 200714 | 6/16a | 1 | Amiodarone in 3 and sotalol in 3 |
| 14/26b | 1 | Amiodarone in 8, sotalol in 5, and botolal in 1 | |
| Bunch, 200815 | Not reported | Not reported | Not reported |
| Lu, 200916 | 3/7a | 1 | Amiodarone in 3 |
| 6/11b | 1 | Amiodarone in 3 and propafenone in 3 | |
| Di Donna, 201017 | 22/41a | 1 | Amiodarone in 15, sotalol in 3, and flecainide in 4 |
| 31/61b | Not reported | Amiodarone in 24, sotalol in 3, and flecainide in 4 | |
| Derejko, 201318 | 11/16a | 1 | Amiodarone in 7, sotalol in 3, and dronedarone in 1 |
| Santangeli, 201319 | 6/39a,c | Not reported | Not reported |
| Hayashi, 201422 | 8/17b | Not reported | Amiodarone or d,l-sotalol |
| Mussigbrodt, 201423 | 6/22b | Not reported | Amiodarone in 4 and sotalol in 2 |
| Bassiouny, 201526 | 4/23a,d | 1 | Not reported |
| Contreras-Valdes, 201527 | 5/24a | Not reported | Not reported |
| 18/40b | Not reported | Not reported |
aThe proportion of patients who were taking drugs in the group maintaining sinus rhythm successfully.
bThe proportion in the overall study population.
cThe time point at 1-year follow-up.
dLast follow-up after the initial ablation.
Single-procedure efficacy of catheter ablation
Outcome data regarding the efficacy of CA for AF were reported in all of the studies examined. Most studies provided single-procedure success rates, while four studies19,22,23,26 reported cumulative freedom from recurrent atrial arrhythmia at 12 months and the pooled success rate was 46.5% (95% CI: 38.8–54.1%, I2 = 0.0%, Figure 2A). In nine studies,13,14,16–19,22,23,26 outcome at the latest follow-up was reported, and eight studies13,14,16,17,21,25–27 reported these data for patients that were not treated with AAD. The pooled success rate was 45.5% (95% CI: 34.8–56.2%, I2 = 73.2%, Figure 2B) and 32.9% (95% CI: 21.7–44.1%, I2 = 81.3%, Figure 4A), with the follow-up time 23.4 ± 12.0 and 29.4 ± 10.2 months, respectively.
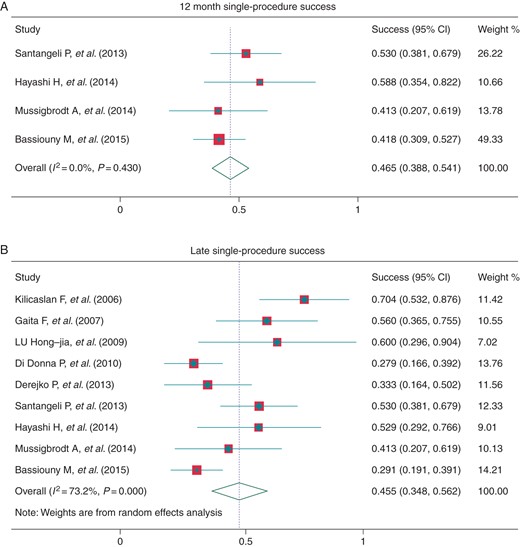
Single-procedure success at 12 months post-procedure (A) and at late follow-up (B).
