-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Scaglione, Domenico Caponi, Elisa Ebrille, Paolo Di Donna, Francesca Di Clemente, Alberto Battaglia, Cristina Raimondo, Manuela Appendino, Fiorenzo Gaita, Very long-term results of electroanatomic-guided radiofrequency ablation of atrial arrhythmias in patients with surgically corrected atrial septal defect, EP Europace, Volume 16, Issue 12, December 2014, Pages 1800–1807, https://doi.org/10.1093/europace/euu076
Close - Share Icon Share
Abstract
Atrial tachycardias are common after repair of atrial septal defect (ASD). Although ablation has shown promising results in the short and mid-term follow-up, little data regarding the very long-term success exist. Our aim was to assess very long-term follow-up in patients who have undergone electroanatomic-guided radiofrequency (RF) ablation of late-onset atrial arrhythmias after ASD surgery.
Forty-six consecutive patients with surgically repaired ASD were referred for atrial tachycardia ablation. Electrophysiological (EP) study and ablation procedure with the aid of an electroanatomic mapping (EAM) system were performed. Mean age was 49 ± 13 years (females 61%). The presenting arrhythmias were typical atrial flutter (48%), atypical atrial flutter (35%), and atrial tachycardia (17%). In 41% of patients, atrial fibrillation was also present. The EP study showed a right atrial macroreentrant circuit in all the patients. In 12 of 46 (26%), the circuit was localized in the cavo-tricuspid isthmus, whereas in the remaining 34 patients (74%) was atriotomy-dependent. Acute success was 100%. Clinical arrhythmia recurred in 24% of the patients. Nine patients underwent a second and two a third ablation procedure, reaching an overall efficacy of 87% (40 of 46) at a mean follow-up of 7.3 ± 3.8 years since the last procedure. With antiarrhythmic drugs the success rate increased to 96% (44 of 46). No complications occurred.
In patients with surgically corrected ASD, EAM-guided RF ablation of late-onset macroreentrant atrial arrhythmias demonstrated a high success rate in a very long-term follow-up. Therefore, RF ablation could be considered early in the management of late-onset macroreentrant atrial tachycardias.
Our study reported a very long-term follow-up (>7 years) of a quite large sample of patients with surgically repaired atrial septal defect (ASD) and late-onset atrial tachycardia who underwent radiofrequency ablation.
Results showed a very high success rate (100% acute success, 87% overall efficacy at long-term follow-up). The high success rate could be ascribed to the use of high-density electroanatomic mapping (crucial for accurately localizing the entire reentrant circuit and to design the ablation strategy) and to the preventive cavo-tricuspid isthmus ablation, even if not directly involved in the mechanism of the clinical arrhythmia.
Introduction
Atrial tachycardias are common after repair or palliation of many types of complex congenital heart disease (CHD).1 In addition, patients with simple CHD, such as an atrial septal defect (ASD), usually exhibit atrial tachycardias during late follow-up of surgical repair.2,3 The most common late-onset atrial arrhythmias in these patients are cavo-tricuspid isthmus (CTI)-dependent atrial flutter, incisional atrial reentrant tachycardia, atrial fibrillation (AF) and less commonly focal atrial tachycardia.4,5 Although some patients with incisional atrial reentrant tachycardia are minimally symptomatic, associated morbid events may include haemodynamic deterioration, thromboembolism, and death. Arrhythmia mechanisms are related to surgical incisions, atrial dilatation, and structural remodelling with conduction slowing creating the substrate for macroreentry.6–8 Efficacy of antiarrhythmic drugs (AAD) in this type of arrhythmias has been unsatisfactory.9 Conversely, some studies have shown a high acute success rate of radiofrequency (RF) catheter ablation in this patient population. Currently, little data regarding long-term clinical follow-up after ablation exist.5,10–13
The aim of our study was to evaluate a very long-term follow-up in a large population of patients who underwent RF catheter ablation guided by electroanatomic mapping (EAM) of late-onset atrial arrhythmias following surgical closure of ASD.
Methods
Study population
The study population included 46 consecutive patients with a history of surgically corrected ASD who underwent electrophysiological (EP) study and transcatheter ablation at our Institution for atrial arrhythmias refractory to at least one AAD. Each patient underwent clinical evaluation with electrocardiogram (ECG), echocardiography, and a subsequent EP study.
