-
PDF
- Split View
-
Views
-
Cite
Cite
Federico Guerra, Matilda Shkoza, Lorena Scappini, Marco Flori, Alessandro Capucci, Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis, EP Europace, Volume 16, Issue 3, March 2014, Pages 347–353, https://doi.org/10.1093/europace/eut304
Close - Share Icon Share
Abstract
Electrical storm (ES) is a devastating and life-threatening event in clinical practice, but its real weight as a risk factor and its clinical predictors remain unclear. Our objective was to evaluate ES as a mortality and morbidity risk factor and to define the clinical variables associated with ES.
The meta-analysis was performed according to the PRISMA guidelines. At the end of the selection process, 13 studies were collected and included in the quantitative analysis. Mortality and morbidity due to ES were assessed. The most acknowledged ES predictors were taken into account in separate sub-analyses. The whole cohort included 5912 patients (857 with ES). Risk of death was increased in the ES group [risk ratio (RR) 3.15; 95% confidence interval (CI) 2.22–4.48]. Electrical storm was also associated with increased composite risk of all-cause death, cardiac transplantation, and hospitalization for acute heart failure (RR 3.39; 95% CI 2.31–4.97). These results were confirmed by comparing the ES group with patients with or without previous unclustered episodes of ventricular arrhythmias. Moreover, implantable cardioverter-defibrillator (ICD) for secondary prevention, lower ejection fraction, monomorphic ventricular tachycardia as triggering arrhythmia, and class I anti-arrhythmic drugs therapy were all associated with ES.
Electrical storm is a strong mortality risk factor and it is associated with an increased combined risk of death, heart transplantation, and hospitalization for heart failure. Implantable cardioverter-defibrillator for secondary prevention, monomorphic ventricular tachycardia as triggering arrhythmia, lower ejection fraction, and class I anti-arrhythmic drugs therapy are all associated with ES and could be used to define specific populations with higher risk to develop ES.
Electrical storm (ES) is a devastating event in everyday clinical practice, but its importance as a mortality risk factor varies widely in current evidence. The present meta-analysis shows that ES accounts for a nearly 3-fold increased risk of death [risk ratio (RR) 3.15; 95% confidence interval (CI) 2.22–4.48] and is associated with a 3.39-fold increased risk for the composite endpoint of death, heart transplantation, and hospitalization for heart failure (RR 3.39; 95% CI 2.31–4.97).
Among the plethora of clinical predictors associated with a higher incidence of ES in the current literature, implantable cardioverter-defibrillator for secondary prevention, lower ejection fraction, monomorphic ventricular tachycardia as triggering arrhythmia, and class I anti-arrhythmic drugs therapy were all confirmed as having a significant association with ES and could be used to define a subset of patients at higher risk.
Introduction
Electrical storm (ES) is usually defined as a clustering of destabilizing episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) in a short period of time, requiring multiple cardioversions or defibrillations. Despite the fact that there is still no consensus regarding an official definition of ES, the generally accepted definition in clinical practice and recent literature is the occurrence of three or more distinct episodes of VT or VF in a 24 h time period.1,2
While ES represents an increasing problem in terms of epidemiology, especially in patients with heart failure (HF) and an implantable cardioverter-defibrillator (ICD), there is conflicting evidence whether ES could be considered a risk factor for mortality and morbidity or rather an ‘innocent bystander’.1 Moreover, little is known regarding the clinical variables associated with ES and, although some studies have shown potential candidates as ES predictors, the literature remains controversial.
The aims of this meta-analysis are to assess the real weight of ES as a mortality and morbidity risk factor and to define the clinical variables associated with ES or specific populations, in which ES is more common.