Multiple-procedure efficacy of catheter ablation
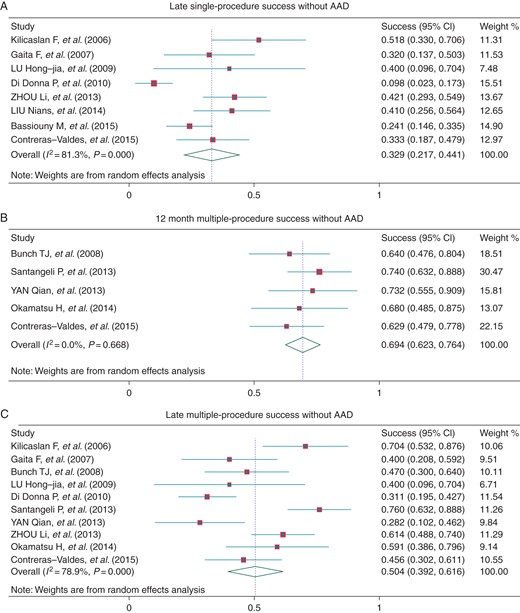
All of the studies provided long-term outcome data for the use of multiple procedures except two.25,26 The pooled 12-month success rate was 76.7% (95% CI: 65.3–88.2%, I2 = 83.8%, Figure 3A). Three studies reported outcomes for PAF patients and NPAF patients separately, and the 12-month success rate was 85.6% (95% CI: 67.2–100%, I2 = 77.5%) and 64.9% (95% CI: 38.0–91.9%, I2 = 80.5%) (Figure 3A). At the latest follow-up, the overall success rate was 66.1% (95% CI: 55.3–76.9%, I2 = 78.5%), and the success rate in the PAF and NPAF patients was 71.8% (95% CI: 61.6–82.0%, I2 = 0.0%) and 47.5% (95% CI: 36.0–59.0%, I2 = 16.0%) (Figure 3B), with the mean follow-up time 22.6 ± 11.5, 27.3 ± 14.0, and 27.3 ± 14.0 months, respectively. The average number of procedures in overall patients at 12 months and the latest follow-up was 1.52 (95% CI: 1.35–1.70) and 1.48 (95% CI: 1.28–1.67). Without AAD treatment, the success rate at 1 year and at the latest follow-up (30.0 ± 10.6 months) was 69.4% (95% CI: 62.3–76.4%, I2 = 0.0%) and 50.4% (95% CI: 39.2–61.6%, I2 = 81.0%), respectively (Figure 4B and C).
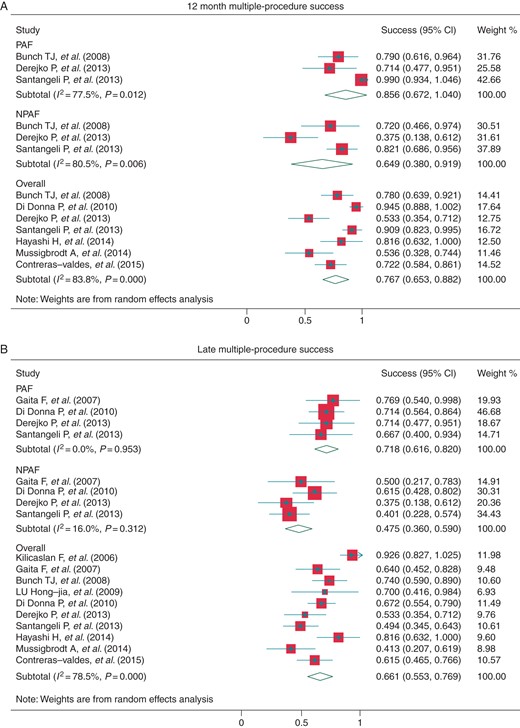
Multiple-procedure success at 12 months post-procedure (A) and at late follow-up (B). AF, atrial fibrillation; PAF, paroxysmal AF; NPAF, non-paroxysmal AF.
Timed ablation success
To evaluate the timing of recurrence, pooled estimates of single- and multiple-procedure arrhythmia-free success rates were evaluated for a subset of studies that provided follow-up data at 3-month intervals up to 36 months from the index ablation (Figure 5). After a single procedure, the 3-month success rate in these studies was 79.0% (95% CI: 71.6–86.3%), and this decreased to 41.7% (95% CI: 28.3–55.2%) at 18 months. Regarding the multiple-procedure success rates, the 3-month success rate was 98.8% (95% CI: 97.1–100%), the 18-month success rate was 74.5% (95% CI: 61.3–87.7%), and the 3-year success rate was 56.1% (95% CI: 42.3–69.9%). Overall, the single-procedure success rate declined more quickly than the multiple-procedure success rate as time goes on.
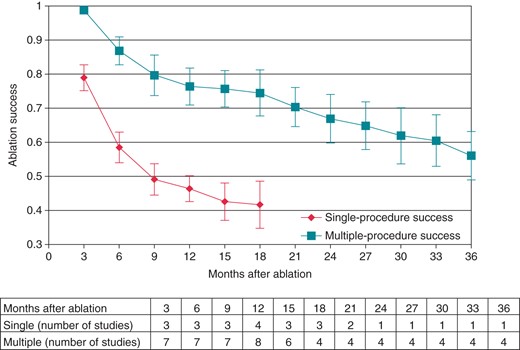
Monthly single- and multiple-procedure arrhythmia-free success were calculated (subtable: number of studies at each month after ablation). Meta-analysis for single-procedure success was not performed beyond 21 months, because only two studies reported at this duration of follow-up.
