-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Sticherling, Francisco Marin, David Birnie, Giuseppe Boriani, Hugh Calkins, Gheorghe-Andrei Dan, Michele Gulizia, Sigrun Halvorsen, Gerhard Hindricks, Karl-Heinz Kuck, Angel Moya, Tatjana Potpara, Vanessa Roldan, Roland Tilz, Gregory Y.H. Lip, ESC Scientific Document Group , Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS), EP Europace, Volume 17, Issue 8, August 2015, Pages 1197–1214, https://doi.org/10.1093/europace/euv190
Close - Share Icon Share
Introduction
Since the advent of the non-vitamin K antagonist oral anticoagulant (NOAC) agents, which act as direct thrombin inhibitors or inhibitors of Factor Xa, clinicians are provided with valuable alternatives to vitamin K antagonists (VKAs). At the same time, electrophysiologists frequently perform more invasive procedures, increasingly involving the left chambers of the heart. Thus, they are constantly faced with the dilemma of balancing the risk for thromboembolic events and bleeding complications. These changes in the rapidly evolving field mandate an update of the European Heart Rhythm Association (EHRA) 2008 consensus document on this topic.1 The present document covers the antithrombotic management during different ablation procedures, implantation or exchange of cardiac implantable electronical devices (CIEDs), as well as the management of peri-interventional bleeding complications.
The document is not a formal guideline and due to the lack of prospective randomized controlled trials (RCTs) for many of the clinical situations encountered, the recommendations are often ‘expert opinion’. The document strives to be practical for which reason we subdivided it in the three main topics: ablation procedure, CIED implantation or generator change, and issues of peri-interventional bleeding complications on concurrent antiplatelet therapy. For quick reference, every subchapter is followed by a short section on consensus recommendations.
Many RCTs are ongoing in this field and it is hoped that this document will help to prompt further well-designed studies.
Antithrombotic management in patients undergoing ablation procedures
Ablation of atrial fibrillation, left atrial arrhythmias and right sided atrial flutter
In patients with symptomatic paroxysmal or even persistent atrial fibrillation (AF), catheter ablation is indicated when antiarrhythmic drugs have failed in controlling recurrences or even as a first-line therapy in selected patients.2–4
Patients with AF have an increased risk of thromboembolic events, which varies according to the presence of several risk factors.5,6 Apart from their intrinsic thromboembolic risks, ablation in these patients increases thromboembolic risk due to the introduction and manipulation of one or more catheters and long sheaths into the left atrium, and also due to endocardial lesions produced during ablation. Cerebral imaging studies have shown embolic events post-ablation without clinical overt cognitive deficits.7
The management of anticoagulation during ablation for the prevention of thromboembolic events may, on the other hand, increase the risk of bleeding complications during the procedure (Table 1).
Data from the literature on stroke/TIA and tamponade rates during AF ablation
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | References . |
|---|---|---|---|---|---|
| Stabile (CACAF) 2006 | RCT | 68 | 1.5 | 1.5 | 8 |
| Wazni (RAAFT) 2005 | RCT | 33 | 0 | 0 | 9 |
| Oral 2006 | RCT | 130 | 0 | 0 | 10 |
| Pappone 2006 | RCT | 99 | 1 | 0 | 11 |
| Jais (A4) 2008 | RCT | 155 | 0 | 1.2 | 12 |
| Wilber (Thermocool-AF) 2010 | RCT | 106 | 0 | 0.9 | 13 |
| Nielsen (MANTRA PAF) 2012 | RCT | 146 | 1.3 | 2.1 | 3 |
| Packer (STOP AF) 2013 | RCT | 163 | 4.2 | 0.6 | 14 |
| Cappato 2010 | Survey | 16′309 | 0.9 | 1.3 | 15 |
| Deshmukh 2013 | Survey | 93′801 | 1.0 | 1.5 | 16 |
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | References . |
|---|---|---|---|---|---|
| Stabile (CACAF) 2006 | RCT | 68 | 1.5 | 1.5 | 8 |
| Wazni (RAAFT) 2005 | RCT | 33 | 0 | 0 | 9 |
| Oral 2006 | RCT | 130 | 0 | 0 | 10 |
| Pappone 2006 | RCT | 99 | 1 | 0 | 11 |
| Jais (A4) 2008 | RCT | 155 | 0 | 1.2 | 12 |
| Wilber (Thermocool-AF) 2010 | RCT | 106 | 0 | 0.9 | 13 |
| Nielsen (MANTRA PAF) 2012 | RCT | 146 | 1.3 | 2.1 | 3 |
| Packer (STOP AF) 2013 | RCT | 163 | 4.2 | 0.6 | 14 |
| Cappato 2010 | Survey | 16′309 | 0.9 | 1.3 | 15 |
| Deshmukh 2013 | Survey | 93′801 | 1.0 | 1.5 | 16 |
RCT, randomized controlled trial; A4, atrial fibrillation ablation versus antiarrhythmic drugs; CACAF, catheter ablation for the cure of atrial fibrillation; MANTRA-PAF, medical antiarrhythmic treatment or radiofrequency ablation in paroxysmal atrial fibrillation; RAAFT, radiofrequency ablation atrial fibrillation trial; STOP AF, sustained treatment of paroxysmal atrial fibrillation; TIA, transient ischemic attack.
Data from the literature on stroke/TIA and tamponade rates during AF ablation
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | References . |
|---|---|---|---|---|---|
| Stabile (CACAF) 2006 | RCT | 68 | 1.5 | 1.5 | 8 |
| Wazni (RAAFT) 2005 | RCT | 33 | 0 | 0 | 9 |
| Oral 2006 | RCT | 130 | 0 | 0 | 10 |
| Pappone 2006 | RCT | 99 | 1 | 0 | 11 |
| Jais (A4) 2008 | RCT | 155 | 0 | 1.2 | 12 |
| Wilber (Thermocool-AF) 2010 | RCT | 106 | 0 | 0.9 | 13 |
| Nielsen (MANTRA PAF) 2012 | RCT | 146 | 1.3 | 2.1 | 3 |
| Packer (STOP AF) 2013 | RCT | 163 | 4.2 | 0.6 | 14 |
| Cappato 2010 | Survey | 16′309 | 0.9 | 1.3 | 15 |
| Deshmukh 2013 | Survey | 93′801 | 1.0 | 1.5 | 16 |
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | References . |
|---|---|---|---|---|---|
| Stabile (CACAF) 2006 | RCT | 68 | 1.5 | 1.5 | 8 |
| Wazni (RAAFT) 2005 | RCT | 33 | 0 | 0 | 9 |
| Oral 2006 | RCT | 130 | 0 | 0 | 10 |
| Pappone 2006 | RCT | 99 | 1 | 0 | 11 |
| Jais (A4) 2008 | RCT | 155 | 0 | 1.2 | 12 |
| Wilber (Thermocool-AF) 2010 | RCT | 106 | 0 | 0.9 | 13 |
| Nielsen (MANTRA PAF) 2012 | RCT | 146 | 1.3 | 2.1 | 3 |
| Packer (STOP AF) 2013 | RCT | 163 | 4.2 | 0.6 | 14 |
| Cappato 2010 | Survey | 16′309 | 0.9 | 1.3 | 15 |
| Deshmukh 2013 | Survey | 93′801 | 1.0 | 1.5 | 16 |
RCT, randomized controlled trial; A4, atrial fibrillation ablation versus antiarrhythmic drugs; CACAF, catheter ablation for the cure of atrial fibrillation; MANTRA-PAF, medical antiarrhythmic treatment or radiofrequency ablation in paroxysmal atrial fibrillation; RAAFT, radiofrequency ablation atrial fibrillation trial; STOP AF, sustained treatment of paroxysmal atrial fibrillation; TIA, transient ischemic attack.
The antithrombotic strategy in patients undergoing AF ablation includes three different stages: pre-procedural treatment, peri-procedural anticoagulation, and post-procedural strategy.
Pre-procedural treatment
Based on the 2012 Heart Rhythm Society/European Heart Rhythm Association/ European Cardiac Arrhythmia Society (HRS/EHRA/ECAS) consensus document of AF Ablation, the minimum criteria concerning anticoagulation at the time of AF ablation are those that apply to cardioversion of AF.2 All patients undergoing AF ablation who present in AF for the procedure should be anticoagulated for at least 3 weeks prior to AF ablation. If they have not been anticoagulated prior to ablation, a transoesophageal echocardiography (TEE) should be performed. In addition to adhering to these well-established anticoagulation guidelines that apply to cardioversion, the 2012 HRS/EHRA/ECAS consensus document recommends that all patient being anticoagulated during AF ablation with heparin to achieve an activated clotting time (ACT) of at least 300 s. This writing group fully supports these prior minimum recommendations for anticoagulation.
Despite the lack of controlled trials, there is a general trend to consider starting antithrombotic treatment before ablation,17 even in patients who present for ablation in sinus rhythm. The higher the patient's stroke risk profile, the lower the threshold is to start anticoagulation prior to ablation. This approach is in keeping with current anticoagulation guidelines that apply to all AF patients. The current guidelines recommend that the initial step is to identify low-risk patients (CHA2DS2-VASc score = 0 for males and 1 for females) who do not need any antithrombotic therapy (Step 1). Subsequent to this step, it is recommended that all patients with a CHA2DS2-VASc score of ≥2 should be anticoagulated, and that anticoagulation should also be considered for males with a CHA2DS2-VASc score of 1 (Step 2).18 For those patients treated with a VKA or a NOAC, the recommendation is to have at least 3 weeks of effective stable international normalized ratio (INR) at therapeutic levels (between 2 and 3 for VKA).19–23 Patients on a VKA should aim for an average therapeutic target range (TTR) of >70% within the target INR of 2.0–3.0, to minimize the risks of thromboembolism and bleeding.24,25 For those treated with a NOAC, 3 weeks of anticoagulation is recommended in a patient previously anticoagulation naive. Attention to drug adherence and counselling of patients may help to emphasize the importance of treatment.26
While effective oral anticoagulation is readily achieved when starting a NOAC, the initiation of a VKA after pulmonary vein isolation (PVI) requires bridging with a low-molecular-weight heparin (LMWH) until therapeutic INRs are obtained. This is not an issue if the ablation is performed on therapeutic INRs. Achievement of an adequate TTR of >70% is dependent on many factors, and use of the SAMe-TT2R2 score can aid decision-making on whether patients are likely to do well on VKA with a high TTR (SAMe-TT2R2 score 0–2) or those patients where labile INRs are likely (with a low TTR) and a NOAC may be a better therapeutic option (SAMe-TT2R2 score >2).27
Several studies have compared the strategy of discontinuation of VKA for 3–5 days before the ablation, with bridging therapy with LMWH (until the evening before the procedure) with a strategy consisting on performing ablation without interrupting VKA agents with INR between 2 and 3.5. Of note, the available randomized data does not compare bridging with unfractionated heparins.
Several non-controlled studies have shown that performing ablation with an uninterrupted VKA maintaining therapeutic INR levels is not only safe but also decreases the rate of thromboembolic and haemorrhagic complications.28–30 One recent controlled multicentre study compared a strategy of discontinuing warfarin 2–3 days before ablation with bridging therapy with LMWH with a strategy consisting of performing ablation without interruption of warfarin.22 Patients with an INR of >3.5 were postponed and those with an INR between 3 and 3.5 received fresh frozen plasma (which we do not recommend) before the ablation. In this trial, patients in whom the ablation was performed with therapeutic INR levels had a lower rate of thromboembolic complications (0.25 vs. 4.9%, P < 0.001) without significant differences in major bleeding complications (<1%).
