-
PDF
- Split View
-
Views
-
Cite
Cite
Shervin A. Sadrpour, Deepa Srinivasan, Ashish A. Bhimani, Seungyup Lee, Kyungmoo Ryu, Ivan Cakulev, Celeen M. Khrestian, Alan H. Markowitz, Albert L. Waldo, Jayakumar Sahadevan, Insights into new-onset atrial fibrillation following open heart surgery and implications for type II atrial flutter, EP Europace, Volume 17, Issue 12, December 2015, Pages 1834–1839, https://doi.org/10.1093/europace/euv019
Close - Share Icon Share
Abstract
Postoperative atrial fibrillation (POAF), new-onset AF after open heart surgery (OHS), is thought to be related to pericarditis. Based on AF studies in the canine sterile pericarditis model, we hypothesized that POAF in patients after OHS may be associated with a rapid, regular rhythm in the left atrium (LA), suggestive of an LA driver maintaining AF. The aim of this study was to test the hypothesis that in patients with POAF, atrial electrograms (AEGs) recorded from at least one of the two carefully selected LA sites would manifest a rapid, regular rhythm with AEGs of short cycle length (CL) and constant morphology, but a selected right atrial (RA) site would manifest AEGs with irregular CLs and variable morphology.
In 44 patients undergoing OHS, AEGs recorded from the epicardial surface of the RA, the LA portion of Bachmann's bundle, and the posterior LA during sustained AF were analysed for regularity of CL and morphology. Sustained AF occurred in 15 of 44 patients. Atrial electrograms were recorded in 11 of 15 patients; 8 of 11 had rapid, regular activation with constant morphology recorded from at least one LA site; no regular AEG sites were present in 3 of 11 patients.
Atrial electrograms recorded during sustained POAF frequently demonstrated rapid, regular activation in at least one LA site, consistent with a driver maintaining AF.
Areas of rapid, regular atrial electrogram (AEG) activation were identified during new-onset postoperative atrial fibrillation (POAF) following open heart surgery
Areas of rapid, regular AEGs are a common occurrence in new-onset POAF
Recorded AEGs and ECGs during ‘Type II atrial flutter’ exhibit characteristics which mirror those of new-onset POAF
Introduction
Atrial fibrillation (AF) occurs frequently in patients following open heart surgery (OHS), and is related importantly to obligatory postoperative pericardial inflammation.1,2 However, the mechanism(s) that sustains postoperative AF (POAF) (i.e. new-onset AF after OHS) in patients is not well understood. The canine sterile pericarditis (CSP) model of AF and atrial flutter (AFL)3,4 which has a clinical counterpart on which it is based, namely postoperative AF and AFL, was developed more than three decades ago. In the CSP model, we have identified two mechanisms of induced AF. One is due to multiple, unstable, reentrant circuits of very short cycle length (CL), which drive the atria, thereby producing AF.4 The second and most common mechanism is due to a single, stable, reentrant circuit of very short CL that circulates around one or more pulmonary veins.5,6 In AF due to this latter reentrant mechanism, high-density sequence of activation mapping of the atria from 420–512 simultaneously recorded electrodes5,6 has demonstrated atrial electrograms (AEGs) in the reentrant circuit manifested a regular, short CL with constant morphology. Activation of areas close to the reentrant circuit were also able to follow the reentrant driver with a 1:1 response. However, most of the right atrium (RA) and parts of the left atrium (LA) could not follow the driver in a 1:1 fashion, resulting in fibrillatory conduction. Having understood this latter model of AF, we thought it likely that during POAF in patients following OHS, the atria in some patients would also manifest areas with regular AEGs of very short CL, i.e. a putative AF driver. However, we recognized that it was not possible to map the atria during POAF in patients using the several hundred electrodes that we apply in studies in our CSP model. Nevertheless, based on our mapping studies in the CSP model, we had important insight regarding where in the LA we were most likely to find and record AEGs of regular, short CL in POAF. And again, knowing that we were limited in the number of temporary epicardial electrodes we could place after OHS, based on our CSP model, we had a well substantiated rational for where to place the limited number of temporary epicardial electrodes in order to record regular AEGs of short CL and constant morphology in patients with POAF. In light of the above, we designed a study to test the hypothesis that in patients with POAF, AEGs recorded from one or both of two carefully selected LA sites, would likely manifest a rapid, regular rhythm with AEGs of very short CL and constant morphology suggestive of an LA driver maintaining AF, but the third carefully selected site on the RA would manifest AEGs with irregular CLs and variable morphology consistent with our findings in the CSP model.