Predictors of recurrent arrhythmia
Eleven studies reported predictors of AF recurrence following CA based on univariate and/or multivariate analyses (Table 4). Structural or electrical characteristics of the LA were found to be the most important factor for AF recurrence after ablation.13,17,20,23–25,27 In three studies,17,18,25 maximum LV wall thickness failed to show a statistical association with radiofrequency CA outcome. In one of these studies,17 LV outflow obstruction had no impact on the success rate, yet in the other two studies,21,27 non-obstructive HCM predicted success. An association between LV diastolic dysfunction and difficult rhythm control after CA was observed in two studies.24,26 In three case–control studies,20,22,27 the outcomes of AF patients with HCM vs. patients with AF alone were compared. The results were inconsistent, with two studies20,27 reporting that HCM patients were more likely to experience recurrence of AF, while the other study22 reported that there was no significant difference between the groups with AAD used more frequently in HCM patients. Another study23 compared HCM patients with secondary cardiac hypertrophy patients (those with hypertensive disease). The results showed that with more CA procedures and frequently additional AAD therapy in HCM patients, success rate of the two groups had no significant difference.
Risk factors for recurrence or success after AF ablation were presented for 11 studies
| First author, year . | Predictive model . | Covariates predictive of reurrence/success . |
|---|---|---|
| Kilicaslan, 200613 | None, just observation | Electrically silent areas in the LA predicted recurrence |
| Di Donna, 201017 | Multivariate Cox regression model | Increased LA volume and NYHA functional class predicted recurrence |
| Derejko, 201318 | Multivariate Cox regression model | PAF predicted success |
| Santangeli, 201319 | None, just observation | Non-PV triggers seem to be responsible of late recurrences |
| Yan, 201320 | Multivariate Cox regression model | HCM and LA size were independent predictors of recurrence |
| Zhou, 201321 | Single-factor analysis | Non-obstructive HCM and PAF predicted success |
| Mussigbrodt, 201423 | Single-factor analysis | LA diameter greater than 45 mm predicted recurrence |
| Okamatsu, 201424 | Univariate and multivariate Cox regression model | Univariate: increased E/e′, duration of AF and LA volume predicted recurrence Multivariate: increased E/e′ predicted recurrence |
| Liu, 201425 | Multiple factors logistic stepwise regression and multivariate Cox regression model | Short QTc and small LA diameter predicted success |
| Bassiouny, 201526 | Univariate and multivariate logistic regression analysis | Higher baseline ejection fraction and male gender predicted success; persistent or long-standing AF, log of AF duration and diastolic dysfunction predicted recurrence |
| Contreras-Valdes, 201527 | Not reported | LV outflow obstruction was an independent predictor of recurrence; LA pressure ≥12 mmHg and dilated LA were associated with recurrence only in univariate analysis |
| First author, year . | Predictive model . | Covariates predictive of reurrence/success . |
|---|---|---|
| Kilicaslan, 200613 | None, just observation | Electrically silent areas in the LA predicted recurrence |
| Di Donna, 201017 | Multivariate Cox regression model | Increased LA volume and NYHA functional class predicted recurrence |
| Derejko, 201318 | Multivariate Cox regression model | PAF predicted success |
| Santangeli, 201319 | None, just observation | Non-PV triggers seem to be responsible of late recurrences |
| Yan, 201320 | Multivariate Cox regression model | HCM and LA size were independent predictors of recurrence |
| Zhou, 201321 | Single-factor analysis | Non-obstructive HCM and PAF predicted success |
| Mussigbrodt, 201423 | Single-factor analysis | LA diameter greater than 45 mm predicted recurrence |
| Okamatsu, 201424 | Univariate and multivariate Cox regression model | Univariate: increased E/e′, duration of AF and LA volume predicted recurrence Multivariate: increased E/e′ predicted recurrence |
| Liu, 201425 | Multiple factors logistic stepwise regression and multivariate Cox regression model | Short QTc and small LA diameter predicted success |
| Bassiouny, 201526 | Univariate and multivariate logistic regression analysis | Higher baseline ejection fraction and male gender predicted success; persistent or long-standing AF, log of AF duration and diastolic dysfunction predicted recurrence |
| Contreras-Valdes, 201527 | Not reported | LV outflow obstruction was an independent predictor of recurrence; LA pressure ≥12 mmHg and dilated LA were associated with recurrence only in univariate analysis |
AF, atrial fibrillation; PAF, paroxysmal AF; LA, left atrial; HCM, hypertrophic cardiomyopathy; E, mitral inflow early filling velocity; e′, velocity of early medial mitral annular ascent.