Methods
Search strategy and study selection
Two big medical databases (MEDLINE and ISI Web of Science) were systematically searched to include all available papers showing mortality, morbidity, and clinical characteristics related to ES. MEDLINE was searched using the following query: ‘electrical storm’ [Mesh] OR ‘arrhythmic storm’ [Mesh] OR ‘recurrent ventricular arrhythmias’ [Mesh] OR ‘ventricular tachycardia clusters’ [Mesh] OR ‘electrical instability’ [Mesh]. ISI Web of Science was searched using the following query: title contains ‘electrical storm’ OR ‘arrhythmic storm’ OR ‘recurrent ventricular arrhythmia’ OR ‘ventricular tachycardia clusters’ OR ‘electrical instability’. Figure 1 shows the study selection flowchart according to the PRISMA statement.3 Two authors (M.S. and L.S.) screened all the records independently, and were blinded to the other's decisions. After each selection phase, all divergences regarding exclusion of studies from the process were resolved by consensus between three authors (F.G., M.S., and L.S.). Reasons for study exclusion are shown in Figure 1. Interobserver concordance was optimal during the whole selection process (k=0.81). At the end of the selection process, 12 studies comparing mortality in patients with or without ES were found and included in the meta-analysis.4–15 One more study16 did not present data regarding mortality but showed data related to ES predictors and was therefore included in the relative sub-analyses.
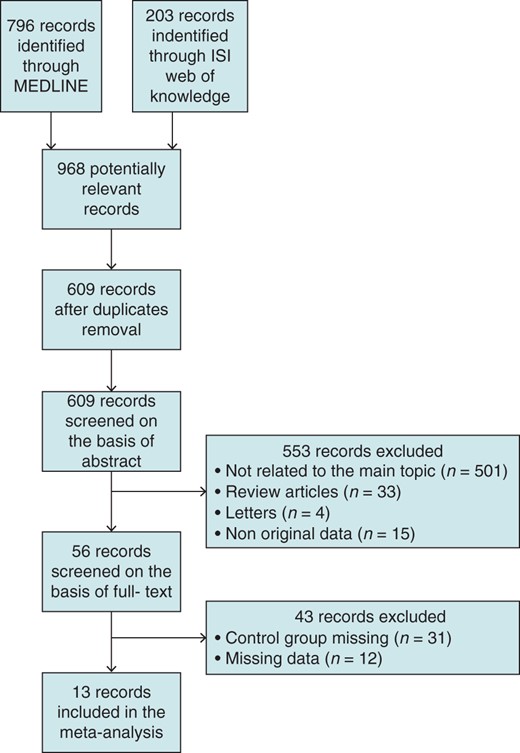
Flow chart of the study selection according to PRISMA statement.
Data extraction
Two authors (F.G. and M.F.) independently extracted all the data from the 13 papers selected for the meta-analysis and the differences were resolved by consensus. From all the studies, the total number of patients with ES, the total number of patients without ES, and the total number of deaths in each group were recorded. If feasible, patients without ES were divided according to the previous history of ventricular arrhythmias (no previous arrhythmias vs. one or more ventricular arrhythmias). In 8 out of 12 studies, data regarding heart transplantation or admission for severe HF and cardiogenic shock were available and recorded. The following variables were taken into account as risk factors for ES and recorded if present: age, gender, ischaemic cardiomyopathy, idiopathic dilated cardiomyopathy, ICD implanted for secondary prevention, monomorphic VT as triggering arrhythmia, polymorphic VT as triggering arrhythmia, VF as triggering arrhythmia, ejection fraction (EF), chronic therapy with beta-blockers, chronic therapy with amiodarone, and chronic therapy with class I anti-arrhythmic drugs (AADs). The whole cohort included 5912 patients (857 with ES, 5055 controls). Clinical features of the included papers are shown in Supplementary material online, Table S1.
Quality assessment
Quality assessment of included papers has been performed using the Newcastle–Ottawa quality assessment scale for non-randomized studies.17 Two authors (M.S. and M.F.) assessed independently all the papers and divergences were resolved by consensus. Interobserver concordance was optimal (k=0.86). Results of the quality assessment are shown in Supplementary material online, Table S2.
