-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Kubuš, Pavel Vít, Roman A. Gebauer, Libor Zaoral, Petr Peichl, Martin Fiala, Jan Janoušek, Long-term results of paediatric radiofrequency catheter ablation: a population-based study, EP Europace, Volume 16, Issue 12, December 2014, Pages 1808–1813, https://doi.org/10.1093/europace/euu087
Close - Share Icon Share
Abstract
We aimed to evaluate long-term utilization and results of paediatric radiofrequency catheter ablation (RFCA) in a population-based study.
Data from all three centres performing paediatric RFCA for the whole population of the Czech Republic between 1993 and 2010 were retrospectively reviewed. A total of 708 ablation procedures in 633 consecutive patients <18 years for 716 different substrates were tracked, with accessory pathways = 439 (61.3%) and atrioventricular nodal reentry tachycardia (AVNRT) = 205 (28.6%) being most frequent. Incidence of RFCA reached 0.049 per 1000 children <18 years of age in the recent era (2006–10). Indications included patient preference (68.0%), drug refractoriness (15.5%), asymptomatic Wolff–Parkinson–White pre-excitation (8.4%), and malignant arrhythmia (6.1%). Median follow-up was 13.7 (interquartile range 5.7–21.5) months. Overall acute/long-term success of the primary procedure was 89.1/77.2% (accessory pathways 87.2/77.7%, AVNRT 98.5/84.4%). Re-ablation was performed in 73 of 163 substrates after a primary unsuccessful ablation resulting in a long-term cumulative efficacy of 96.3%. Between 1993–2005 and 2006–10, procedure/fluoroscopy time decreased from median 154/24 to 105/14 min. (P < 0.001 for both). Serious complications occurred in nine patients (1.4%).
This population-based study could replicate data from previous single- or multi-centre reports confirming RFCA as a safe method of arrhythmia treatment in children with long-term cumulative efficacy exceeding 90% and significant decrease in the procedure and fluoroscopy time during the study period. The need for RFCA can be estimated at ∼0.05/1000 children <18 years using current indication criteria.
Radiofrequency catheter ablation (RFCA) is currently widely used for the treatment of tachyarrthmias in both the adult and paediatric population. Despite this fact, population-based data on long-term efficacy and safety of RFCA in children is still lacking.
The present study evaluated long-term results of all paediatric RFCA performed on the territory of one country over a long time interval.
Incidence of RFCA per 1000 children <18 years of age has been calculated giving important information to predict resource utilization while planning delivery of this highly specialized care.
Introduction
Radiofrequency catheter ablation (RFCA) has been reported to be an effective and safe method of tachyarrhythmia treatment in children and adolescents with favourable acute1,2 and long-term3,4 efficacy in both retrospective1,3,4 and prospective2,5 studies. Radiofrequency catheter ablation is nowadays considered the standard of care for the majority of paediatric patients with supraventricular tachycardia (SVT).6–8 However, population-based data on its safety and long-term efficacy is lacking. Published single-centre and multi-centre studies may reflect selected populations treated in highly experienced centres. The results reported may thus be different from those in an unbiased population. The aim of this study was to evaluate long-term results of all children undergoing RFCA in one country.
Methods
Patients and procedures
The study population was identified retrospectively from the clinical databases of all three tertiary care centres (two exclusively paediatric) providing paediatric RFCA for the whole territory of the Czech Republic (10.5 million inhabitants). Between 1993 (introduction of catheter ablation) and 2010, a total of 708 ablation procedures were performed in 633 consecutive patients (331 boys, 302 girls) <18 years of age. The median age at first ablation was 14.9 [interquartile range (IQR) 12.6–16.5] years (Table 1). To assess the annual incidence of catheter ablation, the number of children <18 years of age was retrieved from respective annual Statistical Yearbooks published by the Czech Statistical Office. All procedures were performed using RF energy under conscious sedation or general anaesthesia according to the specific centre protocols and age of the patients. In all cases, heparin was administered with 100 IU/kg body weight after catheter insertion and added as necessary to maintain the activated clotting time between 200 and 300 s. The endpoint for atrioventricular nodal reentry tachycardia (AVNRT) ablation was slow pathway elimination/modification (up to one echo beat allowed). Diagnostic and ablation (non-irrigated) catheters routinely used were 4–6F and 5–7F, respectively, according to body size. In general, 4–5F diagnostic and 5–6F ablation catheters were preferred in children weighing <30 kg. For the substrates on the left side of the heart (N = 259), retrograde approach (222 of 259, 85.7%) or transseptal puncture (37 of 259, 14.3%) was used. To achieve better catheter stability, long steerable introducers were used on an individual basis for right-sided accessory pathways. For the same purpose, short controlled apnoea during general anaesthesia was sometimes utilized to prevent catheter instability during the respiratory cycle. Non-fluoroscopic navigation (LocaLisa®) was routinely used in one of the participating centres during the last 54 consecutive procedures for accessory pathways, AVNRT, and atrial flutter. The CARTO system was used in a total of nine patients (structural heart disease in eight of nine) and the substrates targeted were ventricular tachycardia in six, intra-atrial reentrant tachycardia in two, and atrial ectopic tachycardia in one patient.