Risk factors for recurrence or success after AF ablation were presented for 11 studies
| First author, year . | Predictive model . | Covariates predictive of reurrence/success . |
|---|---|---|
| Kilicaslan, 200613 | None, just observation | Electrically silent areas in the LA predicted recurrence |
| Di Donna, 201017 | Multivariate Cox regression model | Increased LA volume and NYHA functional class predicted recurrence |
| Derejko, 201318 | Multivariate Cox regression model | PAF predicted success |
| Santangeli, 201319 | None, just observation | Non-PV triggers seem to be responsible of late recurrences |
| Yan, 201320 | Multivariate Cox regression model | HCM and LA size were independent predictors of recurrence |
| Zhou, 201321 | Single-factor analysis | Non-obstructive HCM and PAF predicted success |
| Mussigbrodt, 201423 | Single-factor analysis | LA diameter greater than 45 mm predicted recurrence |
| Okamatsu, 201424 | Univariate and multivariate Cox regression model | Univariate: increased E/e′, duration of AF and LA volume predicted recurrence Multivariate: increased E/e′ predicted recurrence |
| Liu, 201425 | Multiple factors logistic stepwise regression and multivariate Cox regression model | Short QTc and small LA diameter predicted success |
| Bassiouny, 201526 | Univariate and multivariate logistic regression analysis | Higher baseline ejection fraction and male gender predicted success; persistent or long-standing AF, log of AF duration and diastolic dysfunction predicted recurrence |
| Contreras-Valdes, 201527 | Not reported | LV outflow obstruction was an independent predictor of recurrence; LA pressure ≥12 mmHg and dilated LA were associated with recurrence only in univariate analysis |
| First author, year . | Predictive model . | Covariates predictive of reurrence/success . |
|---|---|---|
| Kilicaslan, 200613 | None, just observation | Electrically silent areas in the LA predicted recurrence |
| Di Donna, 201017 | Multivariate Cox regression model | Increased LA volume and NYHA functional class predicted recurrence |
| Derejko, 201318 | Multivariate Cox regression model | PAF predicted success |
| Santangeli, 201319 | None, just observation | Non-PV triggers seem to be responsible of late recurrences |
| Yan, 201320 | Multivariate Cox regression model | HCM and LA size were independent predictors of recurrence |
| Zhou, 201321 | Single-factor analysis | Non-obstructive HCM and PAF predicted success |
| Mussigbrodt, 201423 | Single-factor analysis | LA diameter greater than 45 mm predicted recurrence |
| Okamatsu, 201424 | Univariate and multivariate Cox regression model | Univariate: increased E/e′, duration of AF and LA volume predicted recurrence Multivariate: increased E/e′ predicted recurrence |
| Liu, 201425 | Multiple factors logistic stepwise regression and multivariate Cox regression model | Short QTc and small LA diameter predicted success |
| Bassiouny, 201526 | Univariate and multivariate logistic regression analysis | Higher baseline ejection fraction and male gender predicted success; persistent or long-standing AF, log of AF duration and diastolic dysfunction predicted recurrence |
| Contreras-Valdes, 201527 | Not reported | LV outflow obstruction was an independent predictor of recurrence; LA pressure ≥12 mmHg and dilated LA were associated with recurrence only in univariate analysis |
AF, atrial fibrillation; PAF, paroxysmal AF; LA, left atrial; HCM, hypertrophic cardiomyopathy; E, mitral inflow early filling velocity; e′, velocity of early medial mitral annular ascent.