Statistical analysis
Risk ratio (RR) was used as the main effect size. Owing to the large variety in patients' characteristics, both in the ES and in the control groups, a random-effects model was used to calculate RR even in the case where no heterogeneity was found across studies. Log RR and variance were computed to yield a summary effect size and its confidence interval (CI), which were subsequently converted back to linear measures for data presentation. Statistical heterogeneity was tested by Q statistics,18 and a P<0.01 (two-sided) was considered significant for the presence of heterogeneity, which was then quantified using the I2 statistics. Single group summaries were used to compare age and EF between the ES and the non ES group. The interaction between clinical features and ES-related mortality, heart transplantation, and hospitalization for HF was tested by performing a random-effect meta-regression, with weighting provided by the inverse of the variance of each study, the natural logarithm of the individual RRs as dependent variable and any potentially relevant variable as random factors (age, male gender, ischaemic cardiomyopathy, idiopathic dilated cardiomyopathy, ICD implanted for secondary prevention, monomorphic VT as triggering arrhythmia, polymorphic VT as triggering arrhythmia, VF as triggering arrhythmia, EF, chronic therapy with beta-blockers, chronic therapy with amiodarone, and chronic therapy with class I AADs).19 The same random-effect meta-regression models and the same random factors were used to test the interaction between ES incidence and its predictors. All the tests were two-tailed and a P<0.05 was considered statistically significant. Review Manager 5.1 (The Cochrane Collaboration) and Comprehensive Meta-Analysis 2.0 (Biostat Inc.) were used for the analysis.
Results
Mortality
We included in the meta-analysis 12 studies comparing death incidence between patients with and without ES during a median follow-up of 32 months (interquartile range 21–36 months). In the first analysis (Figure 2), the control group included all patients who never experienced ES, from patients with no history of VT/VF to patients with positive history of multiple unclustered ventricular arrhythmias. Our data show that ES accounted for a nearly three-fold increased risk of death (RR 3.15; 95% CI 2.22–4.48).
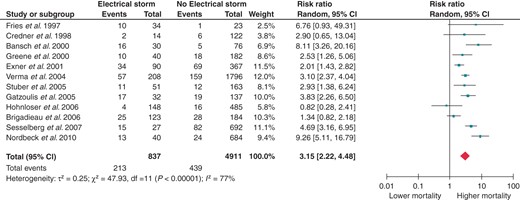
Effect of ES on all-cause mortality. CI, confidence interval; df, degrees of freedom.
The next step was to split the control group into two different sub-groups: patients with a positive history of at least one VT or VF but no previous ES, and patients without any previous history of VT or VF. Electrical storm retained a significantly increased RR for death even when compared with previous unclustered episodes of sustained ventricular arrhythmias (RR 2.51; 95% CI 1.38–4.58, Figure 3). Of course, the risk of death is further increased when the ES patients are compared with patients without any known history of VTs or VFs (RR 3.34; 95% CI 1.52–7.31, Figure 4).
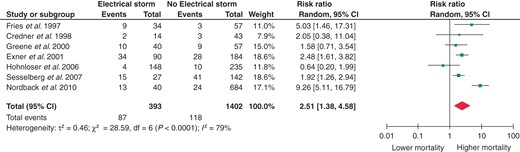
Effect of ES on all-cause mortality when compared with positive previous history of VT/VF but no ES. CI, confidence interval; df, degrees of freedom.
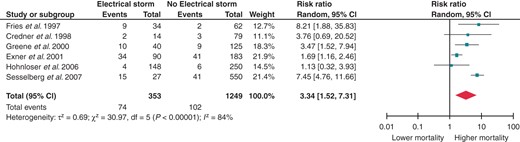
Effect of ES on all-cause mortality when compared with no history of previous VT/VF. CI, confidence interval; df, degrees of freedom.