For patients treated with NOACs, these drugs should be started at least 3 weeks before ablation, and treatment adherence emphasized to the patient as there is no easy way to measure drug compliance.31
One recent prospective randomized controlled trial compared uninterrupted rivaroxaban to uninterrupted VKA and found similar low rates of bleeding and thrombembolic events.32 Several observational and non-controlled trials, have also analysed the role of NOACs, specifically dabigatran and rivaroxaban, in patients undergoing catheter ablation. The strategy of using NOACs in published series is not homogenous: the last dose of dabigatran before ablation varies depending on the different publications between 12 and 36 h, and some authors even performed the ablation without interrupting dabigatran.33,34 For rivaroxaban, the last dose is usually administered 24–36 h before the ablation.31,35–37 Data on the safety about the use of NOACs in ablation have been contradictory, but in general, thromboembolic and bleeding risks are probably similar when comparing NOACs with an uninterrupted VKA strategy.34,37,38
In patients receiving VKA agents, it seems reasonably not to stop VKA administration and performing the ablation with INR levels between 2.0 and 3.0 or even 3.5. For NOACs, RCTs are ongoing, but it seems reasonable that, in patients treated with dabigatran or rivaroxaban, ablation can be performed either by stopping one or two doses before the ablation or even with uninterrupted rivaroxaban.32,39,40 A TEE should be performed in all patients in whom there is a doubt about the appropriate anticoagulation in the 3 weeks before the intervention.2 Indeed, studies have shown that 1.6–2.1% of patients who have been fully anticoagulated undergoing PVI demonstrate a left atrial thrombus or sludge.41–43 Some operators advise a TEE in all patients undergoing AF ablation regardless of the presenting rhythm or stroke risk profile.
Peri-procedural anticoagulant strategy
Regardless of the peri-procedural anticoagulant treatment, all patients should receive full anticoagulation with intravenous heparin during ablation.
A first loading dose of intravenous heparin of 5000–15 000 units (or 90–200 U/kg) should be administered at the beginning of the procedure. It has been shown that patients on VKA require lower heparin doses than those on a NOAC.44 Some operators give this first loading dose immediately after venous puncture just before transseptal puncture (TSP),20,22,28,29,45 whereas others give a half dose before and the remaining dose after completion of TSP,46 and the rest administer the loading dose immediately after TSP.20,23,31,47 There are no controlled data comparing these different strategies. In an European survey, which includes data from 78 centres in 20 different countries in Europe, 69% of the centres administer the first loading dose after TSP, 18% before, and the remaining 13% partly before and partly afterwards.17
All sheaths should be continuously flushed with heparinized saline solution, with a suggested dose of 2000 units per 250 mL.19,48
After the first loading dose of heparin, continuous heparin infusion at an initial rate of 1000–1500 U/kg/h can be started depending on the levels of ACT. Others tailor the administration to achieve the target ACT by intermittently administering heparin between 2500 and 7500 U. The first ACT measurement should be performed 10–15 min after the loading dose and thereafter every 20–30 min. It must be borne in mind that the uninterrupted use of VKAs or NOACs has an influence on the ACT and the time needed to reach the target ACT.49–51 The optimal target ACT is >300 s, which decreases the rate of thromboembolic events without an increase in bleeding complications.30 At the end of the ablation, it is recommended to remove the vascular sheaths when ACT levels are at least <250 s. Protamine may be administered for this purpose.20,22,30,35,46 Accordingly, we recommend the administration of a loading dose of 10 000–15 000 U of heparin before or immediately after TSP followed by either continuous intravenous heparin infusion or repeated heparin boli targeting ACT levels >300 s.
Post-procedural management
Once the ablation has been finished and before initiating anticoagulant treatment, it can be useful to perform transthoracic echocardiography in order to rule out pericardial effusion or cardiac tamponade. If intracardiac echocardiography (ICE) was employed, it can be used at the and of the procedure to rule pericardial effusion.
In those patients in whom the procedure has been performed with brief interruption of a NOAC, the next dose should be administered after 3–4 h once haemostasis has been achieved. In those patients who discontinued a VKA or had a low INR at the time of ablation, LMWH should be administered at 4–6 h once haemostasis has been achieved along with reinitiating VKA agents, maintaining the administration of LMWH until therapeutic INR levels have been achieved.30,47
Oral anticoagulation should be continued for at least 2 months after ablation, since there is evidence that the vast majority of thromboembolic events occurs in the first 4 weeks after ablation.52 Subsequently, the decision for oral anticoagulation depends on the patient's stroke risk profile and not on the perceived success or failure of ablation. Currently, there are insufficient data to support the concept that AF ablation reduces stroke risk post AF ablation.
The role of left atrial appendage occluder devices in the peri-procedural setting has not been studied, and is not recommended pending new data.
Right-sided atrial flutter
The pre- and post-interventional anticoagulation management described for patients undergoing PVI or ablation for left-sided atrial flutter also applies for patients with right-sided, mostly cavotricuspid-dependent, atrial flutter who present for ablation in atrial flutter. The procedural risk for bleeding and thromboembolism is lower, since the catheters remain in the venous circulation only and there is no need for TSP or another access to the systemic circulation. For this reason, it has become common practice to perform catheter ablation of right-sided flutter in patients while on a VKA with a therapeutic INR (INR: 2.0–3.0) and also in patients who are taking a NOAC without interruption prior to ablation. In patients who have not been anticoagulated before and present in atrial flutter, a TEE should be done. After ablation of patients with isolated atrial flutter and a CHA2DS2-VASc score of ≥2, an oral anticoagulant (OAC) may be continued like in AF patients since there is evidence of a very high incidence of subsequent AF in these patients.53,54
Antithrombotic management in patients undergoing atrial fibrillation catheter ablation for the maintenance of sinus rhythm: consensus recommendations
| All patients undergoing AF catheter ablation who present for the procedure in AF should be anticoagulated with a NOAC, or a VKA with a therapeutic INR of 2.0–3.0 for 3 weeks prior to the procedure; or undergo a TEE to screen for thrombi prior to the procedure; post procedure, patients should receive anticoagulation for at least 2 months. |
| In patients receiving a VKA, the ablation should be performed without interruption of VKA therapy. |
| During the ablation procedure, patients should receive unfractionated heparin with an ACT of >300 s. |
| Transoesophageal electrocardiography can be useful before the intervention to rule our left atrial thrombi in all patients with a CHA2DS2-VASc score of ≥2. |
| In patients presenting in atrial flutter and undergoing right-sided atrial flutter ablation of the cavotricuspid isthmus only, therapy with a VKA and a NOAC should not be interrupted and continued for at least 4 weeks after a successful ablation. |
| For patients with AF who present for ablation in sinus rhythm, pre-procedural TEE or initiation of anticoagulation ≥3 weeks prior to ablation can be useful, especially in those patients with a CHA2DS2-VASc score of ≥2. |
| Transoesophageal electrocardiography can be useful in patients who present for ablation in AF and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| In patients receiving a NOAC and with normal renal function, it is reasonable to give the last dose 24 h before the ablation. For patients on dabigatran and renal impairment, this period of interruption is longer. |
| Uninterrupted NOAC therapy may be considered in some patients undergoing ablation. |
| For patients in sinus rhythm and a CHA2DS2-VASc score of 0 (males) or 1 (females), it may be considered starting a NOAC on the day of the procedure, post-ablation. |
| Transoesophageal electrocardiography may be considered in patients who present for ablation in sinus rhythm and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| Ablation is not recommended in patients in whom no anticoagulation can be administered during and after the procedure. |
| In patients on a VKA and an INR of >2–3, the VKA should not be stopped and no bridging with a low molecular weight should be instituted. |
| All patients undergoing AF catheter ablation who present for the procedure in AF should be anticoagulated with a NOAC, or a VKA with a therapeutic INR of 2.0–3.0 for 3 weeks prior to the procedure; or undergo a TEE to screen for thrombi prior to the procedure; post procedure, patients should receive anticoagulation for at least 2 months. |
| In patients receiving a VKA, the ablation should be performed without interruption of VKA therapy. |
| During the ablation procedure, patients should receive unfractionated heparin with an ACT of >300 s. |
| Transoesophageal electrocardiography can be useful before the intervention to rule our left atrial thrombi in all patients with a CHA2DS2-VASc score of ≥2. |
| In patients presenting in atrial flutter and undergoing right-sided atrial flutter ablation of the cavotricuspid isthmus only, therapy with a VKA and a NOAC should not be interrupted and continued for at least 4 weeks after a successful ablation. |
| For patients with AF who present for ablation in sinus rhythm, pre-procedural TEE or initiation of anticoagulation ≥3 weeks prior to ablation can be useful, especially in those patients with a CHA2DS2-VASc score of ≥2. |
| Transoesophageal electrocardiography can be useful in patients who present for ablation in AF and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| In patients receiving a NOAC and with normal renal function, it is reasonable to give the last dose 24 h before the ablation. For patients on dabigatran and renal impairment, this period of interruption is longer. |
| Uninterrupted NOAC therapy may be considered in some patients undergoing ablation. |
| For patients in sinus rhythm and a CHA2DS2-VASc score of 0 (males) or 1 (females), it may be considered starting a NOAC on the day of the procedure, post-ablation. |
| Transoesophageal electrocardiography may be considered in patients who present for ablation in sinus rhythm and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| Ablation is not recommended in patients in whom no anticoagulation can be administered during and after the procedure. |
| In patients on a VKA and an INR of >2–3, the VKA should not be stopped and no bridging with a low molecular weight should be instituted. |
Antithrombotic management in patients undergoing atrial fibrillation catheter ablation for the maintenance of sinus rhythm: consensus recommendations
| All patients undergoing AF catheter ablation who present for the procedure in AF should be anticoagulated with a NOAC, or a VKA with a therapeutic INR of 2.0–3.0 for 3 weeks prior to the procedure; or undergo a TEE to screen for thrombi prior to the procedure; post procedure, patients should receive anticoagulation for at least 2 months. |
| In patients receiving a VKA, the ablation should be performed without interruption of VKA therapy. |
| During the ablation procedure, patients should receive unfractionated heparin with an ACT of >300 s. |
| Transoesophageal electrocardiography can be useful before the intervention to rule our left atrial thrombi in all patients with a CHA2DS2-VASc score of ≥2. |
| In patients presenting in atrial flutter and undergoing right-sided atrial flutter ablation of the cavotricuspid isthmus only, therapy with a VKA and a NOAC should not be interrupted and continued for at least 4 weeks after a successful ablation. |
| For patients with AF who present for ablation in sinus rhythm, pre-procedural TEE or initiation of anticoagulation ≥3 weeks prior to ablation can be useful, especially in those patients with a CHA2DS2-VASc score of ≥2. |
| Transoesophageal electrocardiography can be useful in patients who present for ablation in AF and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| In patients receiving a NOAC and with normal renal function, it is reasonable to give the last dose 24 h before the ablation. For patients on dabigatran and renal impairment, this period of interruption is longer. |
| Uninterrupted NOAC therapy may be considered in some patients undergoing ablation. |
| For patients in sinus rhythm and a CHA2DS2-VASc score of 0 (males) or 1 (females), it may be considered starting a NOAC on the day of the procedure, post-ablation. |
| Transoesophageal electrocardiography may be considered in patients who present for ablation in sinus rhythm and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| Ablation is not recommended in patients in whom no anticoagulation can be administered during and after the procedure. |
| In patients on a VKA and an INR of >2–3, the VKA should not be stopped and no bridging with a low molecular weight should be instituted. |
| All patients undergoing AF catheter ablation who present for the procedure in AF should be anticoagulated with a NOAC, or a VKA with a therapeutic INR of 2.0–3.0 for 3 weeks prior to the procedure; or undergo a TEE to screen for thrombi prior to the procedure; post procedure, patients should receive anticoagulation for at least 2 months. |
| In patients receiving a VKA, the ablation should be performed without interruption of VKA therapy. |
| During the ablation procedure, patients should receive unfractionated heparin with an ACT of >300 s. |
| Transoesophageal electrocardiography can be useful before the intervention to rule our left atrial thrombi in all patients with a CHA2DS2-VASc score of ≥2. |
| In patients presenting in atrial flutter and undergoing right-sided atrial flutter ablation of the cavotricuspid isthmus only, therapy with a VKA and a NOAC should not be interrupted and continued for at least 4 weeks after a successful ablation. |
| For patients with AF who present for ablation in sinus rhythm, pre-procedural TEE or initiation of anticoagulation ≥3 weeks prior to ablation can be useful, especially in those patients with a CHA2DS2-VASc score of ≥2. |
| Transoesophageal electrocardiography can be useful in patients who present for ablation in AF and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| In patients receiving a NOAC and with normal renal function, it is reasonable to give the last dose 24 h before the ablation. For patients on dabigatran and renal impairment, this period of interruption is longer. |
| Uninterrupted NOAC therapy may be considered in some patients undergoing ablation. |
| For patients in sinus rhythm and a CHA2DS2-VASc score of 0 (males) or 1 (females), it may be considered starting a NOAC on the day of the procedure, post-ablation. |
| Transoesophageal electrocardiography may be considered in patients who present for ablation in sinus rhythm and who have been adequately anticoagulated for 3 weeks or longer prior to ablation, especially in those with a CHA2DS2-VASc score of ≥2. |
| Ablation is not recommended in patients in whom no anticoagulation can be administered during and after the procedure. |
| In patients on a VKA and an INR of >2–3, the VKA should not be stopped and no bridging with a low molecular weight should be instituted. |
Ablation of left-sided accessory pathways and focal left atrial tachycardia
Accessory pathways (APs) are located on the left side in more than 50% of cases and their ablation carries a higher acute success and a lower recurrence rate than septal or right-sided accessory pathways.55
Over the past years, the preferred access route for ablation changed from the retrograde aortic access, targeting the ventricular insertion site of the AP, to the antegrade transseptal approach targeting the atrial insertion of the AP. In elderly patients, the antegrade approach also avoids the crossing of potentially calcified aortic valves and the associated embolic risk. Historical rates of cardiac tamponade range from 0.13 to 1.1% and cerebrovascular accidents from 0.15 to 0.49%.56,57
The access route is the same utilized for ablation of AF and left-sided atrial tachycardia (AT). Although there are only limited data concerning the real thromboembolic risk with contemporary ablation equipment, it can be assumed that the actual risk is lower than the rates reported from the 1990s and in the AF/AT population. Patients undergoing AP ablation are also younger and have usually no or few risk factors for thrombembolic events. Furthermore, there is only a single catheter with or without one long sheath in the left atrium or the left ventricle, and the ablation is usually focal resulting in much shorter total ablation times and time spent in the left atrium.