Demographic data and arrhythmogenic substrates according to individual centres providing RFCA
| Demographic data . | Total . | Centre Ia (paediatric) . | Centre IIb (paediatric) . | Centre IIIc (adult+paediatric) . |
|---|---|---|---|---|
| Patients | 633 | 374 | 243 | 16 |
| Age at procedure median (IQR) (years) | 14.9 (12.6–16.5) | 14.5 (12.1–16.4) | 15.3 (13.4–16.8) | 16.4 (15.4–17.0) |
| Ablation procedures | 708 | 412 | 277 | 19 |
| Arrhythmogenic substrates | 716 | 423 | 268 | 25 |
| Accessory pathways | 439 | 276 | 155 | 8 |
| Manifest (asymptomatic) | 310 (62) | 194 (46) | 109 (16) | 7 (0) |
| Concealed | 117 | 73 | 43 | 1 |
| PJRT | 12 | 9 | 3 | 0 |
| AVNRT | 205 | 99 | 103 | 3 |
| Mahaim tachycardia | 20 | 18 | 2 | 0 |
| Focal atrial tachycardia | 16 | 10 | 4 | 2 |
| Ventricular tachycardia | 16 | 7 | 1 | 8 |
| Atrial flutter | 13 | 9 | 1 | 3 |
| IART | 4 | 1 | 2 | 1 |
| Twin AV nodes | 3 | 3 | 0 | 0 |
| Demographic data . | Total . | Centre Ia (paediatric) . | Centre IIb (paediatric) . | Centre IIIc (adult+paediatric) . |
|---|---|---|---|---|
| Patients | 633 | 374 | 243 | 16 |
| Age at procedure median (IQR) (years) | 14.9 (12.6–16.5) | 14.5 (12.1–16.4) | 15.3 (13.4–16.8) | 16.4 (15.4–17.0) |
| Ablation procedures | 708 | 412 | 277 | 19 |
| Arrhythmogenic substrates | 716 | 423 | 268 | 25 |
| Accessory pathways | 439 | 276 | 155 | 8 |
| Manifest (asymptomatic) | 310 (62) | 194 (46) | 109 (16) | 7 (0) |
| Concealed | 117 | 73 | 43 | 1 |
| PJRT | 12 | 9 | 3 | 0 |
| AVNRT | 205 | 99 | 103 | 3 |
| Mahaim tachycardia | 20 | 18 | 2 | 0 |
| Focal atrial tachycardia | 16 | 10 | 4 | 2 |
| Ventricular tachycardia | 16 | 7 | 1 | 8 |
| Atrial flutter | 13 | 9 | 1 | 3 |
| IART | 4 | 1 | 2 | 1 |
| Twin AV nodes | 3 | 3 | 0 | 0 |
PJRT, permanent junctional reciprocating tachycardia; AVNRT, atrioventricular nodal reentry tachycardia; IART, intra-atrial reentry tachycardia; AV, atrioventricular.
aChildren's Heart Centre, Prague.
bPaediatric Cardiology, Brno.
cInstitute for Clinical and Experimental Medicine, Prague.
