-
PDF
- Split View
-
Views
-
Cite
Cite
Stéphane Boulé, Lionel Ovart, Christelle Marquié, Edward Botcherby, Didier Klug, Claude Kouakam, François Brigadeau, Laurence Guédon-Moreau, Ludivine Wissocque, Jonathan Meurice, Dominique Lacroix, Salem Kacet, Pregnancy in women with an implantable cardioverter-defibrillator: is it safe?, EP Europace, Volume 16, Issue 11, November 2014, Pages 1587–1594, https://doi.org/10.1093/europace/euu036
Close - Share Icon Share
Abstract
To describe obstetric/neonatal and cardiac outcomes for a cohort of women carrying implantable cardioverter-defibrillators (ICDs) during pregnancy.
All women in routine follow-up at our institution for ICD implantation who became pregnant between 2006 and 2013 were included in this study. All ICDs were pre-pectoral devices with bipolar endocardial leads. Obstetric/neonatal and cardiac outcomes were assessed during pregnancy and post-partum. Twenty pregnancies were conceived by 12 women carrying ICD devices, 14 of which resulted in live births and none in maternal death. Seven of these women had structural cardiomyopathies and five had channelopathies. No device-related complications were recorded. Twelve shocks (nine transthoracic and three from ICDs) were experienced during pregnancy by two women, one of whom miscarried shortly afterwards at 4 weeks gestation. One stillbirth, three miscarriages and one termination were recorded for women with long QT syndrome, repaired tetralogy of Fallot and repaired Laubry–Pezzi syndrome, respectively. Intrauterine growth restriction, low birth weight, and neonatal hypoglycaemia were recorded in four, three, and five pregnancies, respectively.
Pregnancy had no effect on ICD operation and no evidence was found to link ICD carriage with adverse pregnancy outcomes, although one miscarriage may have been induced by ICD shock therapy. A worsening of cardiac condition occurs in specific cardiac diseases and β-blocker therapy should be continued for all women carrying ICDs in pregnancy as the benefits outweigh the risks of taking this medication.
Little is known about the outcome of women carrying implantable cardioverter-defibrillators (ICDs) during pregnancy. This retrospective study supports the hypothesis that the ICD itself should not constitute a contraindication for pregnancy, since outcomes are more related to specific cardiac disease than to the mere presence of an ICD.
One woman experienced a miscarriage after receiving two electric shocks from her ICD during an episode of ventricular fibrillation at 4 weeks gestation. This observation may challenge the commonly held belief that defibrillation shocks do no affect the foetus and potentially suggests that undesirable effects can result from ICD shock therapy in the very early stages of the pregnancy.
Introduction
In recent years, the number of individuals carrying implantable cardioverter-defibrillator (ICD) devices for inherited cardiomyopathy and congenital heart disease has increased dramatically. As a result, a number of women of reproductive age now carry such devices, which raises the question as to whether these patients are more at risk of adverse outcomes or complications during pregnancy because they carry such a device.1,2 In particular, it would be interesting to know whether the morphological and physiological changes occurring during pregnancy affect the device operation and conversely, whether ICD devices affect the obstetric outcome by their mere presence, particularly if they deliver electric shock therapy. Very little is known on the subject at present and no specific guidelines exist due to the sparsity of data in the field. A large prospective cohort study is therefore needed to address these questions fully. In the meantime, data from retrospective studies are of particular interest to describe clinical outcomes for this growing population of patients. We therefore report our experiences of obstetric/neonatal and cardiac outcomes over the past 7 years for all women followed up at our institution for ICD implantation, who conceived pregnancies during this time.
Methods
Study population
All women in routine follow-up at our institution for carrying an ICD who became pregnant between 2006 and 2013 were included in this study. All pregnancies were included, whether they resulted in live births or not. Obstetric/neonatal and cardiology data were collated retrospectively from medical records kept by our institution. From obstetric records, we noted the length of pregnancy, type of labour (spontaneous, induced, neither), type of anaesthesia used during delivery (epidural, general, none), mode of delivery (caesarean section, vaginal), blood loss, birth weight, as well as Apgar score at 5 and 10 min for each pregnancy. From cardiology records, we noted underlying cardiac disease, indication for ICD implantation, whether implantation was for primary or secondary prophylaxis, information of medication regimes, and ICD settings for each patient.
Clinical outcomes
We also examined obstetric/neonatal as well as cardiac outcomes during each pregnancy and for the 6 months post-delivery. The specific obstetric and neonatal outcomes were miscarriage, stillbirth, intrauterine growth retardation (IUGR), pre-term labour (PTL), pre-term delivery (PTD), low birth weight (LBW), and neonatal hypoglycaemia (NHG). The cardiac outcomes were heart failure, thromboembolic events, maternal death, supraventricular and ventricular tachyarrhythmias (VAs) as well as initiation of ICD therapy (antitachycardia pacing, shocks), and device-related complications.
Implantable cardioverter-defibrillators implantation and programming
All ICDs were pre-pectoral devices with bipolar transvenous endocardial leads and a single defibrillation coil. No one was implanted with an epicardial ICD. Patients were fitted with single- or dual-chamber ICDs, according to their indication and the brands used were: Medtronic (n = 8), Saint Jude Medical (n = 4), and Biotronik (n = 1). One patient, who conceived four pregnancies, was switched from a Medtronic to a Saint Jude Medical device between her second and third pregnancies. Each ICD was custom programmed, according to the indication for implantation. Implantable cardioverter-defibrillators in patients deemed to be at risk of developing ventricular tachycardia (VT) were programmed to detect VT and all devices were programmed to detect ventricular fibrillation (VF).
For patients delivering by caesarean section, a magnet was placed over the ICD at the time of operation to avoid provocation of inappropriate shocks by diathermy equipment and other sources of electrical interference. For patients delivering vaginally, it was left to the discretion of the attending clinician as to whether the magnet was applied or not.
