-
PDF
- Split View
-
Views
-
Cite
Cite
Jan Beyer-Westendorf, Independent data about the safety and efficacy of rivaroxaban for prevention of stroke/embolism are needed: Author reply, EP Europace, Volume 18, Issue 1, January 2016, Pages 156–158, https://doi.org/10.1093/europace/euv286
Close - Share Icon Share
We appreciate the comments of Stollberger et al.1 regarding our publication on the persistence with rivaroxaban therapy in the Dresden NOAC registry.2 A number of issues were addressed in their letter, some of which are directly related to our publication while others are more related to an overall concern regarding the safety of non-VKA oral anticoagulants (NOACs) such as rivaroxaban. Unfortunately, word count restrictions do not allow discussing the latter issues in detail here, especially since some of these issues were discussed with Stollberger et al. previously.3,4
More or less directly related to our manuscript are the following questions:
‘How did the authors ascertain that the patients were indeed taking the drug? Whereas adherence to VKA is easily monitored by measuring the international normalized ratio (INR), a readily available test for rivaroxaban effect is not available. Were serum concentrations of rivaroxaban obtained among the participants of the registry?’
First of all, Stollberger et al. need to understand that our publication related to persistence (=the continuation of treatment over time) and not to adherence (=the adherence to regular drug intake according to prescribed drug regimen).5 Regarding our persistence data, we assessed treatment continuation or discontinuation by patient interviews and compared patient-derived information against prescription plans, information from attending physicians or hospital documentation, if applicable. In the case of treatment interruptions or discontinuations, the exact dates and times of last intake (or restart) as well as reasons for intervention were obtained.
Further questions by Stollberger et al. wereThese are certainly interesting and relevant questions but not within the focus of a paper addressing persistence to oral anticoagulation. The authors of the letter may want to refer to further publications from our registry, especially those relating to the peri-procedural management of NOAC patients6 and to the management and outcome of rivaroxaban-associated bleeding.7
We are grateful for this important comment. The ‘numbers at risk’ in our Kaplan–Meier diagrams were correctly submitted to the journal but obviously got changed during the type-setting process, resulting in incorrect numbers in the final publication, which was missed during our proof-reading. We provide our original Kaplan–Meier diagrams with this letter as an Erratum.‘We are confused about the numbers given in Figure 1A and B. The number of subjects at risk in the ‘Rivaroxaban new’ group increases over time, whereas it is expected that it will decrease. In Figure 1B, we expect the number of patients being less than in Figure 1A. However, at 480 days, 587 patients are in the ‘Rivaroxaban new’ group in Figure 1B, compared with 387 patients in that group in Figure 1A at 480 days.’
This is a justified consideration that Stollberger et al. phrase repeatedly.3,8,9 We agree that the influence of drug companies in scientific projects may affect the study results. Consequently, we setup our registry independently from pharma companies and run it by a CRO that is based at our institution, which also serves as the sponsor. While funding for the Dresden NOAC registry is indeed obtained from NOAC manufacturers, contracts made sure that these companies did not have access to any data or influence on the registry publications. Furthermore, all statistical analyses were performed by an independent statistician. Finally, we obtained funding from three NOAC manufacturers, also to avoid the impression that the registry would serve the interest of a specific company.‘The registry was funded by grants from pharmaceutical companies including the manufacturer of rivaroxaban. We know from social psychology that the influence of industry may also be subconscious and unintentional. To assess the clinical value of rivaroxaban and other non-Vitamin K–antagonist anticoagulants, independent studies should be also carried out.’
– How many patients died during the observational period and how many of these patients died because of bleeding or embolic event?
– How many patients underwent emergency surgery and were there any bleeding problems?
– How many of the included patients experienced ischaemic stroke during follow-up, and how many underwent systemic thrombolysis without being aware that these patients were under an anticoagulant therapy?
– It would be of interest to know how many patients in the registry received P-gp- or CYP 3A4-affecting drugs and if therapy with these drugs was associated with bleeding or embolic events.
– Since thrombin exerts many biological functions, long-term thrombin inhibition may also affect the rate of infections, tumour growth, and angiogenesis. Were patients in the registry also investigated for these potential side effects of rivaroxaban?
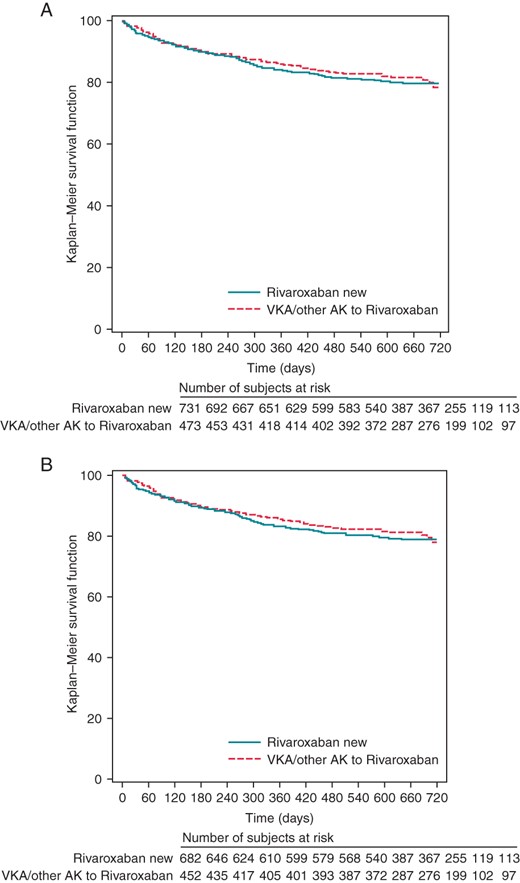
Kaplan–Meier analysis of persistence to rivaroxaban treatment for all patients (A) and for all patients who were observed for at least 12 months (B), according to VKA pre-treatment.
We fully support the transparency policy of all major medical journals and think that our detailed conflict-of-interest statement allows the reader to sufficiently reflect the potential for funding bias. However, since the potential influence of pharma companies on NOAC results seems to have been a major concern of Stollberger et al. over the last years, we would like to encourage them to start collecting data on NOAC safety in daily care independent from industry support.