Since there is no scientific evidence supporting peri-interventional anticoagulation, the potential risks of bleeding have to be taken into account. Prior anticoagulant therapy is not warranted. After arterial access, 5000–15 000 units (or 90–200 U/kg) of intravenous sodium heparin is recommended followed by 1000 U/h during the procedure.1 Long sheaths should be continuously flushed to avoid thrombus formation. There is no evidence, supporting the post-interventional use of oral anticoagulation or aspirin.
Antithrombotic management in patients undergoing focal left atrial ablation of an accessory pathway or a focal atrial tachycardia: consensus recommendations
| During the ablation procedure, it is recommended to give unfractionated heparin with a target ACT of >300 s. |
| After focal left atrial ablation of an accessory pathway or an AT, oral anticoagulation or the use of aspirin is not recommended unless otherwise indicated. |
| During the ablation procedure, it is recommended to give unfractionated heparin with a target ACT of >300 s. |
| After focal left atrial ablation of an accessory pathway or an AT, oral anticoagulation or the use of aspirin is not recommended unless otherwise indicated. |
Antithrombotic management in patients undergoing focal left atrial ablation of an accessory pathway or a focal atrial tachycardia: consensus recommendations
| During the ablation procedure, it is recommended to give unfractionated heparin with a target ACT of >300 s. |
| After focal left atrial ablation of an accessory pathway or an AT, oral anticoagulation or the use of aspirin is not recommended unless otherwise indicated. |
| During the ablation procedure, it is recommended to give unfractionated heparin with a target ACT of >300 s. |
| After focal left atrial ablation of an accessory pathway or an AT, oral anticoagulation or the use of aspirin is not recommended unless otherwise indicated. |
Ablation of right atrial arrhythmias (excluding atrial flutter)
The thromboembolic risk in patients undergoing right atrial ablation is linked to venous access, the ablation procedure itself, and comorbidities. The thrombotic risk is higher in the initial days after ablation.58
The rate of systemic complications in published observational studies on right atrial ablation procedures varies from 0 to 3.2% (see Supplementary material online, Table S8). This includes thromboembolic complications with an overall incidence of 0.6%.59 One study found a 5% incidence rate of asymptomatic deep vein femoral thrombosis in patients who underwent right-sided ablation.60 Risk factors were the use of large sheaths for a prolonged duration. In one of the few randomized studies, comparing a loading dose of 5000 UI heparin with no loading dose, only local in situ thrombosis connected to the catheter was observed; the risk is generally low, and risk factors include the number of the cannulation sites and female gender, but not heparin use.59
Ablation of the right ATs (ATs, right accessory slow pathways, and junctional tachycardias) is considered as low thrombotic risk procedures.1 The management of right-sided atrial flutter differs and is described above.
Antithrombotic management in patients undergoing right atrial ablation procedures (excluding atrial flutter): consensus recommendation
| Unfractionated heparin should be considered during the procedure. |
| It is not recommended to start the patients on oral anticoagulation or aspirin unless otherwise indicated. |
| Unfractionated heparin should be considered during the procedure. |
| It is not recommended to start the patients on oral anticoagulation or aspirin unless otherwise indicated. |
Antithrombotic management in patients undergoing right atrial ablation procedures (excluding atrial flutter): consensus recommendation
| Unfractionated heparin should be considered during the procedure. |
| It is not recommended to start the patients on oral anticoagulation or aspirin unless otherwise indicated. |
| Unfractionated heparin should be considered during the procedure. |
| It is not recommended to start the patients on oral anticoagulation or aspirin unless otherwise indicated. |
Ablation of right-sided ventricular tachycardias
Reported complication rates for ablation of right-sided ventricular tachycardia (VT) are <1% in isolated right-sided procedures.61–65 In a single tertiary centre, Bohnen et al.66 reported a major complication rate of 3.4%, although none of these patients was on oral anticoagulation; also, there was no significant difference between right-sided (3.2%) and left-sided (3.5%) idiopathic VT ablations. Tokuda et al.67 investigated the cardiac perforation rate in 1152 VT ablations of 892 patients between 1999 and 2010, and reported 11 cardiac perforations (1%), which occurred in right ventricular (RV) or RV outflow tract mapping in 7 patients. As expected, the RV seems to be more susceptible to perforation due to the thinner wall than the LV.
Overall, right-sided procedures are at low risk for relevant thromboembolic events. Heparin use seems not to be necessary for right-sided procedures and deliver no clinical benefit, but might be given in special situations (long-lasting procedure, history of previous venous thromboemboli, and/or known risk factors for thrombosis) or in the presence of right to left intracardiac shunts that pose a risk of paradoxical emboli.68 Meticulous sheath management with frequent flushing is required during the procedure, and compression after pulling the sheath should be done with care and only as long as necessary. Should patients require oral anticoagulation or platelet inhibition for another reason, there is no evidence mandating discontinuation of these agents before the RV ablation procedure.
In patients on a VKA and a higher risk for thromboembolism, it is safe to continue oral anticoagulation at an INR between 2 and 3.29,69,70 Studies addressing the NOACs in this context are not available, but it seems reasonable to manage patients with stopping the NOAC the evening before the day of intervention and continue if no bleeding complications occurred 3–4 h after the intervention.71
For planned epicardial access, an oral anticoagulation with a VKA should be withdrawn to achieve an INR of <1.5 and NOACs should be discontinued for at least 48 h (longer for renal impairment, if dabigatran is used). There is no proven benefit of administering post-interventional aspirin or oral anticoagulation unless it is required for another reason.
Antithrombotic management in patients undergoing right ventricular catheter ablation: consensus recommendations
| In patients with structural heart disease undergoing endocardial ablation of a right ventricular tachycardia only, established therapy with a VKA, a NOAC, or platelet inhibitors can be continued. |
| Unfractionated heparin should be considered during the procedure. |
| Before an epicardial ablation, it can be useful to stop NOACs 48 h before the procedure. |
| Before an epicardial ablation, it may be considered to withhold VKA until the INR is <1.5. |
| In patients with structural heart disease undergoing endocardial ablation of a right ventricular tachycardia only, established therapy with a VKA, a NOAC, or platelet inhibitors can be continued. |
| Unfractionated heparin should be considered during the procedure. |
| Before an epicardial ablation, it can be useful to stop NOACs 48 h before the procedure. |
| Before an epicardial ablation, it may be considered to withhold VKA until the INR is <1.5. |
Antithrombotic management in patients undergoing right ventricular catheter ablation: consensus recommendations
| In patients with structural heart disease undergoing endocardial ablation of a right ventricular tachycardia only, established therapy with a VKA, a NOAC, or platelet inhibitors can be continued. |
| Unfractionated heparin should be considered during the procedure. |
| Before an epicardial ablation, it can be useful to stop NOACs 48 h before the procedure. |
| Before an epicardial ablation, it may be considered to withhold VKA until the INR is <1.5. |
| In patients with structural heart disease undergoing endocardial ablation of a right ventricular tachycardia only, established therapy with a VKA, a NOAC, or platelet inhibitors can be continued. |
| Unfractionated heparin should be considered during the procedure. |
| Before an epicardial ablation, it can be useful to stop NOACs 48 h before the procedure. |
| Before an epicardial ablation, it may be considered to withhold VKA until the INR is <1.5. |
Ablation of left-sided ventricular tachycardias
Therapeutic anticoagulation is paramount for the prevention of potentially serious thrombotic complications in the treatment of left-sided ventricular tachyarrhythmias (VT).1,2,68,72,73 Left-sided VT can originate from the endocardium as well as epicardium, and left ventricular (LV) access can be achieved via antegrade transseptal, retrograde transaortic, or subxiphoid epicardial techniques. Specific considerations are therefore needed depending on the access route. Currently, no data comparing different anticoagulation methods before, during, and after LV ablation exist (Table 2).
Data from the literature on stroke/TIA and tamponade rates during ventricular tachyarrhythmia ablation
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | Comment . |
|---|---|---|---|---|---|
| Calkins et al., 200074 | Multicentre | 146 | 2.7 | 2.7 | Internal irrigation |
| Segal et al., 200575 | Single centre | 40 | 2.5 | 7.5 | Catheter mounted non-contact mapping |
| Stevenson et al., 200873 | Multicentre | 231 | 0 | 0 | External irrigation |
| Sacher et al., 201076 | Multicentre | 134 | 0 | 5.1 | Epicardial ± endocardial |
| Della Bella et al., 201177 | Multicentre, survey | 222 | 0 | 3.7 | Epicardial ± endocardial |
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | Comment . |
|---|---|---|---|---|---|
| Calkins et al., 200074 | Multicentre | 146 | 2.7 | 2.7 | Internal irrigation |
| Segal et al., 200575 | Single centre | 40 | 2.5 | 7.5 | Catheter mounted non-contact mapping |
| Stevenson et al., 200873 | Multicentre | 231 | 0 | 0 | External irrigation |
| Sacher et al., 201076 | Multicentre | 134 | 0 | 5.1 | Epicardial ± endocardial |
| Della Bella et al., 201177 | Multicentre, survey | 222 | 0 | 3.7 | Epicardial ± endocardial |
Data from the literature on stroke/TIA and tamponade rates during ventricular tachyarrhythmia ablation
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | Comment . |
|---|---|---|---|---|---|
| Calkins et al., 200074 | Multicentre | 146 | 2.7 | 2.7 | Internal irrigation |
| Segal et al., 200575 | Single centre | 40 | 2.5 | 7.5 | Catheter mounted non-contact mapping |
| Stevenson et al., 200873 | Multicentre | 231 | 0 | 0 | External irrigation |
| Sacher et al., 201076 | Multicentre | 134 | 0 | 5.1 | Epicardial ± endocardial |
| Della Bella et al., 201177 | Multicentre, survey | 222 | 0 | 3.7 | Epicardial ± endocardial |
| Author/year . | Study design . | Size (patients) . | Stroke/TIA (%) . | Tamponade (%) . | Comment . |
|---|---|---|---|---|---|
| Calkins et al., 200074 | Multicentre | 146 | 2.7 | 2.7 | Internal irrigation |
| Segal et al., 200575 | Single centre | 40 | 2.5 | 7.5 | Catheter mounted non-contact mapping |
| Stevenson et al., 200873 | Multicentre | 231 | 0 | 0 | External irrigation |
| Sacher et al., 201076 | Multicentre | 134 | 0 | 5.1 | Epicardial ± endocardial |
| Della Bella et al., 201177 | Multicentre, survey | 222 | 0 | 3.7 | Epicardial ± endocardial |
Pre-procedurally, there is most evidence for anticoagulation management in patients with documented AF (see the section ‘Ablation of atrial fibrillation and left atrial arrhythmias’), and current AF anticoagulation guidelines should be followed due to the increased risk of intra-procedural sustained ventricular arrhythmias and need for cardioversion. Echocardiography to rule out LV thrombus is also warranted in patients with reduced LV ejection fraction.