Results
Study population
Twenty pregnancies were conceived by 12 women carrying an ICD, with a mean age of 28 ± 5 years at the time of conception. Table 1 lists the baseline characteristics for this population. Seven of the women suffered from an underlying cardiomyopathy, namely: hypertrophic cardiomyopathy (HCM, n = 2), arrhythmogenic right ventricular cardiomyopathy (ARVC, n = 2), repaired tetralogy of Fallot (ToF, n = 1), repaired Laubry–Pezzi syndrome with a closed ventricular septal defect and mechanical aortic prosthesis implant (LPS, n = 1) as well as a repaired congenital heart disease, comprising HCM, mitral regurgitation, and an atrial septal defect, which required two surgical myomectomy procedures and a mechanical mitral prosthesis implantation in childhood followed by surgical closure of the atrial septal defect later on in life (CHD, n = 1). The remaining five women did not have structural heart disease and suffered from the following conditions: long QT syndrome (LQTS, n = 1), catecholaminergic polymorphic VT (CPVT, n = 1), and idiopathic VF (IVF, n = 3).
| Patient . | Underlying cardiac disease . | ICD prophylaxis . | Previous ICD shocks . | LVEF (%) . | Age at ICD implant (years) . | Age at first conception (years) . | Medications . | VT zones (b.p.m.) . | VF zone (b.p.m.) . | Pacing mode . | ApVp (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | IVF | Secondary | No | >60 | 36 | 36 | Nadolol | – | >240 | VVI 40 | 0 |
| #2 | ARVC | Secondary | No | >60 | 26 | 29 | Atenolol | – | >210 | VVI 40 | 0 |
| #3 | ARVC | Secondary | No | 50a | 29 | 33 | Nadolol | 140–182/182–260 | >260 | VVI 35 | 0 |
| #4 | HCM | Primary | No | >60 | 22 | 29 | Atenolol | – | >230 | DDD 50 | 30/0 |
| #5 | HCM | Secondary | No | >60 | 29 | 32 | Propanolol | 182–214 | >214 | VVI 40 | 0 |
| #6 | ToF | Secondary | No | >60 | 22 | 24 | Bisoprolol | 181–214/214–260 | >260 | VVI 35 | 0 |
| #7 | LQTS | Secondary | No | >60 | 27 | 29 | Nadolol | – | >222 | AAIR/DDDR 75 | 100/0 |
| #8 | CPVT | Primary | Yes | >60 | 16 | 24 | Nadolol | 200–250 | >250 | VVI 40 | 0 |
| #9 | IVF | Secondary | Yes | >60 | 14 | 21 | Nadolol | 190–220 | >220 | VVI 35 | 0 |
| #10 | IVF | Secondary | No | >60 | 35 | 35 | None | 180–240 | >240 | VVI 40 | 0 |
| #11 | LPS | Primary | No | >60 | 24 | 25 | warfarine | 200–250 | >250 | DDD 50 | 0/100 |
| #12 | CHD | Secondary | No | 50 | 24 | 27 | warfarine | 182–222 | >222 | DDD 60 | 40/100 |
| Patient . | Underlying cardiac disease . | ICD prophylaxis . | Previous ICD shocks . | LVEF (%) . | Age at ICD implant (years) . | Age at first conception (years) . | Medications . | VT zones (b.p.m.) . | VF zone (b.p.m.) . | Pacing mode . | ApVp (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | IVF | Secondary | No | >60 | 36 | 36 | Nadolol | – | >240 | VVI 40 | 0 |
| #2 | ARVC | Secondary | No | >60 | 26 | 29 | Atenolol | – | >210 | VVI 40 | 0 |
| #3 | ARVC | Secondary | No | 50a | 29 | 33 | Nadolol | 140–182/182–260 | >260 | VVI 35 | 0 |
| #4 | HCM | Primary | No | >60 | 22 | 29 | Atenolol | – | >230 | DDD 50 | 30/0 |
| #5 | HCM | Secondary | No | >60 | 29 | 32 | Propanolol | 182–214 | >214 | VVI 40 | 0 |
| #6 | ToF | Secondary | No | >60 | 22 | 24 | Bisoprolol | 181–214/214–260 | >260 | VVI 35 | 0 |
| #7 | LQTS | Secondary | No | >60 | 27 | 29 | Nadolol | – | >222 | AAIR/DDDR 75 | 100/0 |
| #8 | CPVT | Primary | Yes | >60 | 16 | 24 | Nadolol | 200–250 | >250 | VVI 40 | 0 |
| #9 | IVF | Secondary | Yes | >60 | 14 | 21 | Nadolol | 190–220 | >220 | VVI 35 | 0 |
| #10 | IVF | Secondary | No | >60 | 35 | 35 | None | 180–240 | >240 | VVI 40 | 0 |
| #11 | LPS | Primary | No | >60 | 24 | 25 | warfarine | 200–250 | >250 | DDD 50 | 0/100 |
| #12 | CHD | Secondary | No | 50 | 24 | 27 | warfarine | 182–222 | >222 | DDD 60 | 40/100 |
Ap, atrial pacing; ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; IVF, idiopathic ventricular fibrillation; LPS, Laubry–Pezzi syndrome; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; ToF, tetralogy of Fallot; Vp, ventricular pacing; VT, ventricular tachycardia.
aFor Patient #3, baseline LVEF was 50 %. A transient decrease in LVEF down to 35 % was noted 2 months post-partum.