Composite risk for all-cause mortality, heart transplantation, and hospitalization for heart failure
Eight papers presented composite data regarding death, cardiac transplantation, and hospital admission for acute decompensated HF or cardiogenic shock. As shown in Figure 5, ES is associated with a 3.39-fold increased risk for the aforementioned composite endpoint (RR 3.39; 95% CI 2.31–4.97). Splitting the control group according to the previous history of VT or VF still provided significant results. Patients with ES had a 2.85-fold increased risk of either death, heart transplantation, and hospitalization for HF when compared with patients with previous unclustered VTs or VFs (RR 2.85; 95% CI 1.81–4.48; Figure 6) and a 5.42-fold increased risk when compared with patients without previous sustained ventricular arrhythmias (RR 5.42; 95% CI 3.14–9.34; Figure 7).
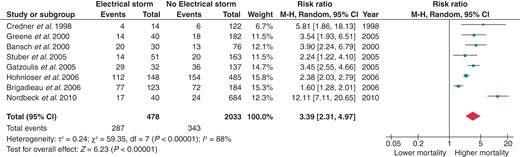
Effect of ES on the composite risk for all-cause mortality, heart transplantation, and hospitalization for acute decompensated HF or cardiogenic shock. CI, confidence interval; df, degrees of freedom.
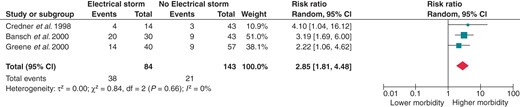
Effect of ES on the composite risk for all-cause mortality, heart transplantation, and hospitalization for acute decompensated HF or cardiogenic shock when compared with positive previous history of VT/VF but no ES. CI, confidence interval; df, degrees of freedom.
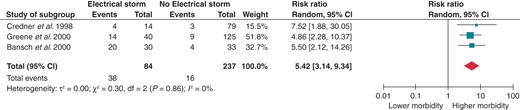
Effect of ES on the composite risk for all-cause mortality, heart transplantation, and hospitalization for acute decompensated HF or cardiogenic shock when compared with no history of previous VT/VF. CI, confidence interval; df, degrees of freedom.
Random-effect meta-regressions disclosed no significant interaction between any of the studied factors and the increased mortality, heart transplantation, and HF-related hospitalization in ES patients.
Clinical variables associated with electrical storm
Thirteen studies have been included in the sub-analyses regarding variables commonly reported as associated with ES. A trend towards an increased prevalence of advanced age and male gender in ES patients was present, although not statistically significant. No significant differences have been found regarding ES prevalence in ischaemic cardiomyopathy or idiopathic dilated cardiomyopathy. Instead, an ICD implant for secondary prevention was associated with a significantly greater prevalence of ES [odds ratio (OR) 3.62; 95% CI 1.08–12.14]. Regarding HF-related clinical variables, patients with ES have on average a lower EF (−3.08%; 95% CI −5.56 to 0.62). Monomorphic VTs as triggering arrhythmias were also shown as significantly related to ES (OR 1.79; 95% CI 1.35–2.39), whereas polymorphic VTs and VFs were not. Amiodarone and beta-blockers were equally distributed between patients with and without ES, whereas class I AADs were associated with a higher incidence of ES (OR 5.20; 95% CI 1.35–20.01). Forest plots for each variable considered are shown in the Supplementary material.
Random-effect meta-regressions disclosed no significant interaction between any of the studied clinical variables and the increased risk of ES associated with either secondary prevention, low EF, higher New York Heart Association, or monomorphic VTs as triggering arrhythmia.