Electrophysiological study and ablation
All antiarrhythmic medications were withheld at least five half-lives prior to the procedure. After written informed consent was obtained, an EP study was performed using the CARTO system (Biosense Webster Inc., Diamond Bar, CA, USA).
A steerable decapolar catheter for recording and pacing was placed in the coronary sinus through a venous femoral approach in all the patients.
A Navistar catheter (Biosense Webster Inc., Diamond Bar, CA, USA) was used for mapping and ablation. In our series, ablation was carried out utilizing different ablation catheters: a 4 mm tip Navistar catheter and a 3.5 mm irrigated-tip Thermocool Navistar catheter or an 8 mm tip Navistar catheter. Radiofrequency was delivered in a temperature control mode with the 4 mm and 8 mm tip catheters (65–70°C up to 50 and 100 W, respectively); in the case of irrigated-tip catheter RF was delivered in a power control mode (30–35 W, 45°C maximum temperature with an irrigation set of 17–30 mL/min).
If atrial arrhythmias were present at the time of the EP study, 3D EAM was performed during tachycardia to clarify the mechanism of arrhythmia. In patients in sinus rhythm, atrial mapping aiming to localize the sinus node and the site of the atriotomy was performed at first. Then an atrial pacing protocol, at baseline or with infusion of isoproterenol, was carried out to induce the clinical arrhythmia. The tachycardia mechanism was then identified using a combination of entrainment mapping technique together with a 3D EAM reconstruction with the CARTO system.
Both activation and voltage maps of the 3D EAM reconstruction were evaluated to define the nature and location of arrhythmia circuit tagging surgical or spontaneous scar, fractionated signals, and double potentials. Scar was defined as the presence of a signal having amplitude <0.05 mV. The atriotomy was defined as a linear lesion with double atrial potentials separated by an isoelectrical line indicating conduction block.
In the case of focal atrial arrhythmia, the target of RF delivery was the site with the earliest bipolar atrial activation and a QS morphology of the unipolar recording. In the presence of intra-atrial reentrant arrhythmia or scar-related atrial circuit, an ablation strategy was designed to create a transmural linear lesion connecting the scar/atriotomy to an anatomical barrier (tricuspid annulus, inferior vena cava or superior vena cava) to eliminate the critical isthmuses. In case of planned RF energy delivery in the right lateral wall, the site was paced before ablation to demonstrate the absence of diaphragmatic stimulation and therefore to avoid phrenic nerve injury. If phrenic nerve capture occurred, the ablation strategy was modified. The complete course of the phrenic nerve was not assessed routinely.
Cavo-tricuspid isthmus-dependent atrial flutter ablation was performed with the following criteria: (i) in patients with typical atrial flutter as the only clinical arrhythmia CTI ablation was the sole ablative strategy; (ii) CTI ablation was not performed in patients with atypical atrial flutter in whom the ablation scheme was also able to reasonably prevent the peri-tricuspid conduction; (iii) in all the remaining patients, despite the absence of clinical documentation and inducibility of typical atrial flutter, CTI ablation was also performed in addition to other atrial lines with the aim of preventing arrhythmia in the follow-up.
In all cases, the strategy of RF delivery was chosen to avoid any sinus node injury or compartmentalization of the atrium.
After ablation, the conduction block along the CTI was validated with pacing and mapping manoeuvers as previously described,14–16 while the completeness of the other ablation lines was assessed with both differential pacing and a post-ablation EAM.
Ablation endpoint was the interruption of the tachycardia during RF delivery and the absence of inducibility of any atrial arrhythmia at the end of the procedure. After the procedure, echocardiographic and Doppler evaluations were performed to exclude pericardial effusion and to assess the atrial contractility. Enoxaparin was given as bridge therapy to warfarin if indicated; if warfarin was not indicated enoxaparin was continued for 20 days after the discharge. Further decision on anticoagulation therapy was left to the referring cardiologist.
In the case of successful ablation, all the AAD were discontinued and the patient was discharged home.
Follow-up
Every patient underwent clinical follow-up with ambulatory visit and 24 h ECG Holter monitoring at 1, 3, 6, and 12 months, and then yearly. In case of recurrence, ECG and immediate clinical evaluation were performed.
Results
Baseline patients and clinical characteristics
Our study population consisted of 46 patients with surgically corrected ASD: 18 males and 28 females, with a mean age of 49 ± 13 years.