| Patient . | Underlying cardiac disease . | ICD prophylaxis . | Previous ICD shocks . | LVEF (%) . | Age at ICD implant (years) . | Age at first conception (years) . | Medications . | VT zones (b.p.m.) . | VF zone (b.p.m.) . | Pacing mode . | ApVp (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | IVF | Secondary | No | >60 | 36 | 36 | Nadolol | – | >240 | VVI 40 | 0 |
| #2 | ARVC | Secondary | No | >60 | 26 | 29 | Atenolol | – | >210 | VVI 40 | 0 |
| #3 | ARVC | Secondary | No | 50a | 29 | 33 | Nadolol | 140–182/182–260 | >260 | VVI 35 | 0 |
| #4 | HCM | Primary | No | >60 | 22 | 29 | Atenolol | – | >230 | DDD 50 | 30/0 |
| #5 | HCM | Secondary | No | >60 | 29 | 32 | Propanolol | 182–214 | >214 | VVI 40 | 0 |
| #6 | ToF | Secondary | No | >60 | 22 | 24 | Bisoprolol | 181–214/214–260 | >260 | VVI 35 | 0 |
| #7 | LQTS | Secondary | No | >60 | 27 | 29 | Nadolol | – | >222 | AAIR/DDDR 75 | 100/0 |
| #8 | CPVT | Primary | Yes | >60 | 16 | 24 | Nadolol | 200–250 | >250 | VVI 40 | 0 |
| #9 | IVF | Secondary | Yes | >60 | 14 | 21 | Nadolol | 190–220 | >220 | VVI 35 | 0 |
| #10 | IVF | Secondary | No | >60 | 35 | 35 | None | 180–240 | >240 | VVI 40 | 0 |
| #11 | LPS | Primary | No | >60 | 24 | 25 | warfarine | 200–250 | >250 | DDD 50 | 0/100 |
| #12 | CHD | Secondary | No | 50 | 24 | 27 | warfarine | 182–222 | >222 | DDD 60 | 40/100 |
| Patient . | Underlying cardiac disease . | ICD prophylaxis . | Previous ICD shocks . | LVEF (%) . | Age at ICD implant (years) . | Age at first conception (years) . | Medications . | VT zones (b.p.m.) . | VF zone (b.p.m.) . | Pacing mode . | ApVp (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | IVF | Secondary | No | >60 | 36 | 36 | Nadolol | – | >240 | VVI 40 | 0 |
| #2 | ARVC | Secondary | No | >60 | 26 | 29 | Atenolol | – | >210 | VVI 40 | 0 |
| #3 | ARVC | Secondary | No | 50a | 29 | 33 | Nadolol | 140–182/182–260 | >260 | VVI 35 | 0 |
| #4 | HCM | Primary | No | >60 | 22 | 29 | Atenolol | – | >230 | DDD 50 | 30/0 |
| #5 | HCM | Secondary | No | >60 | 29 | 32 | Propanolol | 182–214 | >214 | VVI 40 | 0 |
| #6 | ToF | Secondary | No | >60 | 22 | 24 | Bisoprolol | 181–214/214–260 | >260 | VVI 35 | 0 |
| #7 | LQTS | Secondary | No | >60 | 27 | 29 | Nadolol | – | >222 | AAIR/DDDR 75 | 100/0 |
| #8 | CPVT | Primary | Yes | >60 | 16 | 24 | Nadolol | 200–250 | >250 | VVI 40 | 0 |
| #9 | IVF | Secondary | Yes | >60 | 14 | 21 | Nadolol | 190–220 | >220 | VVI 35 | 0 |
| #10 | IVF | Secondary | No | >60 | 35 | 35 | None | 180–240 | >240 | VVI 40 | 0 |
| #11 | LPS | Primary | No | >60 | 24 | 25 | warfarine | 200–250 | >250 | DDD 50 | 0/100 |
| #12 | CHD | Secondary | No | 50 | 24 | 27 | warfarine | 182–222 | >222 | DDD 60 | 40/100 |
Ap, atrial pacing; ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; CPVT, catecholaminergic polymorphic ventricular tachycardia; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; IVF, idiopathic ventricular fibrillation; LPS, Laubry–Pezzi syndrome; LQTS, long QT syndrome; LVEF, left ventricular ejection fraction; ToF, tetralogy of Fallot; Vp, ventricular pacing; VT, ventricular tachycardia.
aFor Patient #3, baseline LVEF was 50 %. A transient decrease in LVEF down to 35 % was noted 2 months post-partum.
The mean age at the time of ICD implantation was 25 ± 7 years (full range: 14–35 years). In all but one patient (#1), ICD implantation was carried out prior to the start of the pregnancy, with the mean time between ICD implantation and conception being 58 ± 42 months (range: 1–124 months).
Outcomes during pregnancy and for the 6 months post-delivery
Obstetric and neonatal outcomes
Twenty pregnancies were conceived by 12 women, 8 having a single pregnancy and 4 having multiple pregnancies under ICD coverage (#1 and #7 having two pregnancies each, #9 and #6 having 4). Of the 20 pregnancies, 2 were achieved by in vitro fertilization methods (#4 and #5) and 14 (70%) resulted in live births. Four miscarriages were experienced by two of the women (#6 and #9), one occurring 7 days after an episode of VF that was terminated by shock therapy from the ICD (second pregnancy of #9). We discuss this in more detail in the next section. The remaining three miscarriages were experienced by the same patient (#6), all between 4 and 6 weeks gestation. The second pregnancy of Patient #7 (LQTS) resulted in a stillbirth at 37 weeks gestation. Subsequent ICD interrogation revealed no arrhythmia at the time of foetal demise and the parents refused an autopsy; therefore, genetic testing for LQTS was never carried out on the foetus so no cause for the death was attributed at the time. Finally, the pregnancy in Patient #11 was terminated following recommendation from a multidisciplinary discussion at 15 weeks gestation, as she had great difficulty in achieving stable anticoagulation and was therefore deemed to be at a very high risk of developing both haemorrhagic complications as well as mitral valve thrombosis. She was also deemed to be at high risk of developing obstetric and cardiac complications due to the persistence of a left ventricular outflow tract gradient. Data regarding premature labour and pre-term delivery occurrence are provided in Table 2. Twelve deliveries were performed under epidural anaesthesia and two under general anaesthesia. The mean gestational age at delivery was 37 weeks, and the mean birth weight was 2690 ± 596 g. Less than 500 mL of blood was lost during each delivery (mean = 239 ± 87 mL), so by definition no patients suffered post-partum haemorrhage. The Apgar scores at 5 and 10 min were 10 for all live births. Of the 14 live births, 5 newborns (36%) suffered from NHG, of which 4 were the offspring of mothers receiving β-blocker therapy during pregnancy. The type and dose of β-blocker used in each case were as follows: nadolol 80 mg/day (#3), atenolol 50 mg/day (#4), bisoprolol 2.5 mg/day (#6), and bisoprolol 10 mg/day (#12).