Although data in patients undergoing left-sided VT ablation are lacking, there is consensus that, in patients on a VKA and a therapeutic INR, OAC should not be interrupted for VT ablation. Should an epicardial approach be likely, interruption of VKA 3–5 days prior to the procedure with bridging therapeutic heparin or LMWH can be considered. Pre-procedural anticoagulation is not required unless otherwise indicated in patients without structural heart disease.
Open irrigation radiofrequency ablation is standard for the treatment of left-sided VT and allows delivery of higher radiofrequency current before the catheter tip temperature reaches the point of coagulum formation.78 Although there are no current consensus anticoagulation recommendations for left-sided VT ablation, due to the thrombotic risk profile similarities with AF ablation, therapeutic intravenous heparin is recommended in patients with and without structural heart disease. When endocardial substrates are suspected, full-dose heparin is generally given once transeptal access is achieved. When epicardial access is needed, full-dose heparin should only be given once this is achieved. If patients had already received heparin, administration of protamine (1 mg per 100 units of unfractionated heparin) before entering the epicardial space can be useful. An initial bolus of 100 U/kg followed by intermittent boluses or a continuous infusion of heparin to maintain an ACT of >300 s is recommended. When only the epicardium is accessed, therapeutic heparin is not required. All intravascular long sheaths should be continuously flushed with heparinized saline to prevent clot formation.72 The epicardial sheath should regularly be aspirated during the procedure to reduce the risk of epicardial clot formation and tamponade.
Post-procedure, aspirin 75–150 mg or oral anticoagulation for 1–3 months may be considered, although commonly used the evidence for aspirin is weak and no antithrombotic therapy also is an option. Anticoagulation is not required in patients without structural heart disease or who have only received epicardial ablation unless otherwise indicated. Epicardial sheaths should only be removed once the ACT is <300 s, and a pericardial drain is often left intra-epicardially for up to 24 h until no further drainage occurs. In patients with indications for anticoagulation or with structural heart disease, a VKA or a NOAC can be started 4–6 h after haemostasis is achieved following endo- and/or epicardial ablation, with bridging heparin, LMWH, or a NOAC. For patients on VKA, bridging with a NOAC will have an impact on the prothrombin time.79
As there are limited well-controlled studies on anticoagulation management in left-sided VT ablation, the recommendations represent expert consensus. Individual patient characteristics and co-morbidities should always be considered, and the thromboembolic risk balanced with the risk of cardiac tamponade, bleeding, and vascular injury.
Antithrombotic management in patients undergoing ablation procedures for left ventricular tachycardia: consensus recommendations
| It is recommended to give unfractionated heparin with a target ACT of >300 s during the procedure. |
| It can be useful not to interrupt oral anticoagulation with a VKA before ablation of a left VT. |
| It is recommended to stop oral anticoagulation with a NOAC at least 24 h before LV ablation (longer for dabigatran, if renal impairment is present). |
| A transthoracic echocardiography can be useful to rule out LV thrombi before the ablation procedure. |
| When switching to an epicardial access during a LV ablation, it may be considered to administer protamine before epicardial access. |
| After LV ablation, oral anticoagulation or aspirin for 4–12 weeks may be considered. |
| In the absence of another indication, oral anticoagulation before LV ablation should not be given. |
| It is recommended to give unfractionated heparin with a target ACT of >300 s during the procedure. |
| It can be useful not to interrupt oral anticoagulation with a VKA before ablation of a left VT. |
| It is recommended to stop oral anticoagulation with a NOAC at least 24 h before LV ablation (longer for dabigatran, if renal impairment is present). |
| A transthoracic echocardiography can be useful to rule out LV thrombi before the ablation procedure. |
| When switching to an epicardial access during a LV ablation, it may be considered to administer protamine before epicardial access. |
| After LV ablation, oral anticoagulation or aspirin for 4–12 weeks may be considered. |
| In the absence of another indication, oral anticoagulation before LV ablation should not be given. |
Antithrombotic management in patients undergoing ablation procedures for left ventricular tachycardia: consensus recommendations
| It is recommended to give unfractionated heparin with a target ACT of >300 s during the procedure. |
| It can be useful not to interrupt oral anticoagulation with a VKA before ablation of a left VT. |
| It is recommended to stop oral anticoagulation with a NOAC at least 24 h before LV ablation (longer for dabigatran, if renal impairment is present). |
| A transthoracic echocardiography can be useful to rule out LV thrombi before the ablation procedure. |
| When switching to an epicardial access during a LV ablation, it may be considered to administer protamine before epicardial access. |
| After LV ablation, oral anticoagulation or aspirin for 4–12 weeks may be considered. |
| In the absence of another indication, oral anticoagulation before LV ablation should not be given. |
| It is recommended to give unfractionated heparin with a target ACT of >300 s during the procedure. |
| It can be useful not to interrupt oral anticoagulation with a VKA before ablation of a left VT. |
| It is recommended to stop oral anticoagulation with a NOAC at least 24 h before LV ablation (longer for dabigatran, if renal impairment is present). |
| A transthoracic echocardiography can be useful to rule out LV thrombi before the ablation procedure. |
| When switching to an epicardial access during a LV ablation, it may be considered to administer protamine before epicardial access. |
| After LV ablation, oral anticoagulation or aspirin for 4–12 weeks may be considered. |
| In the absence of another indication, oral anticoagulation before LV ablation should not be given. |
Antithrombotic management for the implantation of cardiac implantable electronic devices
Management of vitamin K antagonists
In the most recent worldwide survey (2009), there were an estimated 1.25 million pacemaker and 410 000 implantable cardioverter defibrillator operations.80 Between 14 and 35% of patients receiving these devices require chronic OAC,81–84 and their peri-procedural management may present a dilemma to physicians.85 This is particularly true for the subset of patients with a moderate-to-high risk (≥5% per year) of thromboembolic (TE) events.86 In patients with non-valvular AF, this risk corresponds to a CHA2DS2-VASc score of ≥3. Physicians responded to concerns about peri-procedural TE by treating moderate- to high-risk device surgery patients with heparin bridging. Previous guidelines recommended this as standard of care.87 However, it became clear that there is a substantial risk of clinically significant device pocket haematoma related to heparin bridging. Importantly, device pocket haematomas can necessitate prolonged cessation of anticoagulation, with the attendant risk of TE,88,89 they can significantly increase the duration and cost of hospitalization;90 sometimes, reoperation is required.
Finally and perhaps most importantly, there is an association between haematoma formation and subsequent device system infection. For example, in the REPLACE registry,91 patients with infections were 20-fold more likely to have had postoperative haematomas. Device system infections usually require complete system removal, which has significant associated morbidity, mortality, and cost to the healthcare system.
In response to these issues, some centres started performing pacemaker and defibrillator surgery without interruption of warfarin anticoagulation.92–95 Two small randomized trials were inconclusive.96,97 In the first of these, 4 of 51 patients (7.8%) from the bridging arm and 4 of 50 (8.0%) from the VKA arm developed pocket haematoma following the implant. A third, much larger, large clinical trial, BRUISE CONTROL (Bridge or Continue Warfarin for Device Surgery Randomized Controlled Trial),98 patients (n = 681) with an annual risk of TE of 5% or greater were randomly assigned to continued warfarin or heparin bridging (Table 3). The primary outcome was clinically significant haematoma, which was defined as prolonging hospitalization, necessitating interruption of anticoagulation, or requiring reoperation. Clinically significant haematoma occurred in 12 of 343 (3.5%) patients in the continued-warfarin arm and 54 of 338 (16.0%) patients in the heparin-bridging arm [relative risk, 0.19; 95% confidence interval (CI) 0.10–0.36; P < 0.001]. Major surgical and thromboembolic complications were rare and not significantly different between arms. They included one episode of cardiac tamponade and one myocardial infarction in the heparin-bridging arm, and one stroke and one transient ischaemic attack (TIA) in the continued-warfarin arm. It should be noted that exceptions to operating without interruption of warfarin were sub-pectoral implants and lead extraction.98
Importantly, BRUISE CONTROL did not include patients at a lower embolic risk (<5% annual risk of TE).98 Current international thrombosis guidelines suggest temporary discontinuation of warfarin for these patients, without heparin bridging.87 However, physicians may also consider continuing warfarin in these patients, especially if there is any history of previous embolic stroke or TIA (Figure 1). This strategy is corroborated by two recent meta-analyses.99,100
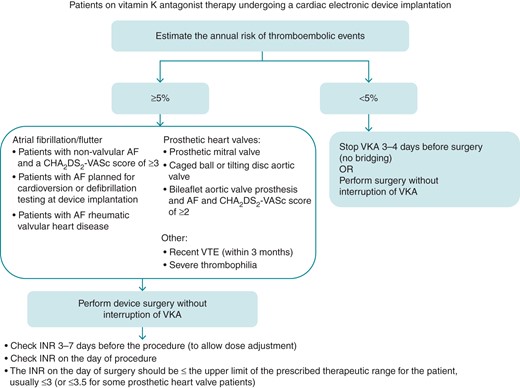
Algorithm for peri-device surgery anticoagulation for patients on a VKA (note exceptions to operating without interruption of VKA include sub-pectoral implants and lead extraction). AF, atrial fibrillation; VKA, vitamin K antagonist; VTE, venous thromboembolism.
Device implantation in patients receiving vitamin K antagonists: consensus recommendation
In the following patient groups with AF, it is recommended to perform device surgery without interruption of VKA.
|
In the following patient groups with prosthetic heart valves, it is recommended to perform device surgery without interruption of VKA.
|
| In patients with recent venous thromboembolism (within 3 months), it is recommended to perform device surgery without interruption of VKA. |
| The INR on the day of surgery should be under the upper limit of the prescribed therapeutic range for the patient (usually ≤3; ≤3.5 for some valve patients. |
| In patients with an annual risk of TE events <5% either perform surgery without interruption of VKA or interrupt VKA 3–4 days before surgery, no heparin bridging is recommended. |
| Interruption of VKA and bridging with an unfractionated heparin or LMWH should be avoided. |
In the following patient groups with AF, it is recommended to perform device surgery without interruption of VKA.
|
In the following patient groups with prosthetic heart valves, it is recommended to perform device surgery without interruption of VKA.
|
| In patients with recent venous thromboembolism (within 3 months), it is recommended to perform device surgery without interruption of VKA. |
| The INR on the day of surgery should be under the upper limit of the prescribed therapeutic range for the patient (usually ≤3; ≤3.5 for some valve patients. |
| In patients with an annual risk of TE events <5% either perform surgery without interruption of VKA or interrupt VKA 3–4 days before surgery, no heparin bridging is recommended. |
| Interruption of VKA and bridging with an unfractionated heparin or LMWH should be avoided. |
Device implantation in patients receiving vitamin K antagonists: consensus recommendation
In the following patient groups with AF, it is recommended to perform device surgery without interruption of VKA.
|
In the following patient groups with prosthetic heart valves, it is recommended to perform device surgery without interruption of VKA.
|
| In patients with recent venous thromboembolism (within 3 months), it is recommended to perform device surgery without interruption of VKA. |
| The INR on the day of surgery should be under the upper limit of the prescribed therapeutic range for the patient (usually ≤3; ≤3.5 for some valve patients. |
| In patients with an annual risk of TE events <5% either perform surgery without interruption of VKA or interrupt VKA 3–4 days before surgery, no heparin bridging is recommended. |
| Interruption of VKA and bridging with an unfractionated heparin or LMWH should be avoided. |
In the following patient groups with AF, it is recommended to perform device surgery without interruption of VKA.
|
In the following patient groups with prosthetic heart valves, it is recommended to perform device surgery without interruption of VKA.
|
| In patients with recent venous thromboembolism (within 3 months), it is recommended to perform device surgery without interruption of VKA. |
| The INR on the day of surgery should be under the upper limit of the prescribed therapeutic range for the patient (usually ≤3; ≤3.5 for some valve patients. |
| In patients with an annual risk of TE events <5% either perform surgery without interruption of VKA or interrupt VKA 3–4 days before surgery, no heparin bridging is recommended. |
| Interruption of VKA and bridging with an unfractionated heparin or LMWH should be avoided. |
Management of non-vitamin K oral anticoagulants
Of the NOACs approved for use for prevention of stroke and systemic embolism in patients with AF, data on general peri-operative experience with dabigatran and rivaroxaban have been published; the key points from these two studies are:101,102 The results of BRUISE CONTROL cannot be applied to patients on NOACs.98 Rowley et al.103 recently published the first report on continuous anticoagulation with a NOAC during implantation of cardiac rhythm devices. Dabigatran was administered uninterrupted with no missed doses in 11 patients, and 1 patient developed a pocket haematoma. Jennings et al.104 reported on 48 patients having device surgery with uninterrupted dabigatran. Bleeding complications occurred in 1 of 48 patients (2.1%; late pericardial effusion).