Methods
Patient recruitment
After obtaining Institutional Review Board approval and informed consent, 44 patients scheduled for OHS were recruited. Exclusion criteria were: age <18 years and a history of prior AF or prior OHS.
Intraoperative placement and postoperative care of temporary epicardial electrodes
For all patients studied, at the time of OHS, using standard techniques,1 a single surgeon placed a pair of temporary, stainless steel, wire electrodes (Medtronic Streamline 6495, Minneapolis, MN, USA) on the epicardium of the superior right atrial (RA) free wall, the left atrial side of Bachmann's bundle (BB), and the posterior LA (PLA) between the four pulmonary veins (PVs) (Figure 1). The electrode placement sites were selected based on observations in the CSP model, in which, during high density, biatrial mapping during sustained AF due to a single, stable LA reentrant circuit of short CL, bipolar AEGs recorded from the left side of BB or the PLA or both, often demonstrated a short, regular CL with constant morphology, whereas bipolar AEGs recorded from the superior RA free wall demonstrated irregular, longer CLs with variable morphologies.5,6 The interelectrode distance of each electrode pair was ≤1 cm. The ends of each pair were brought out through the anterior chest wall in a standard fashion,1 placed in a plastic tube with a cap, and secured to the chest wall with tape, on which the location of each atrial site was marked. Prior to discharge, the electrodes were removed using standard techniques.1 All patients were treated following the Society of Thoracic Surgery guidelines.

Atrial electrode position: shown are the locations of the three temporary bipolar atrial electrodes (black dots) on the epicardial surface of the right atrium (RA), left atrial portion of the Bachmann's bundle (BB), and left atrial surface between the four pulmonary veins (PVs). AO-aorta; BB, Bachmann's bundle; CS, coronary sinus; IVC, inferior vena cava; LPVs, left pulmonary veins; PA, pulmonary artery; PLA, posterior left atrium; RA, right atrium; RPVs, right pulmonary veins; SVC, superior vena cava.
Recording and analysis of atrial electrograms during atrial fibrillation
All 44 patients underwent continuous ECG telemonitoring (postoperative days 0 to 5, or until discharge, if prior to day 5) for the spontaneous development of AF. If AF was detected postoperatively, the investigators were contacted by the nursing staff once the AF episode lasted more than 1 h.
The initial 22 patients
For all recordings in patients in this group, a conventional ECG machine was used to obtain a 12-lead ECG. Subsequently, the right- and left-arm ECG leads were disconnected from the patient to allow for bipolar AEG recordings. This was done as follows: the right-arm ECG lead was connected to one electrode from a selected bipolar pair of the temporary epicardial electrodes, and the other electrode of the pair was connected to the left-arm ECG lead. In this manner, a bipolar AEG was recorded from standard lead I, and unipolar AEGs were recorded from standard leads II and III (Figure 2). Bipolar and unipolar AEGs were recorded sequentially in a random fashion from each of the three recording sites (RA, BB, and PLA). Recordings were obtained at paper speeds of either 25 or 50 mm/s, at a gain of 10 mm/mV, and at a band pass of 0.05–150 Hz. To assure the stability of the AF episode, after an episode of AF lasting more than an hour was identified postoperatively, AEGs were recorded for a minimum of 10 s during AF, and assessed for beat-to-beat CL regularity and morphology. The total time to perform such recordings ranged from 15 to 20 min because the recordings were performed sequentially. Analysis of data consisted of manual measurement of CLs from bipolar AEGs using a standard calliper with a resolution of 10 ms (paper speed of 50 mm/s) or 20 ms (paper speed of 25 mm/s), followed by assessing the morphology. The mean and standard deviation were calculated from the measured CLs.