| Patient . | Underlying cardiac disease . | Age at conception (years) . | Previous gestations . | Delivery mode . | GA . | Live birth . | Obstetric/neonatal outcome . | Cardiac outcome . |
|---|---|---|---|---|---|---|---|---|
| #1 | IVF | 36 | G1P0 | VD | 37 | Yes | 9 transthoracic shocks (VF) and 1 induced ICD shock at 6 WG, NSVT at 13 WG | |
| #1 | IVF | 38 | G2P1 | VD | 37 | Yes | ||
| #2 | ARVC | 29 | G1P1 | VD | 34 | Yes | IUGR, LBW, PTL, PTD | |
| #3 | ARVC | 33 | G3P1 | CS | 37 | Yes | IUGR, LBW, NHG | Transient decrease in LVEF down to 35 % 2 months post-partum |
| #4 | HCM | 29 | G0P0 | VD | 36 | Yes | NHG | |
| #5 | HCM | 32 | G0P0 | CS | 37 | Yes | PTL | |
| #6 | ToF | 24 | G0P0 | – | – | No | Miscarriage (6 WG) | |
| #6 | ToF | 25 | G1P0 | VD | 38 | Yes | IUGR, PTL, NHG | Worsening of PI and RV dilatation post-partum |
| #6 | ToF | 28 | G2P1 | – | – | No | Miscarriage (4 WG) | |
| #6 | ToF | 28 | G3P1 | – | – | No | Miscarriage (6 WG) | |
| #7 | LQTS | 29 | G1P0 | CS | 39 | Yes | ||
| #7 | LQTS | 31 | G2P1 | CS | 37 | No | Stillbirth | |
| #8 | CPVT | 24 | G2P0 | CS | 40 | Yes | ||
| #9 | IVF | 21 | G0P0 | CS | 38 | Yes | ||
| #9 | IVF | 23 | G1P1 | – | – | No | Miscarriage following ICD shocks (4 WG) | 2 ICD shocks [1 appropriate (VF) and 1 inappropriate (T-wave oversensing)] at 4 WG |
| #9 | IVF | 24 | G2P1 | CS | 39 | Yes | ||
| #9 | IVF | 27 | G3P2 | CS | 39 | Yes | ||
| #10 | IVF | 35 | G13P11 | VD | 36 | Yes | PTL, NHG | |
| #11 | LPS | 25 | G0P0 | – | 15 | No | Medical interruption | |
| #12 | CHD | 27 | G0P0 | CS | 38 | Yes | IUGR, LBW, NHG |
| Patient . | Underlying cardiac disease . | Age at conception (years) . | Previous gestations . | Delivery mode . | GA . | Live birth . | Obstetric/neonatal outcome . | Cardiac outcome . |
|---|---|---|---|---|---|---|---|---|
| #1 | IVF | 36 | G1P0 | VD | 37 | Yes | 9 transthoracic shocks (VF) and 1 induced ICD shock at 6 WG, NSVT at 13 WG | |
| #1 | IVF | 38 | G2P1 | VD | 37 | Yes | ||
| #2 | ARVC | 29 | G1P1 | VD | 34 | Yes | IUGR, LBW, PTL, PTD | |
| #3 | ARVC | 33 | G3P1 | CS | 37 | Yes | IUGR, LBW, NHG | Transient decrease in LVEF down to 35 % 2 months post-partum |
| #4 | HCM | 29 | G0P0 | VD | 36 | Yes | NHG | |
| #5 | HCM | 32 | G0P0 | CS | 37 | Yes | PTL | |
| #6 | ToF | 24 | G0P0 | – | – | No | Miscarriage (6 WG) | |
| #6 | ToF | 25 | G1P0 | VD | 38 | Yes | IUGR, PTL, NHG | Worsening of PI and RV dilatation post-partum |
| #6 | ToF | 28 | G2P1 | – | – | No | Miscarriage (4 WG) | |
| #6 | ToF | 28 | G3P1 | – | – | No | Miscarriage (6 WG) | |
| #7 | LQTS | 29 | G1P0 | CS | 39 | Yes | ||
| #7 | LQTS | 31 | G2P1 | CS | 37 | No | Stillbirth | |
| #8 | CPVT | 24 | G2P0 | CS | 40 | Yes | ||
| #9 | IVF | 21 | G0P0 | CS | 38 | Yes | ||
| #9 | IVF | 23 | G1P1 | – | – | No | Miscarriage following ICD shocks (4 WG) | 2 ICD shocks [1 appropriate (VF) and 1 inappropriate (T-wave oversensing)] at 4 WG |
| #9 | IVF | 24 | G2P1 | CS | 39 | Yes | ||
| #9 | IVF | 27 | G3P2 | CS | 39 | Yes | ||
| #10 | IVF | 35 | G13P11 | VD | 36 | Yes | PTL, NHG | |
| #11 | LPS | 25 | G0P0 | – | 15 | No | Medical interruption | |
| #12 | CHD | 27 | G0P0 | CS | 38 | Yes | IUGR, LBW, NHG |
CHD, congenital heart disease (see the text for more details); CPVT, catecholaminergic polymorphic ventricular tachycardia; CS, caesarean section; GA, gestational age at birth; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; IUGR, intrauterine growth restriction; IVF, idiopathic ventricular fibrillation; LBW, low birth weight; LPS, Laubry–Pezzi syndrome (see the text for more details); LQTS, long QT syndrome; NHG, neonatal hypoglycaemia; NSVT, non-sustained ventricular tachycardia; PI, pulmonary insufficiency; PTD, pre-term delivery; PTL, pre-term labour; RV, right ventricle; RVAC, right ventricular arrhythmogenic cardiomyopathy; ToF, tetralogy of Fallot; VD, vaginal delivery; WG, weeks gestation.