Demographic data and arrhythmogenic substrates according to individual centres providing RFCA
| Demographic data . | Total . | Centre Ia (paediatric) . | Centre IIb (paediatric) . | Centre IIIc (adult+paediatric) . |
|---|---|---|---|---|
| Patients | 633 | 374 | 243 | 16 |
| Age at procedure median (IQR) (years) | 14.9 (12.6–16.5) | 14.5 (12.1–16.4) | 15.3 (13.4–16.8) | 16.4 (15.4–17.0) |
| Ablation procedures | 708 | 412 | 277 | 19 |
| Arrhythmogenic substrates | 716 | 423 | 268 | 25 |
| Accessory pathways | 439 | 276 | 155 | 8 |
| Manifest (asymptomatic) | 310 (62) | 194 (46) | 109 (16) | 7 (0) |
| Concealed | 117 | 73 | 43 | 1 |
| PJRT | 12 | 9 | 3 | 0 |
| AVNRT | 205 | 99 | 103 | 3 |
| Mahaim tachycardia | 20 | 18 | 2 | 0 |
| Focal atrial tachycardia | 16 | 10 | 4 | 2 |
| Ventricular tachycardia | 16 | 7 | 1 | 8 |
| Atrial flutter | 13 | 9 | 1 | 3 |
| IART | 4 | 1 | 2 | 1 |
| Twin AV nodes | 3 | 3 | 0 | 0 |
| Demographic data . | Total . | Centre Ia (paediatric) . | Centre IIb (paediatric) . | Centre IIIc (adult+paediatric) . |
|---|---|---|---|---|
| Patients | 633 | 374 | 243 | 16 |
| Age at procedure median (IQR) (years) | 14.9 (12.6–16.5) | 14.5 (12.1–16.4) | 15.3 (13.4–16.8) | 16.4 (15.4–17.0) |
| Ablation procedures | 708 | 412 | 277 | 19 |
| Arrhythmogenic substrates | 716 | 423 | 268 | 25 |
| Accessory pathways | 439 | 276 | 155 | 8 |
| Manifest (asymptomatic) | 310 (62) | 194 (46) | 109 (16) | 7 (0) |
| Concealed | 117 | 73 | 43 | 1 |
| PJRT | 12 | 9 | 3 | 0 |
| AVNRT | 205 | 99 | 103 | 3 |
| Mahaim tachycardia | 20 | 18 | 2 | 0 |
| Focal atrial tachycardia | 16 | 10 | 4 | 2 |
| Ventricular tachycardia | 16 | 7 | 1 | 8 |
| Atrial flutter | 13 | 9 | 1 | 3 |
| IART | 4 | 1 | 2 | 1 |
| Twin AV nodes | 3 | 3 | 0 | 0 |
PJRT, permanent junctional reciprocating tachycardia; AVNRT, atrioventricular nodal reentry tachycardia; IART, intra-atrial reentry tachycardia; AV, atrioventricular.
aChildren's Heart Centre, Prague.
bPaediatric Cardiology, Brno.
cInstitute for Clinical and Experimental Medicine, Prague.
Follow-up
Routine follow-up was scheduled at 1 month and 1 year after the procedure yielding a median follow-up time of 13.7 (IQR 5.7–21.5) months. Recurrence was tracked for each treated arrhythmia substrate and defined as one or more of the following: documented original arrhythmia, symptoms highly suggestive of recurrence and reappearance of pre-excitation. Exact time of recurrence has not been uniformly documented and could thus not be retrospectively assessed. Long-term success of the primary procedure was defined as the absence of recurrence within the reported follow-up interval. Long-term cumulative efficacy was calculated as the long-term success of both the primary procedure and the re-ablation (substrates with a primary unsuccessful procedure not repeatedly targeted were excluded). There were only three patients (all with symptomatic arrhythmias before the procedure) not returning to any of three ablation centres after 1 year. These patients were presumed to be arrhythmia free. Early and recent ablation periods (1993–2005 and 2006–10, respectively) were defined to evaluate the changes in RFCA efficacy and in the procedure and fluoroscopy time.
The study complies with the Declaration of Helsinki. Because of the purely retrospective study design using available institutional clinical records, the absence of impact on management of patients included and completely anonymous data presentation, informed consent of the subjects (or their parents), and ethical approval have not been obtained.
Statistical analysis
Continuous data were displayed as median and IQR. Mann–Whitney rank sum test was used for comparison of continuous variables between two groups. Differences in proportions between two groups were tested by the χ2 test. Values of P < 0.05 were regarded as significant. All statistical analysis was performed using the SigmaPlot for Windows Version 11.0 (Systat Software Inc.).