The mean age at the time of surgery was 25 ± 16 years. Details of patients' characteristics are reported in Table 1.
| Males/females | 18/28 |
| Patients' mean age (years) | 49 ± 13 |
| Patients' mean age at surgery (years) | 25 ± 13 |
| Defect type | |
| Ostium secundum ASD | 41/46 |
| Ostium primum ASD | 5/46 |
| Known correction modality | 28/46 |
| Autologous/synthetic patch | 17/28 |
| Continuous suture | 10/28 |
| Combined approach | 1/28 |
| Second surgical intervention | 6/46 |
| Surgical reintervention on ASD | 3/6 |
| Percutaneous closure of ASD | 1/6 |
| Botallo duct closure | 1/6 |
| Right outflow tract reconstruction | 1/6 |
| Arrhythmia's onset after surgery (years) | 19 ± 12 |
| Symptoms | 40/46 |
| Arrhythmia | |
| Typical atrial flutter | 22/46 |
| Atypical atrial flutter | 16/46 |
| Atrial tachycardia | 8/46 |
| Males/females | 18/28 |
| Patients' mean age (years) | 49 ± 13 |
| Patients' mean age at surgery (years) | 25 ± 13 |
| Defect type | |
| Ostium secundum ASD | 41/46 |
| Ostium primum ASD | 5/46 |
| Known correction modality | 28/46 |
| Autologous/synthetic patch | 17/28 |
| Continuous suture | 10/28 |
| Combined approach | 1/28 |
| Second surgical intervention | 6/46 |
| Surgical reintervention on ASD | 3/6 |
| Percutaneous closure of ASD | 1/6 |
| Botallo duct closure | 1/6 |
| Right outflow tract reconstruction | 1/6 |
| Arrhythmia's onset after surgery (years) | 19 ± 12 |
| Symptoms | 40/46 |
| Arrhythmia | |
| Typical atrial flutter | 22/46 |
| Atypical atrial flutter | 16/46 |
| Atrial tachycardia | 8/46 |
| Males/females | 18/28 |
| Patients' mean age (years) | 49 ± 13 |
| Patients' mean age at surgery (years) | 25 ± 13 |
| Defect type | |
| Ostium secundum ASD | 41/46 |
| Ostium primum ASD | 5/46 |
| Known correction modality | 28/46 |
| Autologous/synthetic patch | 17/28 |
| Continuous suture | 10/28 |
| Combined approach | 1/28 |
| Second surgical intervention | 6/46 |
| Surgical reintervention on ASD | 3/6 |
| Percutaneous closure of ASD | 1/6 |
| Botallo duct closure | 1/6 |
| Right outflow tract reconstruction | 1/6 |
| Arrhythmia's onset after surgery (years) | 19 ± 12 |
| Symptoms | 40/46 |
| Arrhythmia | |
| Typical atrial flutter | 22/46 |
| Atypical atrial flutter | 16/46 |
| Atrial tachycardia | 8/46 |
| Males/females | 18/28 |
| Patients' mean age (years) | 49 ± 13 |
| Patients' mean age at surgery (years) | 25 ± 13 |
| Defect type | |
| Ostium secundum ASD | 41/46 |
| Ostium primum ASD | 5/46 |
| Known correction modality | 28/46 |
| Autologous/synthetic patch | 17/28 |
| Continuous suture | 10/28 |
| Combined approach | 1/28 |
| Second surgical intervention | 6/46 |
| Surgical reintervention on ASD | 3/6 |
| Percutaneous closure of ASD | 1/6 |
| Botallo duct closure | 1/6 |
| Right outflow tract reconstruction | 1/6 |
| Arrhythmia's onset after surgery (years) | 19 ± 12 |
| Symptoms | 40/46 |
| Arrhythmia | |
| Typical atrial flutter | 22/46 |
| Atypical atrial flutter | 16/46 |
| Atrial tachycardia | 8/46 |
The average time of onset of atrial arrhythmia after surgery was 19 ± 12 years. In 13% of the patients, the arrhythmia was an incidental finding, while 87% were symptomatic. The most common presenting symptoms were palpitations (75%), dyspnoea (2.5%), palpitations associated with dyspnoea (20%), and syncope (2.5%). At least one atrial arrhythmia was documented in every patient: in 48% of the patients, the ECG showed a typical atrial flutter, 35% showed an atypical atrial flutter, and 17% an atrial tachycardia. Non-sustained AF was associated with the other arrhythmias in 41% of patients.