| Patient . | Underlying cardiac disease . | Age at conception (years) . | Previous gestations . | Delivery mode . | GA . | Live birth . | Obstetric/neonatal outcome . | Cardiac outcome . |
|---|---|---|---|---|---|---|---|---|
| #1 | IVF | 36 | G1P0 | VD | 37 | Yes | 9 transthoracic shocks (VF) and 1 induced ICD shock at 6 WG, NSVT at 13 WG | |
| #1 | IVF | 38 | G2P1 | VD | 37 | Yes | ||
| #2 | ARVC | 29 | G1P1 | VD | 34 | Yes | IUGR, LBW, PTL, PTD | |
| #3 | ARVC | 33 | G3P1 | CS | 37 | Yes | IUGR, LBW, NHG | Transient decrease in LVEF down to 35 % 2 months post-partum |
| #4 | HCM | 29 | G0P0 | VD | 36 | Yes | NHG | |
| #5 | HCM | 32 | G0P0 | CS | 37 | Yes | PTL | |
| #6 | ToF | 24 | G0P0 | – | – | No | Miscarriage (6 WG) | |
| #6 | ToF | 25 | G1P0 | VD | 38 | Yes | IUGR, PTL, NHG | Worsening of PI and RV dilatation post-partum |
| #6 | ToF | 28 | G2P1 | – | – | No | Miscarriage (4 WG) | |
| #6 | ToF | 28 | G3P1 | – | – | No | Miscarriage (6 WG) | |
| #7 | LQTS | 29 | G1P0 | CS | 39 | Yes | ||
| #7 | LQTS | 31 | G2P1 | CS | 37 | No | Stillbirth | |
| #8 | CPVT | 24 | G2P0 | CS | 40 | Yes | ||
| #9 | IVF | 21 | G0P0 | CS | 38 | Yes | ||
| #9 | IVF | 23 | G1P1 | – | – | No | Miscarriage following ICD shocks (4 WG) | 2 ICD shocks [1 appropriate (VF) and 1 inappropriate (T-wave oversensing)] at 4 WG |
| #9 | IVF | 24 | G2P1 | CS | 39 | Yes | ||
| #9 | IVF | 27 | G3P2 | CS | 39 | Yes | ||
| #10 | IVF | 35 | G13P11 | VD | 36 | Yes | PTL, NHG | |
| #11 | LPS | 25 | G0P0 | – | 15 | No | Medical interruption | |
| #12 | CHD | 27 | G0P0 | CS | 38 | Yes | IUGR, LBW, NHG |
| Patient . | Underlying cardiac disease . | Age at conception (years) . | Previous gestations . | Delivery mode . | GA . | Live birth . | Obstetric/neonatal outcome . | Cardiac outcome . |
|---|---|---|---|---|---|---|---|---|
| #1 | IVF | 36 | G1P0 | VD | 37 | Yes | 9 transthoracic shocks (VF) and 1 induced ICD shock at 6 WG, NSVT at 13 WG | |
| #1 | IVF | 38 | G2P1 | VD | 37 | Yes | ||
| #2 | ARVC | 29 | G1P1 | VD | 34 | Yes | IUGR, LBW, PTL, PTD | |
| #3 | ARVC | 33 | G3P1 | CS | 37 | Yes | IUGR, LBW, NHG | Transient decrease in LVEF down to 35 % 2 months post-partum |
| #4 | HCM | 29 | G0P0 | VD | 36 | Yes | NHG | |
| #5 | HCM | 32 | G0P0 | CS | 37 | Yes | PTL | |
| #6 | ToF | 24 | G0P0 | – | – | No | Miscarriage (6 WG) | |
| #6 | ToF | 25 | G1P0 | VD | 38 | Yes | IUGR, PTL, NHG | Worsening of PI and RV dilatation post-partum |
| #6 | ToF | 28 | G2P1 | – | – | No | Miscarriage (4 WG) | |
| #6 | ToF | 28 | G3P1 | – | – | No | Miscarriage (6 WG) | |
| #7 | LQTS | 29 | G1P0 | CS | 39 | Yes | ||
| #7 | LQTS | 31 | G2P1 | CS | 37 | No | Stillbirth | |
| #8 | CPVT | 24 | G2P0 | CS | 40 | Yes | ||
| #9 | IVF | 21 | G0P0 | CS | 38 | Yes | ||
| #9 | IVF | 23 | G1P1 | – | – | No | Miscarriage following ICD shocks (4 WG) | 2 ICD shocks [1 appropriate (VF) and 1 inappropriate (T-wave oversensing)] at 4 WG |
| #9 | IVF | 24 | G2P1 | CS | 39 | Yes | ||
| #9 | IVF | 27 | G3P2 | CS | 39 | Yes | ||
| #10 | IVF | 35 | G13P11 | VD | 36 | Yes | PTL, NHG | |
| #11 | LPS | 25 | G0P0 | – | 15 | No | Medical interruption | |
| #12 | CHD | 27 | G0P0 | CS | 38 | Yes | IUGR, LBW, NHG |
CHD, congenital heart disease (see the text for more details); CPVT, catecholaminergic polymorphic ventricular tachycardia; CS, caesarean section; GA, gestational age at birth; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; IUGR, intrauterine growth restriction; IVF, idiopathic ventricular fibrillation; LBW, low birth weight; LPS, Laubry–Pezzi syndrome (see the text for more details); LQTS, long QT syndrome; NHG, neonatal hypoglycaemia; NSVT, non-sustained ventricular tachycardia; PI, pulmonary insufficiency; PTD, pre-term delivery; PTL, pre-term labour; RV, right ventricle; RVAC, right ventricular arrhythmogenic cardiomyopathy; ToF, tetralogy of Fallot; VD, vaginal delivery; WG, weeks gestation.