Results
Substrates
A total of 716 different arrhythmogenic substrates were targeted in 633 patients with the majority consisting of atrioventricular (AV) accessory pathway (N = 439, 61.3%) and AVNRT (N = 205, 28.6%). Accessory pathways were manifest (310 of 439, 70.6%), concealed (117 of 439, 26.7%), or right posteroseptal with decremental and exclusively retrograde conduction [permanent junctional reciprocating tachycardia (PJRT), 12 of 439, 2.7%]. Sixty-two of the 310 manifest pathways (20%) were asymptomatic. The accessory pathway localization was left-sided in 231 (52.6%), septal in 130 (29.6%), and right-sided in 78 (17.8%) of the 439 pathways. The ablation of multiple different substrates was performed in 51 of 633 patients (8.1%) during the primary procedure: two substrates in 41 patients, three substrates in 9 patients, and four substrates in 1 patient. Multiple accessory pathways were present in 42 of 390 (10.8%) patients with accessory pathways, the combination of an accessory pathway and AVNRT in 6 of 595 (1.0%) patients with respective arrhythmias. Congenital heart disease was present in 43 of 633 patients (6.8%), the most common structural defects were cardiomyopathy (N = 10), Ebstein anomaly (N = 8), congenitally corrected transposition of great arteries (N = 5), heart defects with single ventricle physiology (N = 5), double outlet right ventricle (N = 4), and tetralogy of Fallot (N = 3). Eleven patients (1.7%) were <6 years at the time of RFCA with the youngest being 1.9 years old.
Indications for radiofrequency catheter ablation
The indication for RFCA (Figure 1) was patient preference in the majority (68.0%), drug refractoriness (15.5%), asymptomatic Wolff–Parkinson–White (WPW) pre-excitation (8.4%), malignant arrhythmia (6.1%), tachycardia-induced cardiomyopathy (1.0%), and planned total cavopulmonary connection (TCPC, 1.0%). The cause of the tachycardia-induced cardiomyopathy was focal atrial tachycardia in three of six, PJRT in two of six, and Mahaim tachycardia in one of six patients. Forty-three per cent of patients had antiarrhythmic drugs before the ablation procedure. Extensive antiarrhythmic drug therapy was used in patients <6 years of age to limit RFCA indication to truly drug-refractory cases. In the subgroup of patients being <6 years at the time of RFCA, 10 of 11 had, on an average, 1.7 (1–3) antiarrhythmic drugs before ablation. The substrate was an accessory pathway in 8 of 11, twin AV node in 2 of 11, and ventricular tachycardia in 1 of 11 patients. The indication for RFCA was planned TCPC completion in 5 of 11, the presence of a malignant arrhythmia in 3 of 11, and tachycardia-induced cardiomyopathy, drug refractoriness, and patient preference in 1 of 11 each.
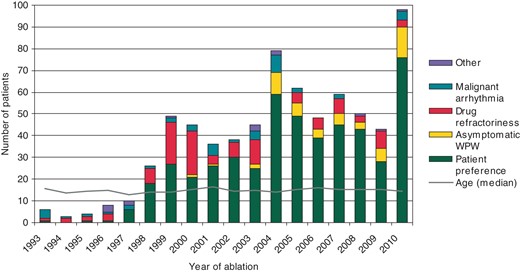
Incidence of catheter ablation increased from 0.029 to 0.049 per 1000 children <18 years of age when comparing the early (1993–2005) and recent (2006–10) ablation periods (P = 0.024) showing increasing utilization of the method over time.
Acute and long-term success
The acute/long-term success of the primary procedure as well as the long-term cumulative efficacy of RFCA for any individual substrate is shown in Table 2. Long-term success has increased for both accessory pathways and AVNRT when comparing the early and recent ablation periods. Re-ablation was performed in 73 of 163 substrates after a primary unsuccessful procedure. In one additional patient, repeated procedure was performed for a de novo substrate. The resulting long-term cumulative efficacy was 96.3% (603 of 626 attempted substrates).