Discussion
This paper, which includes over 5900 patients, is the first meta-analysis performed to evaluate the real effect of ES as a mortality and a morbidity risk marker. Overall, ES is associated with a three-fold increased risk of death. This risk is still significant when the ES patients are compared with patients with previous history of one or more unclustered VTs or VFs. These results generate a few interesting pathophysiological hypotheses. In fact, it is well known that sudden cardiac death occurs in 2% of all patients with ICD and that the death rate is strictly related to the number of episodes.20 One study suggests that the increase in mortality could be due to incessant recurrent VTs, which in turn provoke or worsen the left ventricular dysfunction leading to end-stage HF, cardiogenic shock, and death.4 As most of the patients with ES have an ICD, another possible contribution to the increased risk of death could come from the shocks themselves. A recent paper by Sweeney et al.21 showed that patients with VTs or VFs terminated by shocks have higher mortality (20% increased risk per shocked episode) than patients with no anti-arrhythmic therapies or patients treated only with anti-tachycardia pacing. Moreover, ventricular arrhythmias occurrence rates, durations, and electrical therapy burden were highest among patients who were shocked and died. In this setting, it is feasible to hypothesize that multiple shocks can add their single contribution to transient systolic dysfunction and lead to severe heart decompensation.22 This hypothesis is also supported by the fact that in the AVID and the MADIT-II trials, risk of death after ES reached its peak around 3 months after the acute event and death due to ES were more commonly due to non-sudden cardiac causes.7,13 A similar evidence comes from studies on catheter ablation: in patients experiencing drug-resistant ES, catheter ablation provided optimal survival rates, ranging from 8823 to 91%24 up to 36 months after the acute event. Therefore, it has been hypothesized that catheter ablation, reducing long-term unclustered ventricular arrhythmias as well as ES recurrences, may prevent the associated decline in systolic dysfunction and favourably affect sudden and non-sudden cardiac mortality.
Another main finding of our study is that ES is associated with a 3.39-fold increased combined risk of death, cardiac transplantation, or hospitalization for severe HF or cardiogenic shock. Hospitalization, despite not being mandatory for a proper treatment in all patients, is still needed in ∼80% of patients with ES and hospitalization rates grow higher with each shock delivered, reaching 100% if three or more shocks are needed.4 Hospitalization is in turn associated with a poorer quality of life and higher costs. Cardiac transplantation on the other hand often represents the last line of therapy in patients with recurrent ES, who experienced no improvements from pharmacological, device-related, and ablation treatment. Patients with refractory ES associated with genetic arrhythmia syndromes are also reasonable candidates as these patients are typically young, otherwise healthy individuals.
Of all the variables considered in our study, an ICD implantation for secondary prevention was the strongest predictor of ES, with an OR of 3.62. Stuber et al.14 have already shown that ES was more frequently associated with ICD implanted for secondary prevention rather than for primary prevention, especially when a monomorphic VT was the index event for the ICD implant. This finding has at least two possible explanations. First, ICD can detect the ventricular arrhythmia underlying a clinical episode of dizziness, syncope, or even aborted sudden cardiac death, thus making the diagnosis far easier. Secondly, and most importantly, it can effectively treat the first and second VT or VF, thus saving the patient from sudden cardiac death and making him capable of withstanding more arrhythmic episodes. Of course, patients who have already experienced an arrhythmic episode are more prone to develop further ventricular arrhythmias after ICD implant and, hence, ES.6,7,14,25
The current literature is controversial regarding a possible relationship between ES and EF. Several studies have found that an altered EF is an independent risk factor for ES,5,7 while others showed better systolic function in ES patients.6,11 Overall, our data showed that EF is in fact lower in ES patients, with an absolute reduction of 3%. This finding, which could seem trivial at first sight, is instead quite important in this kind of patients. It must be considered that systolic function was already hampered in the whole population, with EF ranging from 22 to 41%. Unfortunately, in all included papers, EF assessment could have been biased. First, EF was assessed (often retrospectively) by echocardiography, which is often burdened by great intraobserver and interobserver variability. Moreover, some of the included studies5,11 enroled patients with recent VTs or VFs, in whom shock-related transient systolic dysfunction could have had a negative effect on EF at baseline.