Periprocedural complications
Periprocedural complications were reported heterogeneously across the studies examined (Table 5). Serious complications noted in the studies included symptomatic PV stenosis, cerebrovascular accident, cardiac tamponade, serious peripheral haematoma, and thrombo-embolic events requiring medical or surgical intervention, the pool rate was 5.1% (95% CI: 2.8–9.6%). No one died from complications.
| First author, year . | n . | Complications . |
|---|---|---|
| Kilicaslan, 200613 | 27 | 1 had a small haematoma at the femoral puncture site, 4 had pulmonary vein narrowing (2 mild and 2 moderate) |
| Gaita, 200714 | 26 | 5 developed mild pericardial effusion |
| Bunch, 200815 | 33 | 2 had a periprocedural transient ischaemic attack, 1 had symptomatic PV stenosis (one vein) |
| Lu, 200916 | 11 | Not reported |
| Di Donna, 201017 | 61 | 5 developed mild pericardial effusion without haemodynamic compromise |
| Derejko, 201318 | 30 | 2 thrombo-embolic events |
| Santangeli, 201319 | 43 | 2 minor haematoma at the femoral vein access sites |
| Yan, 201320 | 24 | 1 cardiac tamponade need surgery |
| Zhou, 201321 | 57 | Not reported |
| Hayashi, 201422 | 17 | Not reported |
| Mussigbrodt, 201423 | 22 | 1 pulmonary vein stenosis that was subsequently treated with balloon dilation |
| Okamatsu, 201424 | 22 | Not reported |
| Liu, 201425 | 39 | Not reported |
| Bassiouny, 201526 | 79 | 4 developed PV stenosis (2 asymptomatic and 2 symptomatic requiring stenting), 1 developed an embolic stroke, 1 had tamponade requiring pericardiocentesis, and 1 had a large groin haematoma requiring transfusion and surgical intervention |
| Contreras-Valdes, 201527 | 40 | Not reported |
| First author, year . | n . | Complications . |
|---|---|---|
| Kilicaslan, 200613 | 27 | 1 had a small haematoma at the femoral puncture site, 4 had pulmonary vein narrowing (2 mild and 2 moderate) |
| Gaita, 200714 | 26 | 5 developed mild pericardial effusion |
| Bunch, 200815 | 33 | 2 had a periprocedural transient ischaemic attack, 1 had symptomatic PV stenosis (one vein) |
| Lu, 200916 | 11 | Not reported |
| Di Donna, 201017 | 61 | 5 developed mild pericardial effusion without haemodynamic compromise |
| Derejko, 201318 | 30 | 2 thrombo-embolic events |
| Santangeli, 201319 | 43 | 2 minor haematoma at the femoral vein access sites |
| Yan, 201320 | 24 | 1 cardiac tamponade need surgery |
| Zhou, 201321 | 57 | Not reported |
| Hayashi, 201422 | 17 | Not reported |
| Mussigbrodt, 201423 | 22 | 1 pulmonary vein stenosis that was subsequently treated with balloon dilation |
| Okamatsu, 201424 | 22 | Not reported |
| Liu, 201425 | 39 | Not reported |
| Bassiouny, 201526 | 79 | 4 developed PV stenosis (2 asymptomatic and 2 symptomatic requiring stenting), 1 developed an embolic stroke, 1 had tamponade requiring pericardiocentesis, and 1 had a large groin haematoma requiring transfusion and surgical intervention |
| Contreras-Valdes, 201527 | 40 | Not reported |
| First author, year . | n . | Complications . |
|---|---|---|
| Kilicaslan, 200613 | 27 | 1 had a small haematoma at the femoral puncture site, 4 had pulmonary vein narrowing (2 mild and 2 moderate) |
| Gaita, 200714 | 26 | 5 developed mild pericardial effusion |
| Bunch, 200815 | 33 | 2 had a periprocedural transient ischaemic attack, 1 had symptomatic PV stenosis (one vein) |
| Lu, 200916 | 11 | Not reported |
| Di Donna, 201017 | 61 | 5 developed mild pericardial effusion without haemodynamic compromise |
| Derejko, 201318 | 30 | 2 thrombo-embolic events |
| Santangeli, 201319 | 43 | 2 minor haematoma at the femoral vein access sites |
| Yan, 201320 | 24 | 1 cardiac tamponade need surgery |