Procedural and mapping characteristics
Mean duration of the ablation procedure was 110 ± 30 min. Mean X-ray exposure was 30 ± 9 min. In 46% of the patients, the clinical arrhythmia was present at the time of the EP study, whereas 54% were in sinus rhythm and the clinical arrhythmia was induced. Mean right atrium volume was 163 ± 74 mL. The EAM showed a scar or an area of well-separated double atrial potentials indicating a conduction block compatible with the atriotomy incision localized in the right lateral wall in the 63% of the cases (Figure 1), in the posterior wall between superior and inferior vena cava in the 28% of the cases and in the right atrium inferolateral wall (with the inferior part of it reaching the inferior vena cava) in 9% of the cases.
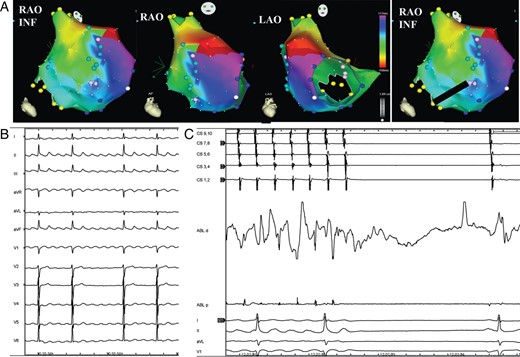
(A) Electroanatomic mapping of macroreentrant right atrial tachycardia. The activation map shows an atriotomy-dependent reentrant circuit. Pale-blue tags indicate the atriotomy site at the lateral wall. The colour code shows the wavefront propagation around the atriotomy site. The red colour indicates the earliest while the purple one the latest wavefront activation. The red bar indicates the ‘early meets late’ automatic interpolation. The black line on RAO INF view is the linear ablation extending from the inferior part of the atriotomy to the inferior vena cava (IVC) with the aim of eliminating the inferior isthmus of conduction. RAO INF, right anterior oblique view from an inferior aspect; RAO, right anterior oblique view; LAO, left anterior oblique view. (B) Surface ECG of the clinical arrhythmia. (C) Interruption of the tachycardia with the restoration of sinus rhythm during linear ablation of the inferior isthmus of conduction. CS 1,2—CS 9,10: bipolar recordings from coronary sinus catheter; ABL d and p: bipolar recordings from distal tip and proximal pair of electrodes of the ablation catheter; I-II-aVL-V1: Surface ECG recordings.
In every patient, the EAM during arrhythmia showed the presence of right atrial macroreentrant circuits only; no focal arrhythmia was observed. The reentrant circuit was found to be CTI dependent in 12 of 46 patients (26%). In the remaining 34 patients (74%), the atriotomy was crucial to create the substrate for the reentrant circuit: the critical isthmus was located from the atriotomy to the superior vena cava in 5 of 34 (15%) (Figure 2), from the atriotomy to the tricuspid valve in 5 of 34 (15%), and from the atriotomy to both superior and/or inferior vena cava in 24 of 34 (70%) (Figure 3). In four patients, phrenic nerve capture from the inferolateral right atrial wall assessed before RF delivery led to change in the planned ablation site.
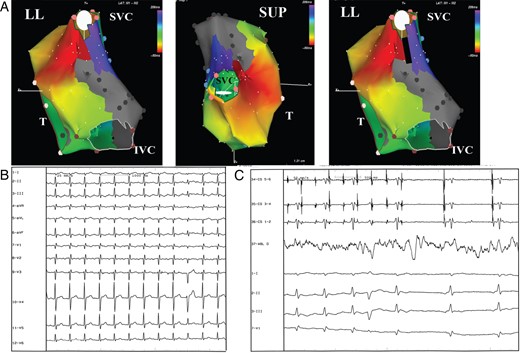
(A) Electroanatomic mapping of macroreentrant right atrial tachycardia. The activation map shows a reentrant circuit around the superior vena cava (SVC). The atriotomy has been performed longitudinally suturing directly the atrial septal defect and extending down to the IVC through the posterior wall. This atriotomy/scar is crucial in creating the isthmus critical for the occurrence of the arrhythmia. The color code shows a wavefront propagation around the atriotomy site. The red bar indicates the ‘early meets late’ automatic interpolation. Gray area indicates scar tissue. The black line indicates the site of the linear ablation extending from the superior part of the atriotomy to the SVC with the aim of eliminating the critical isthmus of conduction. LL, left lateral view; SUP, superior view; T, tricuspid ring. (B) Surface ECG of the clinical arrhythmia. (C) Tachycardia interruption during linear ablation of the critical isthmus of conduction. CS 1-2, 5-6: bipolar recordings from coronary sinus catheter; ABL d: bipolar recordings from distal tip of the ablation catheter; I–II–III–V1: Surface ECG recordings.