However, some studies have shown a higher prevalence of VF as causative arrhythmia,15 the finding that ES is more often triggered by a monomorphic VT was not unexpected. Monomorphic VTs indicate the presence of a reentry due to an electrophysiological substrate, which could trigger and sustain the ES in a majority of cases.1 This could also explain the reported higher prevalence of ES in HF patients, in which a post-ischaemic or idiopathic structural cardiac remodelling could favour reentry pathways around scars or other anatomical barriers.2 Only in less frequent cases is ES due to acute myocardial ischaemia, and in these cases polymorphic VTs of VFs are usually predominant.1
Finally, our analysis showed that chronic therapy with class I AADs is associated with a 5.2-fold increase in ES prevalence, whereas no significant association with ES has been shown for either the amiodarone or beta-blockers. Treatment with class I AADs could also define a sub-population with a higher basal risk of ventricular arrhythmias, pro-arrhythmic effects of these drugs in structural heart disease and systolic dysfunction are well known,26 and could have contributed to the increased ES prevalence.
Study limitations
Limitations of meta-analysis are well known,27 and obviously apply entirely to the present study. Moreover, our meta-analysis is based on observational data, and therefore, risk estimates are even more prone to selection and performance bias. Although the quality assessment performed through the Newcastle–Ottawa Scale17 yielded overall good results, it is not enough to exclude possible bias. Even the random-effect meta-regressions, which showed no significant interactions, cannot cope with the unknown confounders and perform poorly in small studies.
Several other limitations of our meta-analysis must be taken into account. First, there was a great deal of variation between the studied populations regarding underlying structural heart disease, the presence or the absence of ICD, pharmacological therapy, and even different definitions of ES. Although this heterogeneity should not exceedingly limit our results, which are all based on random-effects models to account for a Gaussian distribution of true effects, the heterogeneity makes it difficult to assess possible publication bias through traditional means.
Secondly, the present meta-analysis is based on study level data rather than on patient level data. This is of particular importance when considering the sub-analyses regarding clinical variables associated with ES. Being not able to access individual patient's data means that, for each study included, we were able to account only for those variables that were considered important by the authors, lacking any information on other variables of clinical relevance. This is especially true regarding electrolytic imbalances: despite being considered strongly associated with ES, no included study provided any data regarding potassium or magnesium serum levels, thus ruling them out of any possible sub-analysis.
Thirdly, as all the patients enroled have an ICD implanted, device programming is of the utmost importance for ES diagnosis. Lower heart rates for arrhythmia detection and more aggressive electrical therapies could lead to more detected ventricular arrhythmias and a higher incidence of ES. Moreover, it has recently been demonstrated that programming ICD therapies for higher rates or with prolonged delays was associated with reductions in inappropriate therapy and all-cause mortality during long-term follow-up.28 This could lead to the hypothesis that at least some of the ES episodes documented by earlier studies were in fact episodes of arrhythmias destined to spontaneously self-terminate. Unfortunately, ICD settings were seldom included in the methods of the selected papers and a comparison between those few was not a viable option. Nonetheless, it is not incorrect to speculate that an ICD programming modality specifically tailored to ignore both slower tachyarrhythmias and those of shorter duration, such as the ones tested in the MADIT-RIT trial,28 could be helpful in reducing excessive shocks, hospitalization, morbidity and mortality, and probably ES prevalence as well.
Conclusion
The present meta-analysis sheds some light on the conflicting evidence regarding the role of ES as a strong mortality risk factor. Electrical storm is also associated with a combined risk of death, heart transplantation, and hospitalization for severe acute heart decompensation and cardiogenic shock, leading to a poorer quality of life and increased costs. Among all the considered factors, ICD implantation for secondary prevention, monomorphic VT as triggering arrhythmia, low EF, and chronic therapy with class I AADs are all associated with ES, and could be used as primary endpoints in randomized trials aimed at defining specific populations with higher risk to develop ES.
The results of this study provide a solid argument to improve ES prevention as well as pharmacological and ablation treatment to enhance survival and quality of life in patients at risk.