| Zhou, 201321 | 57 | Not reported |
| Hayashi, 201422 | 17 | Not reported |
| Mussigbrodt, 201423 | 22 | 1 pulmonary vein stenosis that was subsequently treated with balloon dilation |
| Okamatsu, 201424 | 22 | Not reported |
| Liu, 201425 | 39 | Not reported |
| Bassiouny, 201526 | 79 | 4 developed PV stenosis (2 asymptomatic and 2 symptomatic requiring stenting), 1 developed an embolic stroke, 1 had tamponade requiring pericardiocentesis, and 1 had a large groin haematoma requiring transfusion and surgical intervention |
| Contreras-Valdes, 201527 | 40 | Not reported |
| First author, year . | n . | Complications . |
|---|---|---|
| Kilicaslan, 200613 | 27 | 1 had a small haematoma at the femoral puncture site, 4 had pulmonary vein narrowing (2 mild and 2 moderate) |
| Gaita, 200714 | 26 | 5 developed mild pericardial effusion |
| Bunch, 200815 | 33 | 2 had a periprocedural transient ischaemic attack, 1 had symptomatic PV stenosis (one vein) |
| Lu, 200916 | 11 | Not reported |
| Di Donna, 201017 | 61 | 5 developed mild pericardial effusion without haemodynamic compromise |
| Derejko, 201318 | 30 | 2 thrombo-embolic events |
| Santangeli, 201319 | 43 | 2 minor haematoma at the femoral vein access sites |
| Yan, 201320 | 24 | 1 cardiac tamponade need surgery |
| Zhou, 201321 | 57 | Not reported |
| Hayashi, 201422 | 17 | Not reported |
| Mussigbrodt, 201423 | 22 | 1 pulmonary vein stenosis that was subsequently treated with balloon dilation |
| Okamatsu, 201424 | 22 | Not reported |
| Liu, 201425 | 39 | Not reported |
| Bassiouny, 201526 | 79 | 4 developed PV stenosis (2 asymptomatic and 2 symptomatic requiring stenting), 1 developed an embolic stroke, 1 had tamponade requiring pericardiocentesis, and 1 had a large groin haematoma requiring transfusion and surgical intervention |
| Contreras-Valdes, 201527 | 40 | Not reported |
The benefits of restoring sinus rhythm
Six studies reported that restoration of sinus rhythm resulted in marked improvement of symptoms. In five of which,14,16,17,20,26 restoration of sinus rhythm resulted in a decline of New York Heart Association functional class with 0.320–1.27,26 while another study15 reported an improvement of SF 36 quality of life from baseline at 3 and 12 months [physical health 54 ± 18, 62 ± 16, 78 ± 21 (P = 0.002); mental health 53 ± 17, 66 ± 19, 82 ± 24 (P = 0.004)].
Publication bias
To evaluate the included studies for publication bias, funnel plots were constructed for late single-procedure success, for 12-month multiple-procedure success, and for multiple-procedure late success (see Supplementary material online, Appendix S3). There was some suggestion of an association between the log odds of success and the standard error of the log odds, particularly for the 12-month multiple-procedure success rates. However, the larger, more precise studies tended to report higher success rates.
Discussion
To our knowledge, this is the first systematic review to examine the outcome of CA treatment for AF in HCM patients. Based on the results analysed, CA of AF was found to be a relatively effective and safe treatment for the cohorts studied. The principal findings were that: (i) a single ablation procedure was insufficient to achieve freedom from atrial arrhythmia for the cohorts studied (<50% with AAD assistance); (ii) over time, multiple procedures were required to achieve control of AF, which was achieved in 66.1% of the cases at final follow-up; (iii) it was easier to restore sinus rhythm in PAF cases; and (iv) even though with more RFCA procedures, and frequently additional AAD therapy as well, AF was easier to relapse in HCM patients compared with that only experienced AF.