Temporary interruptions for procedures/surgery are common (between 10 and 15% of patients per year).
About 10% of temporary interruptions are for pacemaker or defibrillator surgery.
Even brief temporary interruptions, carefully controlled in the environment of clinical trials, are associated with an approximately three-fold increase in stroke/systemic embolism.
Summary of clinical trials comparing heparin bridging with continued warfarin at time of device surgery
| Author/year . | Design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Tolosana et al. 200997 | RCT | 101 | Four of 51 patients (7.8%) from the heparin-bridging arm and 4 of 50 (8.0%) from the continued-warfarin arm developed pocket haematoma | Underpowered |
| Cheng et al. 201196 | RCT | 100 | Trend for more bleeding events in the heparin-bridging group (2 pocket haematomas, 1 pericardial effusion vs. no event in the continued-warfarin arm) | Underpowered |
| Birnie et al. 201398 | RCT | 681 | Clinically significant haematoma occurred in 12 of 343 (3.5%) patients in the continued-warfarin arm and 54 of 338 (16.0%) patients in the heparin-bridging arm (relative risk, 0.19; 95% CI 0.10–0.36; P < 0.001) |
|
| Author/year . | Design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Tolosana et al. 200997 | RCT | 101 | Four of 51 patients (7.8%) from the heparin-bridging arm and 4 of 50 (8.0%) from the continued-warfarin arm developed pocket haematoma | Underpowered |
| Cheng et al. 201196 | RCT | 100 | Trend for more bleeding events in the heparin-bridging group (2 pocket haematomas, 1 pericardial effusion vs. no event in the continued-warfarin arm) | Underpowered |
| Birnie et al. 201398 | RCT | 681 | Clinically significant haematoma occurred in 12 of 343 (3.5%) patients in the continued-warfarin arm and 54 of 338 (16.0%) patients in the heparin-bridging arm (relative risk, 0.19; 95% CI 0.10–0.36; P < 0.001) |
|
Summary of clinical trials comparing heparin bridging with continued warfarin at time of device surgery
| Author/year . | Design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Tolosana et al. 200997 | RCT | 101 | Four of 51 patients (7.8%) from the heparin-bridging arm and 4 of 50 (8.0%) from the continued-warfarin arm developed pocket haematoma | Underpowered |
| Cheng et al. 201196 | RCT | 100 | Trend for more bleeding events in the heparin-bridging group (2 pocket haematomas, 1 pericardial effusion vs. no event in the continued-warfarin arm) | Underpowered |
| Birnie et al. 201398 | RCT | 681 | Clinically significant haematoma occurred in 12 of 343 (3.5%) patients in the continued-warfarin arm and 54 of 338 (16.0%) patients in the heparin-bridging arm (relative risk, 0.19; 95% CI 0.10–0.36; P < 0.001) |
|
| Author/year . | Design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Tolosana et al. 200997 | RCT | 101 | Four of 51 patients (7.8%) from the heparin-bridging arm and 4 of 50 (8.0%) from the continued-warfarin arm developed pocket haematoma | Underpowered |
| Cheng et al. 201196 | RCT | 100 | Trend for more bleeding events in the heparin-bridging group (2 pocket haematomas, 1 pericardial effusion vs. no event in the continued-warfarin arm) | Underpowered |
| Birnie et al. 201398 | RCT | 681 | Clinically significant haematoma occurred in 12 of 343 (3.5%) patients in the continued-warfarin arm and 54 of 338 (16.0%) patients in the heparin-bridging arm (relative risk, 0.19; 95% CI 0.10–0.36; P < 0.001) |
|
Whether it is better to operate without interrupting these new agents or with temporary cessation is currently unclear, and more data are required. One such clinical trial is ongoing (continued vs. interrupted dabigatran at time of device surgery: BRUISE CONTROL 2, Clinicaltrials.gov NCT# 01675076). Until additional data are available, we recommend interruption of NOACs for device surgery, without heparin bridging. The period of peri-operative discontinuation should be based on the original NOAC clinical trials and as detailed in the respective product monographs (see Table 4 for summary). This recommendation is consistent with the EHRA Practical Guide on the use of NOACs in patients with non-valvular AF.71
Suggested period of NOAC interruption prior to device surgery according to renal function (modified with permission from Heidbuchel et al.71)
| CrCL (mL/min) . | Dabigatran (h) . | Apixaban (h) . | Rivaroxaban (h) . | Edoxaban (h) . |
|---|---|---|---|---|
| ≥80 | ≥24 | ≥24 | ≥24 | ≥24 |
| 50–80 | ≥36 | ≥24 | ≥24 | NA |
| 30–50 | ≥48 | ≥24 | ≥24 | NA |
| 15–30 | Not indicated | ≥36 | ≥36 | NA |
| CrCL (mL/min) . | Dabigatran (h) . | Apixaban (h) . | Rivaroxaban (h) . | Edoxaban (h) . |
|---|---|---|---|---|
| ≥80 | ≥24 | ≥24 | ≥24 | ≥24 |
| 50–80 | ≥36 | ≥24 | ≥24 | NA |
| 30–50 | ≥48 | ≥24 | ≥24 | NA |
| 15–30 | Not indicated | ≥36 | ≥36 | NA |
CrCl, creatinine clearance; NA, no available recommendations.
Suggested period of NOAC interruption prior to device surgery according to renal function (modified with permission from Heidbuchel et al.71)
| CrCL (mL/min) . | Dabigatran (h) . | Apixaban (h) . | Rivaroxaban (h) . | Edoxaban (h) . |
|---|---|---|---|---|
| ≥80 | ≥24 | ≥24 | ≥24 | ≥24 |
| 50–80 | ≥36 | ≥24 | ≥24 | NA |
| 30–50 | ≥48 | ≥24 | ≥24 | NA |
| 15–30 | Not indicated | ≥36 | ≥36 | NA |
| CrCL (mL/min) . | Dabigatran (h) . | Apixaban (h) . | Rivaroxaban (h) . | Edoxaban (h) . |
|---|---|---|---|---|
| ≥80 | ≥24 | ≥24 | ≥24 | ≥24 |
| 50–80 | ≥36 | ≥24 | ≥24 | NA |
| 30–50 | ≥48 | ≥24 | ≥24 | NA |
| 15–30 | Not indicated | ≥36 | ≥36 | NA |
CrCl, creatinine clearance; NA, no available recommendations.
There are no data to guide when to restart NOACs after device surgery. In the major NOAC clinical trials, the NOACs were restarted at the physician's discretion when haemostasis was satisfactory.101,102 Physicians are concerned that early resumption of a NOAC, with their rapid onset of action, may have similar effects on postoperative bridging, i.e. result in significant numbers of haematomas. Hence, in patients, with an annual risk of TE >5%, we suggest giving the first dose of NOAC 24 h after surgery. In patients with a lower risk of TE (e.g. <5%), it would seem reasonable to wait for >48 h after surgery. More data are required to refine all of these recommendations regarding NOAC management around device surgery.
Device implantation in patients receiving non-vitamin K oral anticoagulants: consensus recommendations
| Non-vitamin K oral anticoagulants should probably be temporarily discontinued for all device surgery. |
| The period of discontinuation should be based on product characteristics. |
| It is suggested that the first dose of NAOC should be ≥24–48 h after surgery. The timing of the resumption should be based on individual assessment of the competing risks of stroke risk and pocket haematoma. |
| Non-vitamin K oral anticoagulants should probably be temporarily discontinued for all device surgery. |
| The period of discontinuation should be based on product characteristics. |
| It is suggested that the first dose of NAOC should be ≥24–48 h after surgery. The timing of the resumption should be based on individual assessment of the competing risks of stroke risk and pocket haematoma. |
Device implantation in patients receiving non-vitamin K oral anticoagulants: consensus recommendations
| Non-vitamin K oral anticoagulants should probably be temporarily discontinued for all device surgery. |
| The period of discontinuation should be based on product characteristics. |
| It is suggested that the first dose of NAOC should be ≥24–48 h after surgery. The timing of the resumption should be based on individual assessment of the competing risks of stroke risk and pocket haematoma. |
| Non-vitamin K oral anticoagulants should probably be temporarily discontinued for all device surgery. |
| The period of discontinuation should be based on product characteristics. |
| It is suggested that the first dose of NAOC should be ≥24–48 h after surgery. The timing of the resumption should be based on individual assessment of the competing risks of stroke risk and pocket haematoma. |
Management of antiplatelet drugs
There are no randomized trials regarding antiplatelet (AP) management around device surgery. However, there are data from a number of observational studies (Table 5) and a recent meta-analysis (Figure 2).105 The meta-analysis found that the estimated odds of bleeding were increased by 5.0 times (95% CI 3.0–8.3) for dual AP therapy. There was a non-significant trend (OR 1.5; 95% CI 0.9–2.3) for single AP therapy relative to the no therapy group.105 For the 392 patients on dual AP therapy included in this analysis, there were no reports of acute ischaemic events or in-stent thrombosis.