Bipolar and unipolar AEG recording (initial patient configuration): shown are the ECG limb leads (leads I, II, and III) from a 12-lead ECG reconfigured to record AEGs in the initial 22 patients. The right- and left-arm ECG leads were disconnected from the patient, and one electrode from each electrode pair was connected to the right-arm ECG lead, and the other electrode was connected to the left-arm ECG lead. In this manner, bipolar AEGs were recorded from lead I, and unipolar AEGs were recorded from leads II and III. Paper recording speed was 25 mm/s, the gain setting was 10 mm/mV, and the band pass of the recordings was 0.05 to 150 Hz. a, atrial complex; v, ventricular complex; AEGs, atrial electrograms. See text for discussion.
The final 22 patients
Per above, in the first 22 patients, a conventional ECG machine was used for recording. However, it was quickly recognized that it would be advantageous to have a digital recording system because, compared with a standard ECG paper recording machine, we would have the ability to record bipolar AEGs simultaneously from all three electrodes (RA, BB, and PLA) along with the ECG, improved resolution for measuring AEG CLs and paperless recording. Thus, we developed a digital recording system, and we were able to use it in the last 22 patients. Using this system, data were digitally recorded, and processed with an Active Two system (BioSemi, Amsterdam, Netherlands). This enabled us to record all AEGs simultaneously, and improved the measurement resolution for determining CLs during AF. All AEGs were sampled at 1024 Hz and digitized at 24 bits. Data were transferred in real time, and stored on a personal computer for further analysis. In all patients who developed AF, a minimum of 10 s of simultaneous, continuous recording of bipolar AEGs were made. Analysis of the data consisted of measuring consecutive bipolar AEG CLs from each site using digital callipers with a resolution of 1 ms, followed by assessing the AEG morphology. The means and standard deviations were calculated from the measured CLs.
Definition of a regular site
A regular site during AF was defined as follows: (a) in the initial 22 patients, AEG CLs during the duration of recordings had to be within either 10 or 20 ms of each other at paper recording speeds of 50 or 25 mm/s, respectively; (b) in the final 22 patients, the SD of the AEG CLs during the duration of the recordings had to be <10% of the mean CL.
Results
Characterization of bipolar atrial electrograms during atrial fibrillation
During the postoperative period, 15 of the 44 patients had an episode of AF lasting more than 1 h. Eleven of the 15 patients with AF had AEGs recorded during their AF episode (Table 1). Eight of these 11 patients with AF, a rapid, regular rhythm with constant AEG morphology was recorded from one of the LA sites. The RA AEG showed a rapid, regular rhythm with constant morphology in two of these eight patients (Table 1). In the four patients in whom recordings could not be performed, it was due either to spontaneous termination of AF prior to recording or dislodgement of the atrial epicardial wire electrodes.
Cycle lengths (mean and SD) of bipolar AEGs recorded from the RA, BB, and PLA during AF
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
|---|---|---|---|---|---|---|---|---|---|
| RA | CL mean (ms) | 217 | 208 | 200 | 159 | 174 | 169b | 208 | 168b |
| SD (ms) | 53 | 23 | 26 | 31 | 18 | 13 | 28 | 12 | |
| BB | CL mean (ms) | 208b | 190b | 160b | a | 146 | 165b | 182b | 166b |
| SD (ms) | 10 | 19 | c | 19 | 7 | 13 | 12 | ||
| PLA | CL mean (ms) | 200b | 180b | a | 144b | 145b | 168b | 187b | 187 |
| SD (ms) | c | c | 5 | 3 | 3 | 15 | 23 | ||
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
|---|---|---|---|---|---|---|---|---|---|
| RA | CL mean (ms) | 217 | 208 | 200 | 159 | 174 | 169b | 208 | 168b |
| SD (ms) | 53 | 23 | 26 | 31 | 18 | 13 | 28 | 12 | |
| BB | CL mean (ms) | 208b | 190b | 160b | a | 146 | 165b | 182b | 166b |
| SD (ms) | 10 | 19 | c | 19 | 7 | 13 | 12 | ||
| PLA | CL mean (ms) | 200b | 180b | a | 144b | 145b | 168b | 187b | 187 |
| SD (ms) | c | c | 5 | 3 | 3 | 15 | 23 | ||
Patients' AEG characteristics during AF using conventional ECG (Patients 1–3) and digital (Patients 4–8) recordings.