Cardiac outcomes
As indicated in Table 2, two patients (#1 and #9) experienced VAs during pregnancy and overall 12 shocks were delivered to control these episodes. Patient #1, who was not carrying an ICD at the start of pregnancy, was first admitted for an out-of-hospital cardiac arrest. Nine direct-current (DC) transthoracic defibrillation shocks were required before arrival at hospital to control the electrical storm she sustained. Subsequent investigation did not reveal any underlying structural cardiomyopathy, so a diagnosis of IVF was eventually made. The pregnancy was only diagnosed while in intensive care and gestational age was estimated to be 6 weeks on ultrasound. A single-chamber ICD was implanted before discharge, with particular attention being paid to minimizing maternal fluoroscopic exposure by placing a lead apron over the mother's abdomen. Defibrillation testing was performed with an energy of 20 J. No adverse foetal events were observed during implantation of this ICD or while delivering the test shock.
Patient #9 received a shock in response to an episode of spontaneous VF (Figure 1), at 4 weeks gestation during her second pregnancy, which was brought on by emotional distress during a domestic dispute. The patient later admitted that she had not been complying with her β-blocker medication at the time, and ICD interrogation revealed that the episode started with a run of sinus tachycardia at 170 b.p.m., followed by an episode of VF lasting 10 s, which was terminated by a single ICD shock with energy of 30 J. This was followed by a further run of sinus tachycardia (Figure 2), which unfortunately triggered a second inappropriate shock (30 J) moments later due to T-wave oversensing by the ICD. Miscarriage followed 7 days later and the question remains as to whether the shocks she received might have caused her miscarriage. This patient successfully carried three other pregnancies to term without complication.
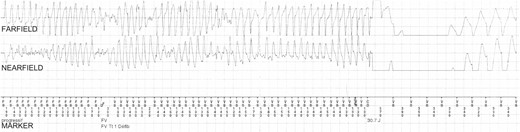
Episode of VF terminated by shock therapy at 4 weeks gestation in Patient #9. This episode of VA occurred in a woman who was 4 weeks pregnant and not complying with β-blocker treatment during a domestic dispute (i.e. in a hyperadrenergic setting). The onset of the VF (not shown) was preceded by a sinus node tachycardia. A short-coupling premature ventricular complex (with coupling interval 160 ms) triggered VF, which was terminated by a shock of 30 J. The total duration of the episode was 10 s. CD, charge delivered; CE, charge ended; FD, fibrillation detected; FS, fibrillation sense; FV, ventricular fibrillation; VS, ventricular sense.
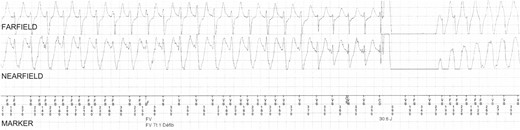
Episode of T-wave oversensing that triggered an inappropriate shock in Patient #9. This episode occurred a few seconds after the appropriate shock shown in Figure 1. Intermittent episodes of oversensing of spontaneous T-waves occurred leading to double counting during sinus rhythm. As a result, an inappropriate ICD shock (30 J) was delivered. CD, charge delivered; CE, charge ended; FD, fibrillation detected; FS, fibrillation sense; FV, ventricular fibrillation; VS, ventricular sense.
In the 6 months that followed delivery, two patients suffered from cardiac events. Patient #3, who suffered from ARVC secondary to a mutation in the desmoplakin gene, was admitted 2 months post-partum with chest pain, a rise in troponin levels, and a significant reduction in LVEF (35%). Treatment was commenced with an angiotensin-converting enzyme inhibitor and 2 months later the LVEF was found to have returned to its pre-pregnancy value of 50%. Patient #6 (repaired ToF) was also admitted 6 months post-delivery for progressive right ventricular dilatation and dysfunction caused by a worsening of her pre-existent pulmonary regurgitation and ultimately had to have her pulmonary valve replaced with a bio-prosthesis.
No other patients suffered any adverse cardiac outcomes in the 6 months following delivery. There were no maternal deaths, no thromboembolic events, no supraventricular tachyarrhythmias, and no device-related complications, such as lead dislodgement, dysfunction, or thrombus for the duration of this study.
Discussion
Very little is known about the outcome for pregnant women carrying ICDs. Beside case reports,3–9 only three retrospective studies10–12 have addressed this issue to date and the main results of these are summarized in Table 3. The first by Natale et al.10 included almost exclusively (95%) patients with abdominal ICDs, and the other two by Schuler et al.11 and Miyoshi et al.12 reported outcomes of 19 pregnancies in 14 women and 6 pregnancies in 6 women with pre-pectoral ICDs, respectively. Two studies10,11 found that ICD-related complications are not uncommon and the remaining study drew the opposite conclusion.12 The impact of ICD shock delivery on the foetal outcome is also relatively undocumented in the wider literature.
