-
PDF
- Split View
-
Views
-
Cite
Cite
Aled R. Jones, David E. Krummen, Sanjiv M. Narayan, Non-invasive identification of stable rotors and focal sources for human atrial fibrillation: mechanistic classification of atrial fibrillation from the electrocardiogram, EP Europace, Volume 15, Issue 9, September 2013, Pages 1249–1258, https://doi.org/10.1093/europace/eut038
Close - Share Icon Share
Abstract
To develop electrocardiogram (ECG) tools to quantify the number of sources for atrial fibrillation (AF), i.e. spatially stable rotors and focal impulses, and whether they lie in right or left atrium. Intracardiac mapping has recently shown that paroxysmal and persistent AF is sustained by rotors or focal sources that are stable in location and thus targets for limited ablation [focal impulse and rotor modulation (FIRM)] to eliminate AF. Importantly, the numbers and locations of concurrent sources determine both the complexity of AF and the approach for ablation.
In 36 AF patients (n = 29 persistent, 63 ± 9 years) in the CONventional ablation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial, we developed phase lock (PL) to quantify spatial repeatability of ECG ‘F-waves’ between leads over time. Phase lock spectrally quantifies the angle θ between F-wave voltages in planes formed by ECG leads I, aVF, and V1 at successive points in time. We compared PL with ECG spectral dominant frequency (DF) and organizational index (OI) to characterize stable rotors and focal sources validated by intracardiac FIRM mapping. Focal impulse and rotor modulation ablation alone at ≤3 sources acutely terminated and rendered AF non-inducible or substantially slowed AF in 31 of 36 patients. Receiver operating characteristics of PL for this endpoint had area under the curve (AUC) = 0.72, and the optimum cut-point (PL = 0.09) had 74% sensitivity, 92% positive predictive value (PPV). Receiver operating characteristics areas for OI and DF were 0.50 and 0.58, respectively. Left (n = 28) or right (n = 3) atrial sources were localized by PL with AUC = 0.85, sensitivity 100%, PPV 30%, and negative predictive value 100%. Spectral DF provided AUC = 0.79. Notably, PL did not comigrate with diagnosis of paroxysmal or persistent AF (P = NS), unlike ECG DF.
The novel metric of ECG PL identifies patients with fewer (≤3) or greater numbers of stable rotors/focal sources for AF, validated by intracardiac FIRM mapping, and localized them to right or left atria. These data open the possibility of using 12-lead ECG analyses to classify AF mechanistically and plan procedures for right- or left-sided FIRM ablation.
This study is based on the CONventional ablation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial, recently validated in several external laboratories, that human atrial fibrillation (AF) is sustained by stable rotors and focal sources where brief ablation [focal impulse and rotor modulation (FIRM)] alone may acutely terminate AF and render it non-inducible. Focal impulse and rotor modulation-guided ablation substantially improved long-term freedom from AF over conventional ablation alone.
This work introduces a new electrocardiogram signal processing index of phase lock (PL), that identifies the number of sources established by intracardiac FIRM mapping and whether they lie in the left or right atrium.
Electrocardiogram PL provides a bedside method to identify AF mechanisms for each patient, with a view to developing more prognostically useful classifications than the current scheme of persistent vs. paroxysmal AF.
Introduction
Atrial fibrillation (AF) is a major public health problem, for which therapies to prevent or treat its sequelae of stroke, hospitalization, heart failure, and death remain suboptimal.1 The electrocardiogram (ECG) is the primary tool for diagnosing AF, yet still has limited value for guiding management or predicting response to therapy in any individual. This limitation partly reflects an incomplete mechanistic understanding of AF, which hinders the design of ECG tools tailored to patient-specific mechanisms.
We2–4 and independent groups5,6 recently used computational analyses of contact AF recordings to show that AF is often sustained by electrical rotors and focal sources, as shown in seminal animal models,7–9 that are stable over time and thus suitable targets for ablation. In the CONventional ablation with or without Focal Impulse and Rotor Modulation (CONFIRM) trial,2 focal impulse and rotor modulation (FIRM) ablation of small regions (∼2–5 cm2 aggregate)4,10 terminated and rendered AF non-inducible or greatly slowed AF in 86% of patients, and improved freedom from AF on rigorous monitoring vs. conventional ablation alone.2 Other groups have also shown stable AF rotors using contact intracardiac electrodes.5,6,11–13 Electrocardiogram imaging, on the other hand, has thus far identified only transient AF rotors14 rather than stable sources, but ECG analyses may indeed define a spectrum from atrial tachycardia to fibrillation,15–18 and spectral analyses19–21 of dominant frequency (DF) and organizational index (OI) track the regularization of AF from medications22 or ablation.23
We hypothesized that the number and location of concurrent stable AF sources should be identifiable from the ECG. Specifically, we reasoned that F-waves should maintain spatial synchrony [‘phase lock’ (PL)] in ECG planes over time in patients with fewer than more AF sources. Moreover, since the success of FIRM ablation relates to elimination of all sources,2 we developed an ECG index to assist in planning FIRM ablation in right (RA) or left atrium (LA).
We tested our hypothesis by developing a novel ECG index to measure PL of F-waves in pseudo-orthogonal ECG planes, then compared it against DF and OI to identify patients in whom FIRM ablation achieved the acute endpoint (AF termination/non-inducibility or substantial slowing) in paroxysmal and persistent AF patients in the CONFIRM trial.