| Substrate . | Acute success of the primary procedure (%) . | Long-term success of the primary procedure (%) . | Cumulative efficacy (%)a . |
|---|---|---|---|
| All substrates (N = 716) | 89.1 | 77.2 | 96.3 |
| Early period (1993–2005, N = 421) | 86.6 | 72.7 | 95.3 |
| Recent period (2006–10, N = 295) | 92.8 | 81.4 | 97.8 |
| P = (early vs. recent period) | 0.077 | 0.040 | |
| Accessory pathways (N = 439) | 87.2 | 77.7 | 97.9 |
| Early period (1993–2005, N = 269) | 84.6 | 74.5 | 97.0 |
| Recent period (2006–10, N = 170) | 91.1 | 83.0 | 98.7 |
| P = (early vs. recent period) | 0.073 | 0.033 | |
| AVNRT (N = 205) | 98.5 | 84.4 | 95.8 |
| Early period (1993–2005, N = 109) | 98.2 | 78.9 | 92.8 |
| Recent period (2006–10, N = 96) | 98.9 | 90.6 | 98.9 |
| P = (early vs. recent period) | 0.912 | 0.034 | |
| Mahaim tachycardia (N = 20) | 90.0 | 65.0 | 88.2 |
| Focal atrial tachycardia (N = 16) | 62.5 | 50.0 | 83.3 |
| Ventricular tachycardia (N = 16) | 75.0 | 43.8 | 70.0 |
| Atrial flutter (N = 13) | 69.2 | 61.5 | 90.0 |
| IART (N = 4) | 50.0 | 25.0 | 100.0 |
| Twin AV nodes (N = 3) | 66.7 | 66.7 | 100.0 |
| Substrate . | Acute success of the primary procedure (%) . | Long-term success of the primary procedure (%) . | Cumulative efficacy (%)a . |
|---|---|---|---|
| All substrates (N = 716) | 89.1 | 77.2 | 96.3 |
| Early period (1993–2005, N = 421) | 86.6 | 72.7 | 95.3 |
| Recent period (2006–10, N = 295) | 92.8 | 81.4 | 97.8 |
| P = (early vs. recent period) | 0.077 | 0.040 | |
| Accessory pathways (N = 439) | 87.2 | 77.7 | 97.9 |
| Early period (1993–2005, N = 269) | 84.6 | 74.5 | 97.0 |
| Recent period (2006–10, N = 170) | 91.1 | 83.0 | 98.7 |
| P = (early vs. recent period) | 0.073 | 0.033 | |
| AVNRT (N = 205) | 98.5 | 84.4 | 95.8 |
| Early period (1993–2005, N = 109) | 98.2 | 78.9 | 92.8 |
| Recent period (2006–10, N = 96) | 98.9 | 90.6 | 98.9 |
| P = (early vs. recent period) | 0.912 | 0.034 | |
| Mahaim tachycardia (N = 20) | 90.0 | 65.0 | 88.2 |
| Focal atrial tachycardia (N = 16) | 62.5 | 50.0 | 83.3 |
| Ventricular tachycardia (N = 16) | 75.0 | 43.8 | 70.0 |
| Atrial flutter (N = 13) | 69.2 | 61.5 | 90.0 |
| IART (N = 4) | 50.0 | 25.0 | 100.0 |
| Twin AV nodes (N = 3) | 66.7 | 66.7 | 100.0 |
aSubstrates with a primary unsuccessful procedure not repeatedly targeted were excluded.
AVNRT, atrioventricular nodal reentry tachycardia; IART, intra-atrial reentry tachycardia.
| Substrate . | Acute success of the primary procedure (%) . | Long-term success of the primary procedure (%) . | Cumulative efficacy (%)a . |
|---|---|---|---|
| All substrates (N = 716) | 89.1 | 77.2 | 96.3 |
| Early period (1993–2005, N = 421) | 86.6 | 72.7 | 95.3 |
| Recent period (2006–10, N = 295) | 92.8 | 81.4 | 97.8 |
| P = (early vs. recent period) | 0.077 | 0.040 | |
| Accessory pathways (N = 439) | 87.2 | 77.7 | 97.9 |
| Early period (1993–2005, N = 269) | 84.6 | 74.5 | 97.0 |
| Recent period (2006–10, N = 170) | 91.1 | 83.0 | 98.7 |
| P = (early vs. recent period) | 0.073 | 0.033 | |
| AVNRT (N = 205) | 98.5 | 84.4 | 95.8 |
| Early period (1993–2005, N = 109) | 98.2 | 78.9 | 92.8 |
| Recent period (2006–10, N = 96) | 98.9 | 90.6 | 98.9 |
| P = (early vs. recent period) | 0.912 | 0.034 | |
| Mahaim tachycardia (N = 20) | 90.0 | 65.0 | 88.2 |
| Focal atrial tachycardia (N = 16) | 62.5 | 50.0 | 83.3 |
| Ventricular tachycardia (N = 16) | 75.0 | 43.8 | 70.0 |
| Atrial flutter (N = 13) | 69.2 | 61.5 | 90.0 |
| IART (N = 4) | 50.0 | 25.0 | 100.0 |
| Twin AV nodes (N = 3) | 66.7 | 66.7 | 100.0 |
| Substrate . | Acute success of the primary procedure (%) . | Long-term success of the primary procedure (%) . | Cumulative efficacy (%)a . |
|---|---|---|---|
| All substrates (N = 716) | 89.1 | 77.2 | 96.3 |
| Early period (1993–2005, N = 421) | 86.6 | 72.7 | 95.3 |
| Recent period (2006–10, N = 295) | 92.8 | 81.4 | 97.8 |
| P = (early vs. recent period) | 0.077 | 0.040 | |
| Accessory pathways (N = 439) | 87.2 | 77.7 | 97.9 |
| Early period (1993–2005, N = 269) | 84.6 | 74.5 | 97.0 |
| Recent period (2006–10, N = 170) | 91.1 | 83.0 | 98.7 |
| P = (early vs. recent period) | 0.073 | 0.033 | |
| AVNRT (N = 205) | 98.5 | 84.4 | 95.8 |
| Early period (1993–2005, N = 109) | 98.2 | 78.9 | 92.8 |
| Recent period (2006–10, N = 96) | 98.9 | 90.6 | 98.9 |
| P = (early vs. recent period) | 0.912 | 0.034 | |
| Mahaim tachycardia (N = 20) | 90.0 | 65.0 | 88.2 |
| Focal atrial tachycardia (N = 16) | 62.5 | 50.0 | 83.3 |
| Ventricular tachycardia (N = 16) | 75.0 | 43.8 | 70.0 |
| Atrial flutter (N = 13) | 69.2 | 61.5 | 90.0 |
| IART (N = 4) | 50.0 | 25.0 | 100.0 |
| Twin AV nodes (N = 3) | 66.7 | 66.7 | 100.0 |
aSubstrates with a primary unsuccessful procedure not repeatedly targeted were excluded.