AF, atrial fibrillation; AEG, atrial electrograms; RA, right atrial appendage; BB, Bachmann's bundle; PLA, posterior left atrium; CL, cycle length; ms, millisecond; SD, standard deviation.
aSites with absent AEG recordings indicative of dislodgement of atrial leads.
bSite with regular cycle lengths (see Methods section for definition of regular CL).
cWithin resolution of measurement with manual callipers.
Cycle lengths (mean and SD) of bipolar AEGs recorded from the RA, BB, and PLA during AF
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
|---|---|---|---|---|---|---|---|---|---|
| RA | CL mean (ms) | 217 | 208 | 200 | 159 | 174 | 169b | 208 | 168b |
| SD (ms) | 53 | 23 | 26 | 31 | 18 | 13 | 28 | 12 | |
| BB | CL mean (ms) | 208b | 190b | 160b | a | 146 | 165b | 182b | 166b |
| SD (ms) | 10 | 19 | c | 19 | 7 | 13 | 12 | ||
| PLA | CL mean (ms) | 200b | 180b | a | 144b | 145b | 168b | 187b | 187 |
| SD (ms) | c | c | 5 | 3 | 3 | 15 | 23 | ||
| Patient . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | |
|---|---|---|---|---|---|---|---|---|---|
| RA | CL mean (ms) | 217 | 208 | 200 | 159 | 174 | 169b | 208 | 168b |
| SD (ms) | 53 | 23 | 26 | 31 | 18 | 13 | 28 | 12 | |
| BB | CL mean (ms) | 208b | 190b | 160b | a | 146 | 165b | 182b | 166b |
| SD (ms) | 10 | 19 | c | 19 | 7 | 13 | 12 | ||
| PLA | CL mean (ms) | 200b | 180b | a | 144b | 145b | 168b | 187b | 187 |
| SD (ms) | c | c | 5 | 3 | 3 | 15 | 23 | ||
Patients' AEG characteristics during AF using conventional ECG (Patients 1–3) and digital (Patients 4–8) recordings.
AF, atrial fibrillation; AEG, atrial electrograms; RA, right atrial appendage; BB, Bachmann's bundle; PLA, posterior left atrium; CL, cycle length; ms, millisecond; SD, standard deviation.
aSites with absent AEG recordings indicative of dislodgement of atrial leads.
bSite with regular cycle lengths (see Methods section for definition of regular CL).
cWithin resolution of measurement with manual callipers.
Eight of the 11 patients had episodes of AF demonstrating an area of regularity. Atrial electrograms with short, regular CLs and constant morphology were recorded from the PLA site in six patients, from the BB site in six patients, and from the RA site in two patients. Five patients had regularity recorded from two sites during AF episodes. There were no atrial complexes recorded from PLA and BB in Patients 3 and 4, respectively, indicating dislodgment of the atrial electrodes. Data from each of these patients are shown in Table 1. Figure 3 is a representative example of AF (Patient 2) demonstrating an area of regularity. A regular AEG CL (180 ms) with constant morphology was recorded only from the PLA, while the RA demonstrated a mean AEG CL of 208 ms (SD 23 ms), and the BB demonstrated a mean AEG CL of 190 ms (SD 19 ms). In Patients 1–3 (Table 1), the recordings were performed using the conventional ECG and the AEGs with constant CL and morphology remained unchanged during the duration of the recording (15–20 min).
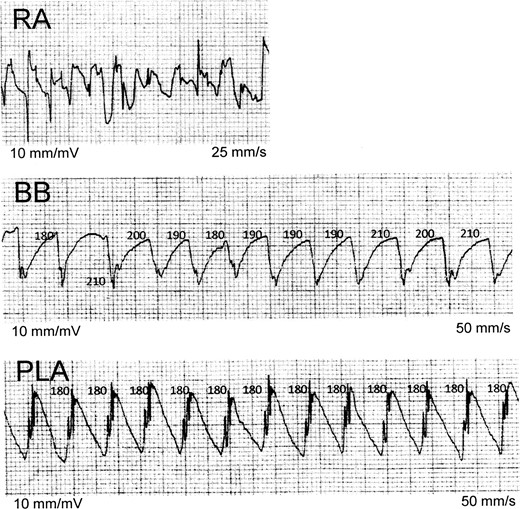
Sequential recordings of bipolar AEGs during AF: this figure, recorded from Patient 1, illustrates sequential recordings of bipolar AEGs during AF. Posterior LA AEGs demonstrate a regular CL (180 ms) with constant morphology, the BB AEGs demonstrate a slightly irregular CL with constant morphology, and the RA AEGs demonstrate an irregular CL with variable morphology. Bachmann's bundle and PLA electrograms demonstrate a prominent current of injury. Note that the RA AEGs demonstrate how difficult it can be to determine precise CL intervals when CLs are grossly irregular and have varying morphology. AEGs, atrial electrograms; AF, atrial fibrillation; BB, Bachmann's bundle; CL, cycle length; PLA, posterior left atrium; RA, right atrium. All numbers are in milliseconds.