Comparisons to the related research
Over the past 2 years, other relevant meta-analyses28–30 had demonstrated that CA is more effective than drug therapy for the treatment of AF. In a recent worldwide study,31 at an intermediate time point during follow-up after CA, the success rates with or without AAD were significantly higher for the PAF patients (83.2 and 74.9%, respectively) than for the patients with persistent AF (75.0 and 64.8%, respectively) and long-lasting AF (72.3 and 63.1%, respectively). Until recently, few series have presented the effect of CA in AF patients complicated with cardiomyopathy. A systematic review and meta-analysis by Ganesan et al.10 showed that CA is an effective and durable long-term therapeutic strategy for some AF patients. Left ventricle systolic dysfunction or heart failure and structural or valvular heart disease were also identified as variables predictive of AF recurrence in this review.10 Another systematic review and meta-analysis conducted by Wilton et al.32 showed that the success rate for achieving freedom from AF in patients with left ventricular systolic dysfunction (LVSD) after one CA treatment was 28–55% lower than that achieved for cases of AF without LVSD. And the success rate increased to 64–96% after a mean of 1.4 procedures were performed, comparable with the results achieved in the patients absence of LVSD. The results of Wilton et al.32 and those of another study33 indicated that AF ablation in patients with LVSD results in significant improvement of LV function. The results of our study are consistent with these previous data.10,32,33
Mechanisms of recurrence
Eight studies14,16,17,19,23,24,26,27 involved the information about PV reconnection in repeat ablations (40–100%). This seems a strong factor of post-operative recurrence. Whether PV reconnection is related to the pathological changes of HCM which needs further research. Contreras-Valdes et al.27 said ‘re-isolation of all chronically reconnected pulmonary veins did not improve arrhythmia control in patients with HCM to the same extent as that in non-affected subjects'. The attribution analysis in some included studies showed structural or electrical abnormalities of LA predicted AF recurrence overwhelmingly (Table 4). The disorders of atrial substrate (fibrosis, scarring) pathophysiologically associated with HCM due to haemodynamics34 or sarcomere protein gene mutations3 might provide the substrate for multiple arrhythmogenic areas beyond the PVs in such patients. Non-PV triggers were demonstrated to be responsible for late AF recurrence in HCM patients by Santangeli et al.19 And Bunch et al.15 found that in patients with an enlarged LA, additional linear ablation was more important for AF control. Thus it can be seen, the role of non-PV triggers may be a potential reason for high AF recurrence rates in HCM patients.
Clinical parameters related to between-study heterogeneity
In the current study, substantial heterogeneity was observed between the studies examined. It has previously been shown that the type of PAF experienced by a patient and AAD therapy is closely related with the success rates associated for these cases. In the present study, subgroup analysis of these two clinical parameters was conducted and both PAF and administration of AAD were found to be accompanied by a high success rate in both the single- and multiple-procedure groups at the 12-month and last follow-up time points. However, heterogeneity still existed in the subgroups. Although there are several clinical differences between the studies and these may account for the observed differences in outcome: (i) there were differences in the patient population that received ablation with respect to patient race, gender, age, AF duration time, degree of myocardial hypertrophy, (ii) differences in the technique and technology used for ablation, (iii) differences in follow-up frequency, intensity, and duration, (iv) differences in the definitions of blanking period, and (v) differences in the time interval of the repeat procedures. Because of small amount of included studies (especially in every subgroup) and relatively more covariates, a meta-regression analysis was not performed to investigate possible reasons for the heterogeneity.
Clinical implications
This meta-analysis provides valuable insight into the clinical implications of CA treatment for AF in HCM patients. The restoration and maintenance of sinus rhythm is more important for HCM patients than patients with AF alone since preservation of atrial systolic function can increase the ventricular filling volume, thereby delaying and relieving LV outflow obstruction in HCM patients. As a supplement of drug therapy, CA is a not bad choice for rhythm control of AF in HCM patients, although multiple procedures may be needed.
Study limitations
Reports of AF ablation in HCM patients represent relatively new data and a controversial field. Moreover, no relevant, randomized controlled study has been published to date. Thus, the present meta-analysis was performed using primarily non-randomized observational data, rather than randomized controlled trial data. Consequently, there were significant limitations in study quality and some risk of bias,9,35 which led to significant heterogeneity in the results. Statistical explanations for this heterogeneity were not provided by detailed subgroup analyses and meta-regression analysis, due to the lack of similarity in the reporting of outcomes and moderator variables, as well as the small number of studies included in this meta-analysis. We specifically acknowledge the limitations and inherent bias that may occur with this approach.
Conclusions
The data presented in this review showed that CA of AF in patients with HCM is feasible and safe, although more repeat procedures and AAD are needed to prevent AF recurrence.
Supplementary material
Supplementary material is available at Europace online.
Conflict of interest: none declared.
References
Author notes
These authors contributed equally.