AVNRT, atrioventricular nodal reentry tachycardia; IART, intra-atrial reentry tachycardia.
The acute/long-term success of the primary procedure for specific accessory pathway localizations is shown in Table 3. In the subgroup of left-sided accessory pathways targeted exclusively by the retrograde approach (N = 217), acute/long-term success of the primary procedure was 94.3/85.6%. The acute/long-term success of the primary procedure for asymptomatic (93.5/85.9%) and symptomatic (88.5/78.6%) manifest accessory pathway did not differ significantly (P = 0.158/0.116). In a subgroup of patients with congenital heart defects (N = 40), a total of 65 substrates were ablated. Major indications were accessory pathways (43 of 65 substrates) with an acute/long-term RFCA success inferior to patients with a structurally normal heart (Table 4). There was no difference in the acute/long-term success for accessory pathways and AVNRT between the two high-volume centres (Table 5).
Acute/long-term success of RFCA for specific localizations of accessory pathways
| Success (%) . | Left-free wall (N = 231) . | Septal (N = 78) . | Right-free wall (N = 130) . | P overall . | P (left-free wall vs. septal) . | P (left-free wall vs. right-free wall) . |
|---|---|---|---|---|---|---|
| Acute | 91.3 | 76.9 | 92.2 | <0.001 | 0.002 | NS |
| Long-term | 83.1 | 62.8 | 72.2 | <0.001 | <0.001 | 0.022 |
| Success (%) . | Left-free wall (N = 231) . | Septal (N = 78) . | Right-free wall (N = 130) . | P overall . | P (left-free wall vs. septal) . | P (left-free wall vs. right-free wall) . |
|---|---|---|---|---|---|---|
| Acute | 91.3 | 76.9 | 92.2 | <0.001 | 0.002 | NS |
| Long-term | 83.1 | 62.8 | 72.2 | <0.001 | <0.001 | 0.022 |
Acute/long-term success of RFCA for specific localizations of accessory pathways
| Success (%) . | Left-free wall (N = 231) . | Septal (N = 78) . | Right-free wall (N = 130) . | P overall . | P (left-free wall vs. septal) . | P (left-free wall vs. right-free wall) . |
|---|---|---|---|---|---|---|
| Acute | 91.3 | 76.9 | 92.2 | <0.001 | 0.002 | NS |
| Long-term | 83.1 | 62.8 | 72.2 | <0.001 | <0.001 | 0.022 |
| Success (%) . | Left-free wall (N = 231) . | Septal (N = 78) . | Right-free wall (N = 130) . | P overall . | P (left-free wall vs. septal) . | P (left-free wall vs. right-free wall) . |
|---|---|---|---|---|---|---|
| Acute | 91.3 | 76.9 | 92.2 | <0.001 | 0.002 | NS |
| Long-term | 83.1 | 62.8 | 72.2 | <0.001 | <0.001 | 0.022 |
Acute/long-term success of accessory pathway ablation in patients with congenital heart disease
| Success (%) . | Congenital heart disease (N = 43) . | Structurally normal heart (N = 396) . | P value . |
|---|---|---|---|
| Acute | 72.4 | 88.3 | 0.029 |
| Long-term | 62.0 | 78.8 | 0.063 |
| Success (%) . | Congenital heart disease (N = 43) . | Structurally normal heart (N = 396) . | P value . |
|---|---|---|---|
| Acute | 72.4 | 88.3 | 0.029 |
| Long-term | 62.0 | 78.8 | 0.063 |
Acute/long-term success of accessory pathway ablation in patients with congenital heart disease
| Success (%) . | Congenital heart disease (N = 43) . | Structurally normal heart (N = 396) . | P value . |