Studies examining the role of antiplatelet therapy on the incidence of bleeding complications in device implantation (modified with permission from Bernard et al.105)
| Author . | Study design . | No therapy . | Single AP therapy . | Dual AP therapy . |
|---|---|---|---|---|
| Tompkins 2010145 | Retrospective observational | 3/255 (1.2%) | 20/536 (3.7%) | 9/139 (6.5%) |
| Kutinsky 2010147 | Prospective observational | 9/164 (5.5%) | 17/327 (5.2%) | 16/66 (24.2%) |
| Thal 2010106 | Retrospective observational | 0/43 (0%) | 1/82 (1.2%) | 3/15 (20%) |
| Dreger et al. 2010107 | Observational prospective and retrospective | 3/318 (0.9%) | 1/109 (0.9%) | |
| Ahmed et al. 2010108 | Retrospective observational | 7/123 (5.7%) | ||
| Cano et al. 2011109 | Prospective observational | 9/375 (2.4%) | 7/220 (3.2%) | 8/63 (12.7%) |
| Author . | Study design . | No therapy . | Single AP therapy . | Dual AP therapy . |
|---|---|---|---|---|
| Tompkins 2010145 | Retrospective observational | 3/255 (1.2%) | 20/536 (3.7%) | 9/139 (6.5%) |
| Kutinsky 2010147 | Prospective observational | 9/164 (5.5%) | 17/327 (5.2%) | 16/66 (24.2%) |
| Thal 2010106 | Retrospective observational | 0/43 (0%) | 1/82 (1.2%) | 3/15 (20%) |
| Dreger et al. 2010107 | Observational prospective and retrospective | 3/318 (0.9%) | 1/109 (0.9%) | |
| Ahmed et al. 2010108 | Retrospective observational | 7/123 (5.7%) | ||
| Cano et al. 2011109 | Prospective observational | 9/375 (2.4%) | 7/220 (3.2%) | 8/63 (12.7%) |
Studies examining the role of antiplatelet therapy on the incidence of bleeding complications in device implantation (modified with permission from Bernard et al.105)
| Author . | Study design . | No therapy . | Single AP therapy . | Dual AP therapy . |
|---|---|---|---|---|
| Tompkins 2010145 | Retrospective observational | 3/255 (1.2%) | 20/536 (3.7%) | 9/139 (6.5%) |
| Kutinsky 2010147 | Prospective observational | 9/164 (5.5%) | 17/327 (5.2%) | 16/66 (24.2%) |
| Thal 2010106 | Retrospective observational | 0/43 (0%) | 1/82 (1.2%) | 3/15 (20%) |
| Dreger et al. 2010107 | Observational prospective and retrospective | 3/318 (0.9%) | 1/109 (0.9%) | |
| Ahmed et al. 2010108 | Retrospective observational | 7/123 (5.7%) | ||
| Cano et al. 2011109 | Prospective observational | 9/375 (2.4%) | 7/220 (3.2%) | 8/63 (12.7%) |
| Author . | Study design . | No therapy . | Single AP therapy . | Dual AP therapy . |
|---|---|---|---|---|
| Tompkins 2010145 | Retrospective observational | 3/255 (1.2%) | 20/536 (3.7%) | 9/139 (6.5%) |
| Kutinsky 2010147 | Prospective observational | 9/164 (5.5%) | 17/327 (5.2%) | 16/66 (24.2%) |
| Thal 2010106 | Retrospective observational | 0/43 (0%) | 1/82 (1.2%) | 3/15 (20%) |
| Dreger et al. 2010107 | Observational prospective and retrospective | 3/318 (0.9%) | 1/109 (0.9%) | |
| Ahmed et al. 2010108 | Retrospective observational | 7/123 (5.7%) | ||
| Cano et al. 2011109 | Prospective observational | 9/375 (2.4%) | 7/220 (3.2%) | 8/63 (12.7%) |
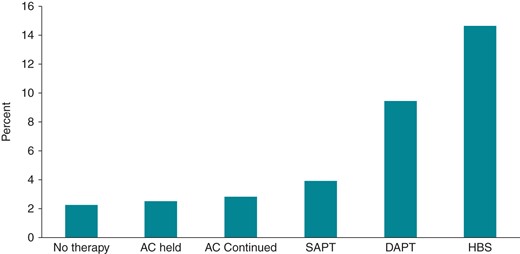
Unadjusted, pooled rates of bleeding complications. Major bleeding defined as any bleeding leading to transfusion, surgical intervention for pocket evacuation or revision, pericardial effusion, haemothorax, or life-threatening bleeding. Minor bleeding was defined as any haematoma requiring conservative management only, blood loss not requiring transfusion, or discontinuation of medication. Bleeding event rates were 33 of 1500 (2.2%) for no therapy, 26 of 1044 (2.5%) for AC held, 34 of 1200 (2.8%) for AC continued, 45 of 1165 (3.9%) for SAPT, 37 of 392 (9.4%) for DAPT, and 99 of 677 (14.6%) for HBS. AC includes vitamin K antagonists; SAPT, single antiplatelet therapy; DAPT, dual antiplatelet therapy; HBS, heparin-bridging strategy (reproduced permission from Bernard et al.105).
For recommendations of the management of antiplatelet therapy at the time of CIED implantation, refer the section ‘Concurrent antiplatelet therapy’ later in this document.
Management of peri-interventional bleeding complications
The management of bleeding in patients while on antithrombotic therapy is defined in relation to its severity as either major, clinically relevant non-major or minor. The definition for major bleeding in surgical patients was defined in 2010 by the International Society of Thrombosis and Haemostasis, and comprises fatal bleeding, bleeding that is symptomatic and occurs in a critical area or organ (intracranial, intraspinal, intraocular, and retroperitoneal), extrasurgical site bleeding causing a fall in haemoglobin level of 20 g/L, surgical site bleeding that requires a second intervention, or is unexpected and prolonged and/or sufficiently large to cause haemodynamic instability.110 Secondly, the management of bleeding is anchored on general measures like fluid resuscitation, red blood cell transfusion, as well as the most important feature, the diagnosis, and the treatment of the bleeding site (Table 6).111
Bleeding risk stratification should be considered as an integral part of anticoagulation treatment decision-making, and the HAS-BLED score112 is a simple practical score which is well validated in various settings. In keeping with international guideline recommendations, the HAS-BLED score should be used for bleeding risk assessment.113 There is scarce information about its use for assessing bleeding risk during interventional procedures. In this setting, the BNK Online bRiDging REgistRy (BORDER) about bridging therapy in patients undergoing oral anticoagulation showed that HAS-BLED score was highly predictive of haemorrhagic complications.114
Antiplatelet therapy
Aspirin, clopidogrel, ticlopidine, and prasugrel inhibit platelet function for the lifetime of the platelet, so its inhibition takes 7–10 days to resolve until new platelets are produced. On the other side, ticagrelor is a reversible inhibitor, so platelet function normalizes after drug clearance, but the AP effect persists for 3–5 days.115
Bleeding in patients while taking antiplatelet therapy should be managed with general haemostatic measures and cessation of the treatment should be done after a carefully assessment of the thrombotic risk [e.g. drug-eluting stent (DES) placed <3 months].116 Platelet transfusion may be considered in case of critical or life-threatening bleeding, but it is important to note that circulating drug or its active metabolites could inhibit transfused platelets. For non-urgent antiplatelet agent reversal, discontinue them for 5–7 days.115
Vitamin-K antagonists (e.g. warfarin, acenocoumarol, or phenprocoumon)
In case of minor or self-limiting bleeding, withholding oral anticoagulation could be sufficient. In case of moderate bleeding, 10 mg of vitamin K can be given intravenously in order to induce a rapid INR reduction (6–8 h), and prothrombin complex concentrates (PCCs) are only added in case of severe bleeding (25–50 U/kg). Recombinant activated factor VII (rFVIIa) is not recommended due to its high rate of thrombotic complications.116,117
Unfractioned heparin and low-molecular-weight heparin
Protamine sulphate will fully reverse the effect of unfractioned heparin (1 mg per 80–100 units), whereas in the case of LMWH, protamine will only reverse about 60% of LMWH, so its effectiveness is very limited and only in the first hour after LWWH administration. Prothrombin complex concentrate or rFVIIa would be recommended in life-threatening bleeding.116
Non-vitamin K antagonist oral anticoagulants (i.e. dabigatran, rivaroxaban, apixaban, and edoxaban)
Owing to the short half-life of these drugs, withholding the next dose would be enough for all mild or self-limiting bleeding. It is important to note the timing of the last pill intake. Consider activated charcoal the two first hours after pill intake. Regarding moderate bleeding, general local haemostatic measures and fluid replacement should be undertaken. If the patient is taking dabigatran, it is important to maintain diuresis and dialysis could be considered. In case of severe or life-threatening bleeding, PCC at 25 U/kg can be considered (but there is no clinical evidence), whereas activated PCC or rFVIIa do not provide additional clinical evidence and the rate of thrombotic complications is higher.71,118
Ongoing studies with direct specific antidotes to the NOACs show promising results (idarucizumab for dabigatran, andexanet alfa for the F.Xa inhibitors, aripazine for factor II, and FXa inhibitors), and these direct antidotes should be licensed in the near future.119–121
Patient's values and preferences
Clinical guidelines for the management of cardiac arrhythmias increasingly advocate attention to patient values and preferences.18,40 Most of the recent focus has been on AF where discussing with the patient on balancing individual stroke risk against bleeding risk associated with oral anticoagulation is part of clinical management and should be integral to the consultation. As clinicians, we should be aware that patient beliefs about their health, their medical conditions, treatment options, and healthcare they receive are key determinants of whether or not treatment is acceptable to the patient, and is highly relevant where antithrombotic therapy is required, and (for example) in AF would require life-long treatment adherence.123 Patients and physicians have different priorities when thromboprophylaxis is considered. Patients are desperate to avoid a stroke, regarding such an outcome as a fate worse than death.124,125 In contrast, physicians placed more emphasis on avoiding bleeding, even if the patient was at risk of stroke. In the study by Lahaye et al.,124 for example, patients were willing to initiate anticoagulation for a minimum annual absolute risk reduction of 0.8% (number needed to treat = 125) and a 15% relative risk reduction in stroke, and would be prepared to suffer 4.4 major bleeds in order to prevent one stroke. Other similar studies focused on patients' preferences for thromboprophylaxis have been published.126–128
We need to emphasize that when considering antithrombotic therapy in the context of arrhythmias and electrophysiological procedures, patients do prefer informative discussions to include individual risk information rather than generic risk.129
Perceptions of risk can be modified considerably by the way in which risk information (benefits and side effects) are presented and explained. Many patients with AF have limited knowledge about their condition and lack understanding of the risks and benefits of using antithrombotic therapy.130–132 Educational intervention can help, as evident by a recent randomized trial showing much improved knowledge and better quality of anticoagulation control, when compared with usual care.133
Health economic considerations
Interventional procedures have the aim to reduce symptoms, morbidity, and possibly, mortality related to arrhythmic events. As shown in cost of illness studies, many arrhythmic conditions induce substantial costs and appropriate interventions may have a positive impact on disease-related hospitalizations, with a consequent favourable economic profile in terms of cost-effectiveness at mid or long term, in the perspective of healthcare systems.134–137
Atrial fibrillation is a costly disease, both in terms of direct and indirect costs, the former being reported as per-patient annual costs in the range of $2000–14 200 in North America and of €450–3000 in Europe.137 The main drivers of costs are arrhythmia-related hospitalizations and stroke events. In AF, OACs are prescribed at long term (to reduce AF-related thromboembolic risk) and acutely during the ablation procedure in order to reduce thromboembolic events, but they carry the risk of haemorrhagic complications which can be serious and absorb important economic resources.138 In other procedures (ablation of supraventricular or ventricular tachyarrhythmias), the use of antithrombotics, as related to the arrhythmic conditions, is usually related to the time of the procedure or a short period after the intervention. In CIEDs, appropriate management of antithrombotics during implants or replacements has a crucial role for minimizing complications, risks, and consequently, costs of the procedure.105
In general, any adverse event (i.e. haemorrhage, haematoma, thromboembolic complication, cardiac tamponade, vascular damage, etc.) is the result of complex interactions including patient characteristics and co-morbidities, type and dosing of antithrombotics, technical aspects of the interventional procedures, and operator's experience.134,139 The cost of complications is related to increased direct costs (lengthening of hospitalization, need for additional diagnostic tests, need for surgical interventions, induction of new hospital admissions, or in-office visits) as well as direct non-medical costs and indirect costs (loss of productivity).134,136 It is worthy to stress that any added day of in-hospital stay has huge costs, ranging from $476–835 in European countries to $4287 (on average) in the USA.140
Concurrent antiplatelet therapy
Many of the patients who are referred for ablation procedures or CIEDs are treated with single or dual antiplatelet therapy (DAPT), due to concurrent coronary heart disease. In some patients, antiplatelet therapy may be used in combination with OACs, and the reader is referred to the recent joint European consensus document on this topic, endorsed by HRS and APHRS.141
Aspirin
Discontinuation of concurrent antiplatelet before the implantation of CIED may increase the thromboembolic risk. In patients who are receiving aspirin for the secondary prevention of cardiovascular disease and require surgery, cessation of aspirin is usually not recommended.86,142 A large meta-analysis in almost 50 000 patients investigating peri-procedural cessation vs. continuation of aspirin revealed a 1.5-fold increased risk of peri-procedural bleeding complications in patients receiving aspirin, however, without any increase in severe bleeding.143 On the other hand, it has been reported that peri-procedural withdrawal of aspirin in patients with coronary artery disease was associated with a three-fold increase in major adverse cardiac events.141 Accordingly, aspirin should be continued for secondary prevention during most CIED implantations. Only if the individual bleeding risk outweighs the potential cardiovascular benefit for secondary prevention, discontinuation of aspirin 5–7 days before the procedure should be considered. In patients on aspirin for primary prevention, aspirin should be stopped 5 days before surgery.