| Study . | Year . | Number of pregnancies . | Number of patients . | Age—mean (full range) . | Structural heart disease—n (%) . | Secondary prophylaxis—n (%) . | Pre-pectoral ICDs—n (%) . | Endocardial leads—n (%) . | Device-related complications . | Number of women who experienced ICD shocks . | Foetal consequences of ICD shocks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natale et al.10 | 1997 | 51 | 44 | 30 (14–36) | 14 (32) | 44 (100) | 2 (5) | 14 (32) | Migration of an abdominal generator, pericarditis related to epicardial patches | 11 | None |
| Schuler et al.11 | 2012 | 19 | 14 | 33 (22–42) | 10 (71) | 5 (36) | 13 (93) | 14 (100) | ICD lead thrombus, atrial lead dysfunction | 1 | None |
| Miyoshi et al.12 | 2013 | 6 | 6 | 28 (25–33) | 3 (50) | 6 (100) | 6 (100) | 6 (100) | None | 0 | – |
| Present study | 2014 | 20 | 12 | 28 (21–38) | 7 (58) | 9 (75) | 12 (100) | 12 (100) | None | 2 | One miscarriage may have been provoked by ICD shocks (4 WG) |
| Study . | Year . | Number of pregnancies . | Number of patients . | Age—mean (full range) . | Structural heart disease—n (%) . | Secondary prophylaxis—n (%) . | Pre-pectoral ICDs—n (%) . | Endocardial leads—n (%) . | Device-related complications . | Number of women who experienced ICD shocks . | Foetal consequences of ICD shocks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natale et al.10 | 1997 | 51 | 44 | 30 (14–36) | 14 (32) | 44 (100) | 2 (5) | 14 (32) | Migration of an abdominal generator, pericarditis related to epicardial patches | 11 | None |
| Schuler et al.11 | 2012 | 19 | 14 | 33 (22–42) | 10 (71) | 5 (36) | 13 (93) | 14 (100) | ICD lead thrombus, atrial lead dysfunction | 1 | None |
| Miyoshi et al.12 | 2013 | 6 | 6 | 28 (25–33) | 3 (50) | 6 (100) | 6 (100) | 6 (100) | None | 0 | – |
| Present study | 2014 | 20 | 12 | 28 (21–38) | 7 (58) | 9 (75) | 12 (100) | 12 (100) | None | 2 | One miscarriage may have been provoked by ICD shocks (4 WG) |
ICD, implantable cardioverter-defibrillator; WG, weeks gestation.
| Study . | Year . | Number of pregnancies . | Number of patients . | Age—mean (full range) . | Structural heart disease—n (%) . | Secondary prophylaxis—n (%) . | Pre-pectoral ICDs—n (%) . | Endocardial leads—n (%) . | Device-related complications . | Number of women who experienced ICD shocks . | Foetal consequences of ICD shocks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natale et al.10 | 1997 | 51 | 44 | 30 (14–36) | 14 (32) | 44 (100) | 2 (5) | 14 (32) | Migration of an abdominal generator, pericarditis related to epicardial patches | 11 | None |
| Schuler et al.11 | 2012 | 19 | 14 | 33 (22–42) | 10 (71) | 5 (36) | 13 (93) | 14 (100) | ICD lead thrombus, atrial lead dysfunction | 1 | None |
| Miyoshi et al.12 | 2013 | 6 | 6 | 28 (25–33) | 3 (50) | 6 (100) | 6 (100) | 6 (100) | None | 0 | – |
| Present study | 2014 | 20 | 12 | 28 (21–38) | 7 (58) | 9 (75) | 12 (100) | 12 (100) | None | 2 | One miscarriage may have been provoked by ICD shocks (4 WG) |
| Study . | Year . | Number of pregnancies . | Number of patients . | Age—mean (full range) . | Structural heart disease—n (%) . | Secondary prophylaxis—n (%) . | Pre-pectoral ICDs—n (%) . | Endocardial leads—n (%) . | Device-related complications . | Number of women who experienced ICD shocks . | Foetal consequences of ICD shocks . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Natale et al.10 | 1997 | 51 | 44 | 30 (14–36) | 14 (32) | 44 (100) | 2 (5) | 14 (32) | Migration of an abdominal generator, pericarditis related to epicardial patches | 11 | None |
| Schuler et al.11 | 2012 | 19 | 14 | 33 (22–42) | 10 (71) | 5 (36) | 13 (93) | 14 (100) | ICD lead thrombus, atrial lead dysfunction | 1 | None |
| Miyoshi et al.12 | 2013 | 6 | 6 | 28 (25–33) | 3 (50) | 6 (100) | 6 (100) | 6 (100) | None | 0 | – |
| Present study | 2014 | 20 | 12 | 28 (21–38) | 7 (58) | 9 (75) | 12 (100) | 12 (100) | None | 2 | One miscarriage may have been provoked by ICD shocks (4 WG) |
ICD, implantable cardioverter-defibrillator; WG, weeks gestation.
Implantable cardioverter-defibrillators shock therapy during pregnancy
Two patients were subjected to defibrillation shocks during our study. Patient #1 was subjected to nine transthoracic shocks and a further ICD shock for episodes of VA in early pregnancy. She subsequently made a full recovery and went on to deliver a healthy neonate at term, demonstrating that shock therapy can be administered during pregnancy without harming the foetus. Patient #9, on the other hand, was subjected to two ICD shocks at 4 weeks gestation and miscarried her pregnancy 7 days later. Although this can be accounted for by typical rates of idiopathic miscarriage in the background population (15%),13 it is, of course, possible that the ICD shocks themselves caused the miscarriage in this case. There are reports in the wider literature of women receiving ICD shocks during pregnancy; however, none of these have ever been associated with an adverse fetal outcome. Natale et al.10 reported 11 patients receiving ICD shocks via epicardial patches during pregnancy and none of these went on to miscarry. In addition, Schuler et al.11 reported the case of a pregnant woman with LQTS receiving an ICD shock (31 J) at 20 weeks gestation, and Bonini et al.4 reported the case of a pregnant woman with IVF receiving an ICD shock at 10 weeks gestation. Neither of these patients miscarried. Although adverse foetal outcomes have never been reported following ICD shocks, they have been following transthoracic shocks.14,15 For instance, Barnes et al.15 reported the case of a woman, who at 28 weeks gestation received a low-energy (50 J) DC transthoracic shock for a supraventricular tachycardia while undergoing continuous foetal monitoring. Following this, a severe and sustained foetal bradycardia was instantly noted, prompting an emergency caesarean section. In theatre, the uterus was found to be tightly contracted and at the time, Barnes hypothesized that this intense uterine contraction and consequent foetal bradycardia had been triggered by the transthoracic shock. Other work16,17 suggests that the uterine muscle is an excellent conductor of electricity and supports this theory. Older studies18,19 on animal models and humans, however, seem to indicate that the foetus itself is relatively invulnerable to electrical activity due to its high cardiac fibrillation threshold and this has led to the commonly held belief that ICD shocks do not cause miscarriage, particularly as defibrillation energy is targeted away from the uterus. Our report may challenge this belief and suggest that undesirable effects can result from ICD shock therapy in the very early stages of the pregnancy. One might also hypothesize that an ‘all-or-nothing’ law applies to complications arising from shock therapy in the first few weeks of pregnancy, as is the case for complications associated with ionizing radiation and drug exposure. None of the other cases of ICD shocks delivered during pregnancy in the wider literature were at such an early stage of pregnancy, which may suggest that the timing of ICD shock therapy is an important determinant of foetal outcome.