|---|---|---|---|
| Acute | 72.4 | 88.3 | 0.029 |
| Long-term | 62.0 | 78.8 | 0.063 |
| Success (%) . | Congenital heart disease (N = 43) . | Structurally normal heart (N = 396) . | P value . |
|---|---|---|---|
| Acute | 72.4 | 88.3 | 0.029 |
| Long-term | 62.0 | 78.8 | 0.063 |
Acute/long-term success of accessory pathway and AVNRT ablation in two high-volume centres
| Success (%) . | Centre Ia (acute/long-term success) (%) . | Centre IIb (acute/long-term success) (%) . | P value . |
|---|---|---|---|
| Accessory pathways (N = 431) | 86.6/76.4 | 88.4/79.4 | 0.700/0.567 |
| AVNRT (N = 202) | 99.0/86.9 | 98.0/80.8 | 0.972/0.400 |
| Success (%) . | Centre Ia (acute/long-term success) (%) . | Centre IIb (acute/long-term success) (%) . | P value . |
|---|---|---|---|
| Accessory pathways (N = 431) | 86.6/76.4 | 88.4/79.4 | 0.700/0.567 |
| AVNRT (N = 202) | 99.0/86.9 | 98.0/80.8 | 0.972/0.400 |
AVNRT, atrioventricular nodal reentry tachycardia.
aChildren's Heart Centre, Prague.
bPaediatric Cardiology, Brno.
Acute/long-term success of accessory pathway and AVNRT ablation in two high-volume centres
| Success (%) . | Centre Ia (acute/long-term success) (%) . | Centre IIb (acute/long-term success) (%) . | P value . |
|---|---|---|---|
| Accessory pathways (N = 431) | 86.6/76.4 | 88.4/79.4 | 0.700/0.567 |
| AVNRT (N = 202) | 99.0/86.9 | 98.0/80.8 | 0.972/0.400 |
| Success (%) . | Centre Ia (acute/long-term success) (%) . | Centre IIb (acute/long-term success) (%) . | P value . |
|---|---|---|---|
| Accessory pathways (N = 431) | 86.6/76.4 | 88.4/79.4 | 0.700/0.567 |
| AVNRT (N = 202) | 99.0/86.9 | 98.0/80.8 | 0.972/0.400 |
AVNRT, atrioventricular nodal reentry tachycardia.
aChildren's Heart Centre, Prague.
bPaediatric Cardiology, Brno.
Procedure and fluoroscopy time
If comparing the early and recent ablation era, the median procedure and fluoroscopy time decreased from 154 to 105 min and from 24 to 14 min (procedures with non-fluoroscopic navigation were excluded), respectively (P < 0.001 for both). After routine implementation of non-fluoroscopic navigation (2010, last 54 procedures) in one of the centres, there was a further decrease in the fluoroscopy time from a median of 14.1–4.2 min (P < 0.001) as compared with procedures performed exclusively with fluoroscopic navigation in the same centre between 2005 and 2009 (N = 98).
Complications
Serious complications occurred in nine patients (1.4%). Complete AV block was induced in 3 patients: 2 of 205 with AVNRT (1.0%) and 1 of 152 patient with a septal accessory pathway (0.7%). The block was transient in one AVNRT case and permanent and requiring pacemaker implantation in the remaining two patients. Neurological complications occurred in two patients: peroneal nerve palsy in one and a central neurological lesion with complete recovery as an anaesthesia complication in another one. Femoral artery pseudoaneurysm and rupture requiring surgical revision occurred in three and one patient, respectively. All patients aged <6 years underwent RFCA without complications. There were no complications in the group of asymptomatic WPW pre-excitation.