| Drug . | Half-life . | Antidote . | Reversal strategies . | Time to restoration of normal haemostatic function . | Laboratory measurements . |
|---|---|---|---|---|---|
| Antiplatelet drugs Aspirin Clopidogrel Prasugrel Ticagrelor | Aspirin: 2–4.5 h Clopidogrel: 7–10 h Prasugrel: 7–10 h Ticagrelor: 7–10 h | NA | Withhold drug Platelet transfusion | Aspirin, clopidogrel, prasugrel: 7–10 h Tigagrelor: 3–5 days 1 apheresis/5–8 blood unit rise platelet count 30 × 109/L | Platelet aggregation Not available for emergency laboratories |
| VKA Warfarin Acenocumarol Phenprocumon | Warfarin: 36–48 h Acenocumarol: 6–8 h Phenprocoumon: 90–140 h | Vitamin K (2–10 mg) Oral and intravenously | Interruption of VKA Vitamin K PCC | 1–7 days 6–24 h 3–5 h | PT |
| UFH | 1–2 h | Protamin (1 mg per 100 U) | Stop UFH Protamin | 4 h Immediately | APTT |
| LMWH Enoxaparin Dalteparin Bemiparin Tinzaparin | Enoxaparin: 4.5 h Dalteparin: 2.2 h Bemiparin: 5.3 h Tinzaparin: 3.9 h | Not useful. Partially reversion | Interruption of LMWH Protamin (only first 8 h) | 12–24 h Not available | Anti-FXa assay. Not available for emergency laboratories |
| NOAC | |||||
| Dabigatran | 14–17 h Renal excretion: 80% | NA | Interruption of dabigatran Oral charcoal (first 2 h) Haemodialysis PCC Idarucizumab (not yet licensed) | 12–24 h (depends on glomerular filtration) – – – | APTT and TT (qualitative measurement) Modified thrombin time (quantitative), usually not available for emergency laboratories |
| Rivaroxaban | 8–9 h Renal excretion: 33% | NA | Interruption of rivaroxaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Prothrombin time (qualitative measurement) Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Apixaban | 7–8 h Renal excretion: 25% | NA | Interruption of apixaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Not available qualitative measurement Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Drug . | Half-life . | Antidote . | Reversal strategies . | Time to restoration of normal haemostatic function . | Laboratory measurements . |
|---|---|---|---|---|---|
| Antiplatelet drugs Aspirin Clopidogrel Prasugrel Ticagrelor | Aspirin: 2–4.5 h Clopidogrel: 7–10 h Prasugrel: 7–10 h Ticagrelor: 7–10 h | NA | Withhold drug Platelet transfusion | Aspirin, clopidogrel, prasugrel: 7–10 h Tigagrelor: 3–5 days 1 apheresis/5–8 blood unit rise platelet count 30 × 109/L | Platelet aggregation Not available for emergency laboratories |
| VKA Warfarin Acenocumarol Phenprocumon | Warfarin: 36–48 h Acenocumarol: 6–8 h Phenprocoumon: 90–140 h | Vitamin K (2–10 mg) Oral and intravenously | Interruption of VKA Vitamin K PCC | 1–7 days 6–24 h 3–5 h | PT |
| UFH | 1–2 h | Protamin (1 mg per 100 U) | Stop UFH Protamin | 4 h Immediately | APTT |
| LMWH Enoxaparin Dalteparin Bemiparin Tinzaparin | Enoxaparin: 4.5 h Dalteparin: 2.2 h Bemiparin: 5.3 h Tinzaparin: 3.9 h | Not useful. Partially reversion | Interruption of LMWH Protamin (only first 8 h) | 12–24 h Not available | Anti-FXa assay. Not available for emergency laboratories |
| NOAC | |||||
| Dabigatran | 14–17 h Renal excretion: 80% | NA | Interruption of dabigatran Oral charcoal (first 2 h) Haemodialysis PCC Idarucizumab (not yet licensed) | 12–24 h (depends on glomerular filtration) – – – | APTT and TT (qualitative measurement) Modified thrombin time (quantitative), usually not available for emergency laboratories |
| Rivaroxaban | 8–9 h Renal excretion: 33% | NA | Interruption of rivaroxaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Prothrombin time (qualitative measurement) Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Apixaban | 7–8 h Renal excretion: 25% | NA | Interruption of apixaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Not available qualitative measurement Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
APTT, activated partial thromboplastin time; FXa, activated Factor Xa; H, hours; NA, not available; PT, prothrombin time; TT, thrombin time; LMWH, low molecular weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist; PCC, prothrombin complex concentrate; NOAC, non-vitamin K oral anticoagulants.
| Drug . | Half-life . | Antidote . | Reversal strategies . | Time to restoration of normal haemostatic function . | Laboratory measurements . |
|---|---|---|---|---|---|
| Antiplatelet drugs Aspirin Clopidogrel Prasugrel Ticagrelor | Aspirin: 2–4.5 h Clopidogrel: 7–10 h Prasugrel: 7–10 h Ticagrelor: 7–10 h | NA | Withhold drug Platelet transfusion | Aspirin, clopidogrel, prasugrel: 7–10 h Tigagrelor: 3–5 days 1 apheresis/5–8 blood unit rise platelet count 30 × 109/L | Platelet aggregation Not available for emergency laboratories |
| VKA Warfarin Acenocumarol Phenprocumon | Warfarin: 36–48 h Acenocumarol: 6–8 h Phenprocoumon: 90–140 h | Vitamin K (2–10 mg) Oral and intravenously | Interruption of VKA Vitamin K PCC | 1–7 days 6–24 h 3–5 h | PT |
| UFH | 1–2 h | Protamin (1 mg per 100 U) | Stop UFH Protamin | 4 h Immediately | APTT |
| LMWH Enoxaparin Dalteparin Bemiparin Tinzaparin | Enoxaparin: 4.5 h Dalteparin: 2.2 h Bemiparin: 5.3 h Tinzaparin: 3.9 h | Not useful. Partially reversion | Interruption of LMWH Protamin (only first 8 h) | 12–24 h Not available | Anti-FXa assay. Not available for emergency laboratories |
| NOAC | |||||
| Dabigatran | 14–17 h Renal excretion: 80% | NA | Interruption of dabigatran Oral charcoal (first 2 h) Haemodialysis PCC Idarucizumab (not yet licensed) | 12–24 h (depends on glomerular filtration) – – – | APTT and TT (qualitative measurement) Modified thrombin time (quantitative), usually not available for emergency laboratories |
| Rivaroxaban | 8–9 h Renal excretion: 33% | NA | Interruption of rivaroxaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Prothrombin time (qualitative measurement) Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Apixaban | 7–8 h Renal excretion: 25% | NA | Interruption of apixaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Not available qualitative measurement Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Drug . | Half-life . | Antidote . | Reversal strategies . | Time to restoration of normal haemostatic function . | Laboratory measurements . |
|---|---|---|---|---|---|
| Antiplatelet drugs Aspirin Clopidogrel Prasugrel Ticagrelor | Aspirin: 2–4.5 h Clopidogrel: 7–10 h Prasugrel: 7–10 h Ticagrelor: 7–10 h | NA | Withhold drug Platelet transfusion | Aspirin, clopidogrel, prasugrel: 7–10 h Tigagrelor: 3–5 days 1 apheresis/5–8 blood unit rise platelet count 30 × 109/L | Platelet aggregation Not available for emergency laboratories |
| VKA Warfarin Acenocumarol Phenprocumon | Warfarin: 36–48 h Acenocumarol: 6–8 h Phenprocoumon: 90–140 h | Vitamin K (2–10 mg) Oral and intravenously | Interruption of VKA Vitamin K PCC | 1–7 days 6–24 h 3–5 h | PT |
| UFH | 1–2 h | Protamin (1 mg per 100 U) | Stop UFH Protamin | 4 h Immediately | APTT |
| LMWH Enoxaparin Dalteparin Bemiparin Tinzaparin | Enoxaparin: 4.5 h Dalteparin: 2.2 h Bemiparin: 5.3 h Tinzaparin: 3.9 h | Not useful. Partially reversion | Interruption of LMWH Protamin (only first 8 h) | 12–24 h Not available | Anti-FXa assay. Not available for emergency laboratories |
| NOAC | |||||
| Dabigatran | 14–17 h Renal excretion: 80% | NA | Interruption of dabigatran Oral charcoal (first 2 h) Haemodialysis PCC Idarucizumab (not yet licensed) | 12–24 h (depends on glomerular filtration) – – – | APTT and TT (qualitative measurement) Modified thrombin time (quantitative), usually not available for emergency laboratories |
| Rivaroxaban | 8–9 h Renal excretion: 33% | NA | Interruption of rivaroxaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Prothrombin time (qualitative measurement) Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
| Apixaban | 7–8 h Renal excretion: 25% | NA | Interruption of apixaban Oral charcoal (first 2 h) PCC Andexanet alfa (not yet lincensed) | 12–24 h (depends on glomerular filtration) – – | Not available qualitative measurement Anti-FXa assay using specific calibrators (qualitative measurement), usually not available for emergency laboratories |
APTT, activated partial thromboplastin time; FXa, activated Factor Xa; H, hours; NA, not available; PT, prothrombin time; TT, thrombin time; LMWH, low molecular weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist; PCC, prothrombin complex concentrate; NOAC, non-vitamin K oral anticoagulants.
Dual antiplatelet therapy
There are no randomized trials studying withdrawal vs. continuation of DAPT before CIED implantations; however, there are some data from cohort studies. In most of these studies (Table 7),109,144–147,149 dual therapy with aspirin and clopidogrel increased the risk of bleeding after EPD implantations compared with aspirin alone. Only the study by Dreger et al.107 did not demonstrate any increased risk of bleeding complications in DAPT patients, but in this study a vacuum drainage system was applied to all patients.
Studies examining the role of clopidogrel therapy in addition to aspirin on the incidence of bleeding complications in electrophysiological device implantation
| Author/year . | Study design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Wiegand et al. 2004144 | Prospective observational | 3165 | Incidence of pocket haematoma: acetylsalicylic acid only 3%, ticlopidine/clopidogrel only 6.7%, DAPT 21.7% | |
| Tompkins et al. 2010145 | Retrospective observational | 1388 | Incidence of bleeding complications: controls 1.6%, DAPT 7.2%, aspirin alone 3.9% | |
| Przybylski et al. 2010146 | Prospective registry | 247 | Bleeding complications (major and minor): 13.9% in the aspirin group vs. 24.5% in the DAPT group | |
| Kutinsky et al. 2010147 | Prospective observational study | 935 | Pocket haematoma: clopidogrel only 11.1%, aspirin only 4.2%, DAP 24.2%. Multivariate analysis: clopidogrel use increased the risk of device-related haematoma (adjusted OR 2.32, 95% CI 1.42–3.79) | |
| Dreger et al. 2010107 | Observational prospective and retrospective study | 424 | Pocket haematoma: 0.9% in the DAPT group, 0.9% in the control group | Electrocautery in all patients, vacuum drainage systems in all patients |
| Cano et al. 2011109 | Prospective observational study | 849 | Increased risk of pocket haematoma in the DAPT vs. single antiplatelet group (13.3 vs. 3.2%) | |
| Lee et al. 2012148 | Retrospective observational study | 260 | Bleeding complications were observed in 8 patients (3.1%), all were receiving heparin bridging during the procedure (P < 0.0001) | Low number of patients on DAPT (n = 25) |
| Author/year . | Study design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Wiegand et al. 2004144 | Prospective observational | 3165 | Incidence of pocket haematoma: acetylsalicylic acid only 3%, ticlopidine/clopidogrel only 6.7%, DAPT 21.7% | |
| Tompkins et al. 2010145 | Retrospective observational | 1388 | Incidence of bleeding complications: controls 1.6%, DAPT 7.2%, aspirin alone 3.9% | |
| Przybylski et al. 2010146 | Prospective registry | 247 | Bleeding complications (major and minor): 13.9% in the aspirin group vs. 24.5% in the DAPT group | |
| Kutinsky et al. 2010147 | Prospective observational study | 935 | Pocket haematoma: clopidogrel only 11.1%, aspirin only 4.2%, DAP 24.2%. Multivariate analysis: clopidogrel use increased the risk of device-related haematoma (adjusted OR 2.32, 95% CI 1.42–3.79) | |
| Dreger et al. 2010107 | Observational prospective and retrospective study | 424 | Pocket haematoma: 0.9% in the DAPT group, 0.9% in the control group | Electrocautery in all patients, vacuum drainage systems in all patients |
| Cano et al. 2011109 | Prospective observational study | 849 | Increased risk of pocket haematoma in the DAPT vs. single antiplatelet group (13.3 vs. 3.2%) | |
| Lee et al. 2012148 | Retrospective observational study | 260 | Bleeding complications were observed in 8 patients (3.1%), all were receiving heparin bridging during the procedure (P < 0.0001) | Low number of patients on DAPT (n = 25) |
DAPT, dual antiplatelet therapy.