Implantable cardioverter-defibrillator-related complications during pregnancy
No device-related complications, such as lead dislodgement, lead dysfunction, or lead thrombus, were reported for any of the patients in our study. This is in agreement with the study by Miyoshi et al.12 who did not observe any problems either when following six women with ICDs in pregnancy. Schuler et al.,11 on the other hand, did report one case of atrial lead dysfunction and lead thrombus in a patient suffering with Factor V Leiden in his study, which he ultimately attributed to her coagulopathy. In the study by Natale et al.,10 complications were reported with epicardial defibrillation systems, including generator migration and episodes of pericarditis brought on by the epicardial patches. For women carrying pre-pectoral ICDs with endocardial leads, however, the results from our study as well as those by Miyoshi et al.12 and Schuler et al.11 seem to suggest that the morphological and haemodynamical changes in pregnancy do not substantially increase the risk of developing device-related complications for women carrying ICDs. Although no device-related complications were recorded in this study, it may yet be interesting to study further the impact of the haemodynamic changes during pregnancy on ICD lead parameters such as impedance, intracardiac signal amplitude, and pacing threshold.
Safety of implantable cardioverter-defibrillators in pregnancy
We found no evidence to suggest that carrying an ICD should be taken as a contraindication to becoming pregnant. Fourteen of the 20 pregnancies (70%) resulted in live births and none resulted in maternal death. The studies by Schuler et al.11 and Miyoshi et al.12 reported higher proportions of live births, recording 18 of 19 (95%) and 6 of 6 (100%) respectively; however, this discrepancy can be explained by the differences in inclusion criteria as well as the great variability that can arise when studying such small populations. In our study, three out of four miscarriages occurred in a single patient (#6, repaired ToF), which clearly had a large influence on our result. The other three foetal deaths were reported in Patient #7 (LQTS), who sustained a stillbirth, Patient #11 (repaired LPS), who underwent termination on medical grounds, and Patient #9 (IVF), whose case was just discussed. Except in the case of Patient #9, where no definitive conclusions could be drawn, it appears that none of the complications recorded in this study were directly attributable to the presence of an ICD, being either idiopathic in origin or the result of the underlying cardiac disease and/or medications the mother was taking at the time.
Post-partum worsening of underlying cardiomyopathy
Two patients experienced worsening of their underlying cardiomyopathy in the months following delivery. Patient #3 (ARVC) sustained an episode of myocarditis with transient worsening of her LVEF down to 35%, which subsequently recovered fully with medical treatment. Patient #6 (repaired ToF), on the other hand, experienced progressive right ventricular dilatation and dysfunction, which ultimately required a bio-prosthetic pulmonary valve replacement 6 months post-delivery. This case is consistent with previous reports in the literature of patients with repaired ToF suffering a deterioration of right ventricular function following pregnancy.20,21 These examples clearly demonstrate that pregnancy can have a profound effect in specific cardiac diseases, and further work is still required to fully characterize these effects.
β-blocker use during pregnancy
A substantial number of foetuses suffered from IUGR (4 of 20, 20%), LBW (3 of 20, 15%), and NHG (5 of 20, 25%) during this study. All of these outcomes are established side-effects of maternal β-blocker use during pregnancy22 but may equally have been idiopathic in origin or attributable to other factors such as maternal smoking. These particular patients were all followed up with regular foetal growth scans during pregnancy as well as with rigorous monitoring of neonatal glycaemic control post-partum. No adverse outcomes were noted during the first 6 months following delivery. Whether β-blockers were responsible for these adverse foetal/neonatal outcomes or not, we know that they considerably reduce the risks of mothers with cardiomyopathy or congenital heart disease from developing episodes of life-threatening VA.23 Indeed, non-compliance to β-blocker medication may well have prompted the episode of VF in Patient #9 in our study. As VAs and ICD shock therapy are considerably harmful for a mother, we believe that for pregnant women with a strong indication for β-blocker therapy, the benefits outweigh the risks of taking this medication both for the mother and for the child. We therefore feel that it is of particular importance to impress on this group of patients the benefits of complying with their β-blocker medication throughout pregnancy.
Limitations
The limitations of the present study are its retrospective design and the small sample size resulting from the low prevalence of pregnant woman carrying ICD devices in the general population.
Conclusions
Overall, ICDs are safe in pregnancy and there is no evidence from our data that women carrying ICDs are at any greater risk of developing adverse complications during pregnancy compared with the background population, solely on the grounds that they carry such a device. Furthermore, the physiological or morphological changes in pregnancy seem to have no bearing on device operation as no device-specific complications or maternal deaths were recorded. Pregnancy can worsen some cardiac conditions; however, these complications are entirely attributable to the underlying cardiac conditions and not on the presence of an ICD. One miscarriage we reported, however, may have been related to ICD shocks received at 4 weeks gestation. This observation may challenge the commonly held belief that defibrillation shocks have no affect on foetal outcome and might potentially suggest that undesirable effects (‘all-or-nothing’ law) can result from ICD shock therapy at the very early stages of the pregnancy. If this is the case, it further emphasizes the importance of strict compliance to β-blocker therapy throughout pregnancy for women carrying ICDs.
As such, carrying an ICD should not be considered a contraindication to becoming pregnant, although some patients who carry ICDs may have specific cardiac conditions that deteriorate as a result of pregnancy. Further work is required to better understand the consequences for patients with these conditions.
Conflict of interest: none declared.