In summary, in 8 of the 11 patients with POAF, a regular AEG CL with constant morphology was recorded from at least one of the LA sites. In contrast, in six of these eight patients, AEGs from the RA site during AF had an irregular CL with variable morphology. In one patient (Patient 8), the RA AEGs also showed a regular CL with constant morphology. In the remaining 3 of these 11 patients with AF, AEGs from the recording sites were irregular in CL, and had variable morphology.
Discussion
This study demonstrates that in the majority (8/11) of patients with new-onset POAF after OHS in our study in whom AEGs were recorded from selected atrial sites, at least one of the LA recording sites demonstrated rapid, regular AEGs of short CLs with constant morphology, while other recording site(s) in the atria demonstrated irregular AEG CLs with variable morphology. In fact, it is striking that although we recorded from at most three selected atrial sites, we found rapid, regular activation present in 8/11 AF episodes, and in 12 more including data from two prior studies (see following).7,8 Per our studies in the CSP model, this pattern is suggestive of a driver mechanism maintaining AF.5,6
Relevance to previous studies
More than three decades ago, in patients studied after OHS, we characterized two types of atrial flutter (AFL): Type I and Type II.6 Type I AFL was what we now call typical AFL. ‘Type II AFL’ demonstrated AEGs of constant morphology and regular, short CLs recorded from temporary epicardial electrodes placed high on the right atrium (the RA portion of BB). In the nine patients with ‘Type II AFL’, the mean constant CLs ranged from 139 to 176 ms, and the overall mean beat-to-beat change in CL was 6 ms. Although we initially labelled this as ‘Type II AFL’,7 in retrospect, the appearance of atrial activity in the ECG was that of so-called coarse AF. Subsequently, while characterizing the onset of Type I (typical) AFL from bipolar AEGs recorded from the sulcus terminalis region of the RA and the LA portion of BB,8 we observed three episodes in which the initiating rhythm was ‘Type II AFL’. However, during the initial ‘Type II AFL’, the ECGs demonstrated AF. At the time, these findings were chance observations made randomly while monitoring the rhythms of patients following OHS.8 From our current study (n = 8) and our prior studies (n = 12),7,8 we, therefore, have reported a total of 20 patients with AEGs demonstrating a regular short CL with constant morphology during POAF. These data suggest that this phenomenon during POAF is not uncommon. Furthermore, we suggest these areas of rapid, regular atrial activity recorded in both the present and previous studies may very well represent activation from or near the location of a driver maintaining AF in these patients, much as demonstrated in our sterile canine pericarditis model.5,6
Relevant studies in the canine sterile pericarditis model
Studies using simultaneous multisite mapping during induced AF in the CSP model, again, a model with a clinical counterpart in postoperative open heart surgical patients, provide insights into our POAF in patients. Following creation of the CSP model, sustained AF usually can be induced by burst atrial pacing.4–6 As indicated earlier, analysis of high density, simultaneous, multisite mapping of both atria has demonstrated two mechanisms of AF in this model. In the most common mechanism, AF is due to a single, stable LA reentrant circuit of short CL causing fibrillatory conduction to much of the rest of the atria.5,6 In the latter example, AEGs recorded in the area of the reentrant circuit and its immediate neighbouring sites are regular in CL, and constant in morphology. However, in parts of the LA and most of the RA, fibrillatory conduction is present, manifested by AEGs of longer and irregular CLs with variable morphology. Extrapolating to patients from our studies in the CSP model, again we suggest that the AEG recordings of rapid, regular activation in patients very well may represent AF due to a stable driver causing fibrillatory conduction.