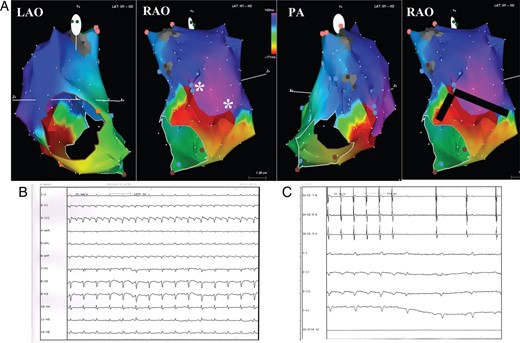
(A) Electroanatomic mapping of macroreentrant right atrial tachycardia. The activation map shows a figure of ‘8’ reentrant circuit around two pivot points (*) in the right lateral wall. The atriotomy has been performed longitudinally in superior/posterior intercaval line and lateral right atrial wall. It presents some conduction gaps creating the electroanatomic substrate for the figure of ‘8’ circuit. The colour code shows the wavefront propagation during tachycardia. The red colour indicates the earliest while the purple one the latest wavefront activation. The red bar indicates the ‘early meets late’ automatic interpolation. Gray area indicates scar tissue. The black line indicates the site of the linear ablation extending from the pivot points to the lateral tricuspid ring and to the IVC with the aim of eliminating the isthmus of conduction. LAO, left anterior oblique view; RAO, right anterior oblique view; PA: postero-anterior oblique view. (B) Surface ECG of the clinical arrhythmia. (C) Tachycardia interruption during linear ablation extending from the pivot points to the lateral tricuspid ring and to the IVC with the aim of eliminating the isthmus of conduction. CS 3-4, 7-8: bipolar recordings from coronary sinus catheter; I–II–III–V1: Surface ECG recordings.
The pattern of the arrhythmia on the surface ECG did not predict the localization of the reentrant circuit found with the EAM. For example, an ECG pattern compatible with isthmus-dependent atrial flutter was actually associated with an atriotomy-dependent reentrant mechanism in some cases (Figure 3). In the 12 patients with clinical typical atrial flutter, the ablation of the CTI was successful in all the patients. Conversely, in all patients with atriotomy-dependent tachycardia, the application of RF eliminating the conduction gaps from the atriotomy to the closest non-conductive structure was able to interrupt the arrhythmia and restoring stable sinus rhythm (Figures 1–3).
At the end of ablation, the EP study showed the re-induction of a sustained clinical arrhythmia in six patients. A repeat EAM demonstrated a conduction gap in the linear lesions previously created in all the six patients. The closure of the conduction gaps was able to interrupt the arrhythmia in all but two patients; in those the tachycardia degenerated in AF requiring electrical cardioversion to restore sinus rhythm. No major complications occurred.
Follow-up
After the first ablation procedure, 35 out of 46 patients (76%) remained free from their clinical arrhythmia.
In 11 of 46 (24%) patients, an atrial arrhythmia recurred (median elapsed time between the first ablation procedure and the recurrence was 15 months, range 2–93): three patients had typical atrial flutter, six patients atypical atrial flutter, and two patients AF. Nine out of 11 patients underwent a second EP evaluation (median elapsed time between the first and the second procedure was 31 months, range 6–117). Two patients, one with AF and one with atypical atrial flutter, denied the consent to a second EP study and the arrhythmia persisted.
Second electrophysiological study
The three patients with typical atrial flutter underwent a successful CTI ablation. Moreover, in two of them a repeat activation map during sinus rhythm demonstrated also a conduction gap in the linear lesion from the inferior vena cava to the atriotomy in one case and from the atriotomy to both the inferior and the superior vena cava in the other one. The conduction gaps were successfully eliminated with RF energy application. A left atrial procedure of pulmonary vein isolation combined to mitral isthmus ablation was successfully performed in the patient with AF.