Discussion
In total, ∼1% of all catheter ablations are performed in children.9 The procedure has become first-line therapy in the majority of patients with SVT starting from the age of 5–6 years, leading to symptom elimination and quality-of-life improvement.10 As current Class IIA indication, it is considered to be an alternative to successfull antiarrhythmic drug therapy in children >5 years of age.11 It can be performed with reasonable success and safety in smaller children with drug-refractory arrhythmias and/or the presence of significant clinical symptoms,12 high risk for haemodynamic compromise during tachycardia,9 and the risk for tachycardia-induced cardiomyopathy.13
The aim of the present study was to analyse population-based data on the results of RFCA from one country to exclude potential bias introduced by single- or multi-centre studies2 treating patients from an undefined territory. Such data are so far lacking. Given the uniform well developed and centralized nation-wide system of care for paediatric patients with heart disease in the Czech Republic with a widespread use of the current indication criteria for RFCA, the reported incidence (in the recent ablation period) of 0.049 ablation procedure per 1000 children <18 years of age is probably approaching the steady state. This may serve as important information to predict resource utilization while planning delivery of this highly specialized care.
In alignment with other paediatric reports, the accessory pathways and AVNRT were the most common arhythmogenic substrates.1,3,14 The incidence of structural congenital heart disease was also comparable.1,7 The acute/long-term success rate of RFCA in our study corresponds to other paediatric reports.1,3,4 In patients with accessory pathways, higher efficacy has been noted for the left-free wall than right-free wall2 and septal locations as well as in the absence of structural heart disease.1,5,15 An increase in success rates and decrease in the procedure and fluoroscopy time has been noted if comparing the early and recent ablation periods reflecting a significant learning curve. Small children (particularly <15 kg) are generally at greater risk for the procedural complications,9,16,17 although favourable outcomes and low complication rates in small children after RFCA,18 even in those with less frequent substrates,19 have been reported. No complications were observed in our limited group of patients <6 years of age. However, according to current guidelines, catheter ablation in small children should be still preferentially reserved for those with significant symptoms and life-threatening arrhythmias refractory to antiarrhythmic drugs.11,20 Low number of patients ablated at the age <6 years in this population-based study confirms indirectly the rarity of such an indication if an extensive antiarrhythmic drug usage is applied in this patient cohort.
As previously reported, the use of non-fluoroscopic navigation is associated with a significant reduction of the fluoroscopy time without a compromise in safety and efficacy of the procedure.21–24 A non-fluoroscopic navigation system (LocaLisa®) was routinely used in combination with fluoroscopy in one of the centres starting from 2010 for the following arrhythmogenic substrates: accessory pathways, AVNRT, Mahaim tachycardia, and atrial flutter. The ‘ALARA’ (As Low As Reasonably Achievable) safety radiation principle25 was applied. This led to a significant decrease in the fluoroscopy time to a median value of 4.2 min. Exclusive use of a non-fluoroscopic navigation has been shown to be feasible for at least a subset of paediatric patients,22 but has not been applied in our population. Further studies are needed to define its safety. The CARTO system found limited use in the studied population because of unavailability in the two larger paediatric centres. Patients with complex substrates (ventricular tachycardia, intra-atrial reentry tachycardia) were preferentially referred to the third mainly adult centre with CARTO availability.
The number of complication was low in our study and corresponds to other paediatric reports on RFCA.1,3,18 Pacemaker implantation due to persistent complete AV block was necessary in two patients with a septal accessory pathway and AVNRT, respectively. In several reports, cryoenergy was used in children to minimize the risk of AV block.6,26,27 Given the low incidence of AV block in AVNRT ablation using RFCA, statistically significant difference between radiofrequency ablation and cryoablation is not likely to be reported in any single study and we still consider RF ablation to be safe and feasible in the vast majority of patients with accessory pathways and AVNRT. However, cryoablation may be preferred in individual cases considered to be at higher risk to limit the incidence of AV block to virtually zero.27,28 Cryoablation may be also used on an individual basis to limit the coronary artery injury (patients with Ebstein's anomaly, ablations inside the coronary sinus).29–31
Limitations
Retrospective characteristics of the study and some variation in technique of RFCA (including the anaesthesia management) among the three centres providing paediatric catheter ablations were the main limitations of the study. Coronary angiography was not routinely performed to exclude potential RF energy-induced coronary artery lesions.14,32 However, none of the patients showed any signs of myocardial ischaemia during the post-procedural non-invasive evaluation. The introduction of the non-fluoroscopic navigation in one of the centres in the last year of the study was associated with shorter follow-up of patients undergoing this type of procedure.
Conclusion
This population-based study could replicate data from previous single- or multi-centre reports confirming RFCA as a safe method of arrhythmia treatment in children with long-term cumulative efficacy exceeding 90% and a highly significant decrease in the procedure and fluoroscopy time during the study period. The need for RFCA can be estimated at ∼0.05/1000 children <18 years using current indication criteria giving important information to predict resource utilization while planning delivery of this highly specialized care.
Funding
This study was supported by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic).
Conflict of interest: none declared.