Studies examining the role of clopidogrel therapy in addition to aspirin on the incidence of bleeding complications in electrophysiological device implantation
| Author/year . | Study design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Wiegand et al. 2004144 | Prospective observational | 3165 | Incidence of pocket haematoma: acetylsalicylic acid only 3%, ticlopidine/clopidogrel only 6.7%, DAPT 21.7% | |
| Tompkins et al. 2010145 | Retrospective observational | 1388 | Incidence of bleeding complications: controls 1.6%, DAPT 7.2%, aspirin alone 3.9% | |
| Przybylski et al. 2010146 | Prospective registry | 247 | Bleeding complications (major and minor): 13.9% in the aspirin group vs. 24.5% in the DAPT group | |
| Kutinsky et al. 2010147 | Prospective observational study | 935 | Pocket haematoma: clopidogrel only 11.1%, aspirin only 4.2%, DAP 24.2%. Multivariate analysis: clopidogrel use increased the risk of device-related haematoma (adjusted OR 2.32, 95% CI 1.42–3.79) | |
| Dreger et al. 2010107 | Observational prospective and retrospective study | 424 | Pocket haematoma: 0.9% in the DAPT group, 0.9% in the control group | Electrocautery in all patients, vacuum drainage systems in all patients |
| Cano et al. 2011109 | Prospective observational study | 849 | Increased risk of pocket haematoma in the DAPT vs. single antiplatelet group (13.3 vs. 3.2%) | |
| Lee et al. 2012148 | Retrospective observational study | 260 | Bleeding complications were observed in 8 patients (3.1%), all were receiving heparin bridging during the procedure (P < 0.0001) | Low number of patients on DAPT (n = 25) |
| Author/year . | Study design . | Size (patients) . | Summary of findings . | Comment . |
|---|---|---|---|---|
| Wiegand et al. 2004144 | Prospective observational | 3165 | Incidence of pocket haematoma: acetylsalicylic acid only 3%, ticlopidine/clopidogrel only 6.7%, DAPT 21.7% | |
| Tompkins et al. 2010145 | Retrospective observational | 1388 | Incidence of bleeding complications: controls 1.6%, DAPT 7.2%, aspirin alone 3.9% | |
| Przybylski et al. 2010146 | Prospective registry | 247 | Bleeding complications (major and minor): 13.9% in the aspirin group vs. 24.5% in the DAPT group | |
| Kutinsky et al. 2010147 | Prospective observational study | 935 | Pocket haematoma: clopidogrel only 11.1%, aspirin only 4.2%, DAP 24.2%. Multivariate analysis: clopidogrel use increased the risk of device-related haematoma (adjusted OR 2.32, 95% CI 1.42–3.79) | |
| Dreger et al. 2010107 | Observational prospective and retrospective study | 424 | Pocket haematoma: 0.9% in the DAPT group, 0.9% in the control group | Electrocautery in all patients, vacuum drainage systems in all patients |
| Cano et al. 2011109 | Prospective observational study | 849 | Increased risk of pocket haematoma in the DAPT vs. single antiplatelet group (13.3 vs. 3.2%) | |
| Lee et al. 2012148 | Retrospective observational study | 260 | Bleeding complications were observed in 8 patients (3.1%), all were receiving heparin bridging during the procedure (P < 0.0001) | Low number of patients on DAPT (n = 25) |
DAPT, dual antiplatelet therapy.
In patients receiving DAPT due to a coronary stent or a recent acute coronary syndrom (ACS), current guidelines recommend deferring surgery until DAPT is no longer necessary.86,143 If delaying surgery is not possible, it is recommended to stop clopidogrel 5 days before surgery, but consider resuming clopipogrel as soon as possible after the procedure. We recommend this procedure also for CIED implantations. The exception is if a coronary stent has been implanted within 30 days [bare metal stent (BMS)] or 3 months (new-generation DES), and it is not possible to defer the implantation, then the procedure should be performed on continued DAPT. Apparently, coronary stenting procedures should better follow implantation of a CIED whenever possible. In patients who are on oral anticoagulation in addition to DAPT, special considerations apply with respect to a minimum duration of DAPT.141
In the setting of CIED implantation, there are no data with the newer antiplatelet agents prasugrel or ticagrelor. There may be similar or even higher bleeding risk with these agents compared with clopidogrel, but until more data are available, the management approach should be similar to clopidogrel, if deferring implantation is not possible.
Patients undegoing cardiac implantable electronical device implantation while being treated with antiplatelet therapy: consensus recommmendations
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during CIED implantations. |
| In patients on DAPT (i.e. aspirin plus clopidogrel or other P2Y12 agent) requiring device surgery within 4 weeks of BMS or within 6 months of DES implantation (within 3 months with new-generation DES), it is recommended to continue both AP agents. |
| In patients on DAPT, it should be considered to defer elective device implantations until DAPT is no longer necessary. |
| In patients on DAPT after ACS requiring device surgery >4 weeks after BMS implantation or >6 months after DES (>3 months after new-generation DES), it should be considered to stop the P2Y12 inhibitor for 5–7 days before surgery, but consider resuming a P2Y12 inhibitor as soon as possible after the procedure. A multidisciplinary approach for the individual patient is recommended. |
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during CIED implantations. |
| In patients on DAPT (i.e. aspirin plus clopidogrel or other P2Y12 agent) requiring device surgery within 4 weeks of BMS or within 6 months of DES implantation (within 3 months with new-generation DES), it is recommended to continue both AP agents. |
| In patients on DAPT, it should be considered to defer elective device implantations until DAPT is no longer necessary. |
| In patients on DAPT after ACS requiring device surgery >4 weeks after BMS implantation or >6 months after DES (>3 months after new-generation DES), it should be considered to stop the P2Y12 inhibitor for 5–7 days before surgery, but consider resuming a P2Y12 inhibitor as soon as possible after the procedure. A multidisciplinary approach for the individual patient is recommended. |
Patients undegoing cardiac implantable electronical device implantation while being treated with antiplatelet therapy: consensus recommmendations
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during CIED implantations. |
| In patients on DAPT (i.e. aspirin plus clopidogrel or other P2Y12 agent) requiring device surgery within 4 weeks of BMS or within 6 months of DES implantation (within 3 months with new-generation DES), it is recommended to continue both AP agents. |
| In patients on DAPT, it should be considered to defer elective device implantations until DAPT is no longer necessary. |
| In patients on DAPT after ACS requiring device surgery >4 weeks after BMS implantation or >6 months after DES (>3 months after new-generation DES), it should be considered to stop the P2Y12 inhibitor for 5–7 days before surgery, but consider resuming a P2Y12 inhibitor as soon as possible after the procedure. A multidisciplinary approach for the individual patient is recommended. |
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during CIED implantations. |
| In patients on DAPT (i.e. aspirin plus clopidogrel or other P2Y12 agent) requiring device surgery within 4 weeks of BMS or within 6 months of DES implantation (within 3 months with new-generation DES), it is recommended to continue both AP agents. |
| In patients on DAPT, it should be considered to defer elective device implantations until DAPT is no longer necessary. |
| In patients on DAPT after ACS requiring device surgery >4 weeks after BMS implantation or >6 months after DES (>3 months after new-generation DES), it should be considered to stop the P2Y12 inhibitor for 5–7 days before surgery, but consider resuming a P2Y12 inhibitor as soon as possible after the procedure. A multidisciplinary approach for the individual patient is recommended. |
Ablation procedures in patients on antiplatelet therapy
For the introduction and manoeuvring of sheaths and catheters, the risk of peripheral bleeding or complications when using aspirin and/or clopidogrel is low.1 However, most patients with AF or atrial flutter are treated with oral anticoagulation in addition to DAPT, and the risk of bleeding with DAPT in addition to oral anticoagulation is known to be much higher.150
The other problem may be the management of cardiac tamponade or pericardial effusion because of perforation when the patient is on DAPT. There are no relevant data in the literature on this specific question; therefore, no definite answer can be given. However, it can be assumed that bleeding is more severe and more difficult to be managed when the patient is on DAPT. This is especially the case if the patient is on oral anticoagulation in addition to DAPT, as in AF and atrial flutter. It is therefore recommended to postpone ablation of AF to a time when DAPT can be safely discontinued.
Patients undergoing the ablation procedure while being treated with antiplatelet therapy: consensus recommendations
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during the ablation procedure. |
| In patients on DAPT in addition to OAC, it is recommended to defer ablation procedures until DAPT is no longer necessary. |
| In patients on DAPT, it may be considered to continue DAPT during right-sided procedures and uncomplicated left-sided procedures. |
| In patients on single antiplatelet therapy (aspirin) in addition to OAC, it should be considered to continue aspirin during the procedure. |
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during the ablation procedure. |
| In patients on DAPT in addition to OAC, it is recommended to defer ablation procedures until DAPT is no longer necessary. |
| In patients on DAPT, it may be considered to continue DAPT during right-sided procedures and uncomplicated left-sided procedures. |
| In patients on single antiplatelet therapy (aspirin) in addition to OAC, it should be considered to continue aspirin during the procedure. |
Patients undergoing the ablation procedure while being treated with antiplatelet therapy: consensus recommendations
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during the ablation procedure. |
| In patients on DAPT in addition to OAC, it is recommended to defer ablation procedures until DAPT is no longer necessary. |
| In patients on DAPT, it may be considered to continue DAPT during right-sided procedures and uncomplicated left-sided procedures. |
| In patients on single antiplatelet therapy (aspirin) in addition to OAC, it should be considered to continue aspirin during the procedure. |
| In patients on single antiplatelet therapy (aspirin or clopidogrel) for secondary prevention, it is recommended to continue aspirin during the ablation procedure. |
| In patients on DAPT in addition to OAC, it is recommended to defer ablation procedures until DAPT is no longer necessary. |
| In patients on DAPT, it may be considered to continue DAPT during right-sided procedures and uncomplicated left-sided procedures. |
| In patients on single antiplatelet therapy (aspirin) in addition to OAC, it should be considered to continue aspirin during the procedure. |
Conclusions
The antithrombotic management of patients undergoing electrophysiological procedures has witnessed major changes due to an increase in the number of procedures and in the knowledge about the role of VKAs and NOACs. Thus, therapy with VKA is usually not interrupted in patients undergoing ablation procedures like PVI. Likewise, patients on VKA requiring implantation of a CIED are operated on a VKA unless they are at very low risk for a thromboembolic event. In this case, VKA can be paused and reinitiated after surgery without heparin bridging. The formerly commonly practiced ‘bridging therapy’ with unfractionated heparin or LMWH must not be used since it significantly increases bleeding complications.
At the same time, numerous NOACs have been approved for the prevention of thromboembolic complications in patients with non-valvular AF, or with a previous pulmonary embolism or deep vein thrombosis. As patients undergoing electrophysiological procedures are increasingly treated with these agents, our knowledge about their use is increasing future adjustments in the current consensus recommendations are likely.
Supplementary material
Supplementary material is available at Europace online.
Acknowledgements
EHRA Scientific Documents Committee: Gregory Y.H. Lip (EHRA Scientific Documents Committee Chair), Bulent Gorenek (EHRA Scientific Documents Committee Co-Chair), Christian Sticherling, Laurent Fauchier, Hein Heidbuchel, Angel Moya Mitjans, Mark A. Vos, Michele Brignole, Gheorghe-Andrei Dan, Michele Gulizia, Francisco Marin, Giuseppe Boriani, Deirdre Lane, and Irene Savelieva.
Conflict of interest: Detailed conflict of interest statement is available as Supplementary material online.
ESC Scientific Document Group: Bulent Gorenek (Reviewer Coordinator; Turkey), Julia H. Indik (USA), Paulus Kirchhof (UK), Chang-Shen Ma (China), Calambur Narasimhan (India), Jonathan Piccini (USA), Andrea Sarkozy (Belgium), Dipen Shah (Switzerland), and Irene Savelieva (on behalf of EP-Europace, UK)
References