Among the five patients with recurrent atypical atrial flutter, the second EP study showed the following results: in one patient no conduction gaps were found, a typical atrial flutter was induced and a CTI ablation was successfully performed. In another patient, a right atrium macroreentrant circuit was demonstrated at the EP study with a conduction gap in the previously created linear lesion. The closure of the gap successfully interrupted the arrhythmia and stable sinus rhythm was restored. In two patients, the macroreentrant circuit was in the left atrium: one patient denied the consent to transeptal puncture so sinus rhythm was restored by means of electrical cardioversion. The patient remained free of arrhythmias on Class IC AAD during the follow-up. In the second patient, the EAM showed a macroreentrant circuit around the mitral isthmus. Left isthmus ablation was ineffective in interrupting the arrhythmia; the patient was discharged home on Class IC AAD. In the last patient with atypical atrial flutter recurrence, the arrhythmia was not inducible at the EP study. During the follow-up, another episode of paroxysmal atypical atrial flutter occurred and the patient was started on Class IC AAD, maintaining stable sinus rhythm since then.
Third electrophysiological study
Two patients, who underwent a successful second ablation procedure, experienced a third atypical atrial flutter recurrence. The third EP study showed a conduction gap along the line previously created in one patient. Sinus rhythm was restored closing the conduction gap. The second patient, who also suffered from paroxysmal AF, refused to undergo a third EP study and was put on Class IC AAD. No more recurrences were documented during the follow-up.
Considering the first procedure, the success rate was 76% (35 of 46). The success rate with the addition of a second and, in two cases, a third ablation procedure increased to 87% (40 of 46) at a mean follow-up of 7.3 ± 3.8 years since the last procedure. With the addition of AAD, 96% (44 of 46) of success rate was achieved (Table 2).
No complications or other adverse events were observed during the follow-up. One patient in sinus rhythm and initial sick sinus syndrome underwent pacemaker implantation 4 months after ablation for progression of the sinus node dysfunction unrelated to the procedure.
Discussion
The improvement in surgical techniques for patients with CHD resulted in significant life prolongation. As these patients reach adulthood, they become highly susceptible to late complications associated with the reparative surgery, including atrial arrhythmias.1,17 These arrhythmias generally include CTI-dependent atrial flutter, incisional atrial reentrant tachycardia, AF, and less commonly focal atrial tachycardia.4,5 These arrhythmias generally occur ≥10 years after surgery and can develop even in patients with simple CHD such as ASD.2,3 The pharmacological treatment of these arrhythmias in patients with surgically corrected ASD has been unsatisfactory. On the other side, in the literature RF ablation has shown to be very effective in the short or mid-term follow-up. Few data exist regarding the long-term follow-up results of late-onset arrhythmia in patients who underwent ASD repair.5,9–13
Based on these considerations we evaluated the role and the success rate of RF ablation of late-onset atrial arrhythmias after surgical repair of an ASD.