Relevant studies in patients
There is only one published study, of which we are aware that is at all similar to ours. Swartz et al.9 studied 44 patients with no prior history of AF who underwent cardiac surgery. The hypothesis of their study was that AF is maintained by high-frequency sources in the LA resulting from ion channels and structural features that differ from the RA. In a subset of 27 patients, unipolar AEGs were recorded from leads sutured to the epicardial surface of two RA sites and two LA sites during AF. Using fast Fourier transformation (FFT) analysis, the dominant frequency (DF) from the RA and LA AEGs was determined. The DF of the unipolar AEGs from the LA was greater than the RA. However, there was no presentation of data on nor discussion of AEG morphology or regularity of beat-to-beat CL. Furthermore, the representative AEGs shown from both the LA and RA were clearly irregular in morphology and CL. Our findings differ in that in 8 of our 11 patients, we found AEGs with both short, regular CLs and constant morphology. We suggest that the difference between these two studies may be due to the site of placement of the epicardial electrodes. As indicated earlier, we placed our recording electrodes strategically based on data from mapping studies during AF in our CSP model,5,6 which we believe is an experimental counterpart of the postoperative patient.
Significance of the study
The mechanism sustaining AF was long thought to be due to multiple wavelet reentry, a self-sustaining rhythm, as proposed by Moe et al. in the 1950s from observations from the canine vagal AF model10 and a computer simulation model.11 Multiple wavelet reentry as a mechanism of AF was subsequently seemingly confirmed experimentally by Allessie et al.12 in 1985 using relatively high-density mapping (from 192 electrodes) of AF in a different model, a Langendorff perfused, acetylcholine infused, canine model. Recently, our group using high-density epicardial mapping (512 electrodes) in the Moe et al.10 canine vagally induced AF model failed to demonstrate multiple reentrant wavelets as the mechanism of AF.13 Rather, AF was due to several focal drivers firing at short, but different CLs, producing collision and fusion of wave fronts, but with no evidence of meaningful reentry. The first definite mechanism of paroxysmal non-postoperative AF in humans came from the seminal observations made by Haissaguerre et al.14 in 1998 that AF was triggered by rapid ectopic firing from the pulmonary veins. In the last decade, we15,16 and others17–21 have shown evidence from mapping and ablation during AF that the mechanism sustaining AF in persistent AF is likely due to one or more drivers maintaining AF. To our knowledge, our present study is the first to demonstrate that new-onset POAF in patients following OHS may be due to a driver maintaining AF.
Clinical implications
In patients with POAF, i.e. without a prior history of AF, our data are consistent with the notion that one mechanism of AF may be due to a driver of short, regular CL causing fibrillatory conduction and, thereby, clinically manifest AF. Also, the nomenclature of two types of AFL needs reconsideration, as ‘Type II AFL’ really is about AF, unless one considers that ‘Type II AFL’ is just a more rapid form of AFL which causes AF.
Limitations
One limitation of our study is that bipolar AEGs were only recorded from a maximum of three atrial sites. Had we recorded AEGs from more sites, we may have identified rapid, regular activity in more patients with AF, and perhaps in other areas of the atria. However, as we indicated, we think it is striking that with at most three strategically placed electrode pairs, we were able to demonstrate regular AEG CL and constant morphology in 73% of our patients with POAF. We believe that this is an important new finding. Although determining the mechanism of the driver was not part of this study's objective, we do point out the similarity of this finding with this type of AF with our very well-studied CSP model, which supports the notion that this type of AF in patients may be due to a single reentrant driver of short CL that produces fibrillatory conduction. Finally, since we only recorded for relatively short intervals, we did not determine whether the rapid, regular rhythm we recorded was constant during the entire course of AF in each episode, or whether, in fact, it changed.
Conclusions
In the majority of patients studied with new-onset POAF, a regular, rhythm with short AEG CLs and constant AEG morphology was present, primarily in the LA. On re-examination of our previous reports in 12 patients with ‘Type II AFL’, the regular AEGs of short CLs and the ECG recordings mirrored the findings of new-onset POAF in the present study. Extrapolating from a well-studied animal model counterpart, all this is consistent with the notion that a driver maintaining AF may have been present in or near the area of rapid, regular activation.
Funding
The research was supported in part by Jennie Zoline, Blue Dot, Glenstone, and MCJ Amelior Foundation.
Conflict of interest: none declared.