In our cohort of patients, the EP study showed that in the majority of the cases the mechanism of the arrhythmia was a reentrant circuit localized around the atriotomy. In 85% of them, the reentrant circuit extended from the atriotomy to the superior and/or inferior vena cava. Applying RF energy to create a linear lesion from the atriotomy to the closest anatomical barrier was acutely successful in restoring sinus rhythm in 100% of our patient cohort. This high success rate was achieved with the aid of a precise EAM. In fact, patients with macroreentrant right atrial tachycardia following atriotomy do not only have a discrete linear scar at the site of the atriotomy surrounded by relatively normal atrial myocardium but, most often, these patients have large areas of markedly reduced voltages in the atrium. The classic mapping technique, utilizing the combination of an electrogram exhibiting a mid-diastolic potential plus entrainment pacing showing concealed fusion with a post-pacing interval equal to the tachycardia cycle length, presents some limitations in this population.18 A very high-density EAM seems, therefore, crucial for accurately localizing the entire reentrant circuit and to design the ablation strategy.19 These results are somewhat conflicting with what has been recently reported. In fact, Wasmer et al.5 showed that, in a comparable population, the most common site of reentrant circuit was localized in the CTI (69% of cases). It is difficult to explain the conflicting results of the tachycardia mapping. However, it is interesting to note that the authors used the EAM only in 24% of the cases and this fact, together with the limitations of the entrainment technique above mentioned, may help explain some of the differences. Conversely, the importance of the ablation of the CTI isthmus has been underlined also with our study. In fact, among the 34 patients with incisional tachycardia but without clinical typical atrial flutter, additional successful CTI ablation was performed in 29 of them to prevent the occurrence of the arrhythmia in the follow-up. In the remaining five patients, CTI ablation was not performed because the ablation scheme designed to eliminate the peri-incisional circuit was also able to clearly prevent the peri-tricuspid conduction. This strategy proved to be successful in the long-term. In fact, clinical typical atrial flutter recurred only in 3 of 46 (6.5%) corroborating the conclusion of the other report.5 In our experience, none of the 34 cases of incisional atrial tachycardia converted to common type atrial flutter during the procedure. Only in two patients the incisional tachycardia degenerated into AF, requiring electrical cardioversion to restore sinus rhythm. These results, possibly, may be due to our ablation strategy of performing ablation lines that were able to eliminate the peri-incisional circuit and also to prevent peri-tricuspid conduction. Secondarily, we have to consider that the majority of the patients underwent CTI ablation as part of the ablation strategy.
Our results were obtained with mean X-ray exposure of 30 ± 9 min. Our fluoroscopy time could appear quite long considering the use of an EAM system, but it must be taken into account also that the enrolment of our population extended over more than a decade. In fact, with experience and technology improvement, fluoroscopy time decreased reaching 2–3 min in the last cases.
Another important feature of our study is that no patient was lost to follow-up. In fact, since our institution is a regional referral center for electrophysiology, we have a dedicated outpatient clinic for grown-up CHD. During follow-up arrhythmias recurred in 11 patients (24%). This recurrence rate was quite high but similar to what has been reported in other series.5,13 A possible explanation could be that the creation of a long, continuous, transmural linear atrial lesion may be difficult to achieve with endocardial RF ablation;17,20 in addition, adjacent scars may allow multiple circuits to develop. Nine patients underwent a second and 2 patients a third EP study. It is interesting to note that, in the majority of patients who underwent the second and the third EP study, conduction gaps were located at the sites of previous RF applications. Applying RF energy to close the conduction gaps, the success rate of the ablation procedure increased to 87%. With the addition of AAD in four patients only, the overall efficacy reached 96% and it was maintained during a mean follow-up of 7 years. These results suggest that the primary macroreentrant circuit continued to use the same critical anatomic areas and that the recurrence of macroreentrant atrial tachycardia was likely secondary to failure of RF lesion formation rather than inadequate atrial mapping of the tachycardia.
In our series of post-ASD surgery patients, similarly to what has been previously reported,3,13,21,22 the incidence of AF associated with right atrial macrore-entry was relatively high, 41% of the patients. This finding is not surprising since patients with ASD repair also have some left atrium enlargement and recent studies have demonstrated the presence of extensive left atrium remodelling in addition to remodelling of the right atrium.7,8 Moreover, new left atrial sustained arrhythmias occurred also as recurrence in five patients despite the successful elimination of the macroreentrant right atrial tachycardia with the ablation. It seems that a different substrate causing AF in these patients should be accounted for. Radio frequency ablation was performed in two patients. It was effective in one patient with AF who underwent pulmonary vein isolation and mitral isthmus ablation.
No major complications or other adverse events related to the procedure occurred in the acute setting or during the follow-up. Even if the small sample size of the population has to be taken into account, the high safety profile of the procedure could be due to the high experience of the center and to the fact that the majority of the arrhythmias were right-sided. The high-density EAM allowed us to localize precisely the sinus node and to perform a safe procedure without harming this delicate structure. Only one patient underwent pacemaker implantation due to the progression of a pre-existing sinus node dysfunction.
Conclusion
Atrial arrhythmias post-ASD surgical closure develop late after the repair and are often refractory to medical therapy. The mechanism is usually a macroreentrant right atrial circuit involving the atriotomy in the majority of patients. Radiofrequency ablation with the aid of a high-density EAM showed very good long-term results. Empiric CTI ablation may improve the long-term success. Therefore, in these patients RF ablation could be considered early in the management of late-onset atrial tachycardias.
Conflict of interest: none declared.