Methods
Patient flow
From 107 patients in the CONFIRM trial,2 we included 36 consecutive patients undergoing FIRM ablation for AF at the Veterans Affairs and University of California Medical Centers in San Diego. The study protocol for the CONFIRM trial was approved by our joint Institutional Review Board as described2 (NCT01008722). We consecutively enroll patients with persistent and paroxysmal AF referred for clinically indicated ablation, excluding only patients who refused or were unable to provide consent.
Electrophysiological study
Electrophysiology study was performed >5 half-lives after discontinuing anti-arrhythmic medications (>69 days after amiodarone; Table 1). A decapolar catheter was placed in the coronary sinus, then 64 contact electrode baskets (Constellation; Boston Scientific) were advanced to RA and trans-septally to LA. The ECG was recorded at 0.05–100 Hz, digitized with a 16 bit analogue-to-digital converter at 1000 Hz and exported for analysis. Custom analytical software was developed in Matlab (The Mathworks) on Windows or Mac OS X-based workstations, that input the 12-lead ECG file (ASCII) and automatically generated the ECG analytical indices presented in this study in <10 s with no further user input.
| Characteristic . | . |
|---|---|
| Persistent/paroxysmal AF | 29/7 |
| Age (years) | 63 ± 9 |
| History of AF (months) | 52 (38–110) |
| Left atrial diameter (mm) | 48 ± 7 |
| LVEF (%) | 53 ± 15 |
| CHADS2 score | |
| 0 or 1 | 36% (13) |
| 2 or more | 64% (23) |
| Prior conventional ablation | 42% (15) |
| Number failing amiodarone | 61% (22) |
| Days since amiodarone discontinued | 365 (69–730) |
| Concomitant drug therapy | |
| ACEI/ARB | 58% (21) |
| β-Adrenoceptor antagonists | 67% (24) |
| Statins | 53% (19) |
| Comorbid conditions | |
| Hypertension | 86% (31) |
| Diabetes | 33% (12) |
| Prior stroke/TIA | 17% (6) |
| Coronary disease | 50% (18) |
| Hypercholesterolaemia | 86% (30) |
| Characteristic . | . |
|---|---|
| Persistent/paroxysmal AF | 29/7 |
| Age (years) | 63 ± 9 |
| History of AF (months) | 52 (38–110) |
| Left atrial diameter (mm) | 48 ± 7 |
| LVEF (%) | 53 ± 15 |
| CHADS2 score | |
| 0 or 1 | 36% (13) |
| 2 or more | 64% (23) |
| Prior conventional ablation | 42% (15) |
| Number failing amiodarone | 61% (22) |
| Days since amiodarone discontinued | 365 (69–730) |
| Concomitant drug therapy | |
| ACEI/ARB | 58% (21) |
| β-Adrenoceptor antagonists | 67% (24) |
| Statins | 53% (19) |
| Comorbid conditions | |
| Hypertension | 86% (31) |
| Diabetes | 33% (12) |
| Prior stroke/TIA | 17% (6) |
| Coronary disease | 50% (18) |
| Hypercholesterolaemia | 86% (30) |
Normally distributed variables are listed as mean ± standard deviation; non-normally distributed values are listed as median (interquartile range).
| Characteristic . | . |
|---|---|
| Persistent/paroxysmal AF | 29/7 |
| Age (years) | 63 ± 9 |
| History of AF (months) | 52 (38–110) |
| Left atrial diameter (mm) | 48 ± 7 |
| LVEF (%) | 53 ± 15 |
| CHADS2 score | |
| 0 or 1 | 36% (13) |
| 2 or more | 64% (23) |
| Prior conventional ablation | 42% (15) |
| Number failing amiodarone | 61% (22) |
| Days since amiodarone discontinued | 365 (69–730) |
| Concomitant drug therapy | |
| ACEI/ARB | 58% (21) |
| β-Adrenoceptor antagonists | 67% (24) |
| Statins | 53% (19) |
| Comorbid conditions | |
| Hypertension | 86% (31) |
| Diabetes | 33% (12) |
| Prior stroke/TIA | 17% (6) |
| Coronary disease | 50% (18) |
| Hypercholesterolaemia | 86% (30) |
| Characteristic . | . |
|---|---|
| Persistent/paroxysmal AF | 29/7 |
| Age (years) | 63 ± 9 |
| History of AF (months) | 52 (38–110) |
| Left atrial diameter (mm) | 48 ± 7 |
| LVEF (%) | 53 ± 15 |
| CHADS2 score | |
| 0 or 1 | 36% (13) |
| 2 or more | 64% (23) |
| Prior conventional ablation | 42% (15) |
| Number failing amiodarone | 61% (22) |
| Days since amiodarone discontinued | 365 (69–730) |
| Concomitant drug therapy | |
| ACEI/ARB | 58% (21) |
| β-Adrenoceptor antagonists | 67% (24) |
| Statins | 53% (19) |
| Comorbid conditions | |
| Hypertension | 86% (31) |
| Diabetes | 33% (12) |
| Prior stroke/TIA | 17% (6) |
| Coronary disease | 50% (18) |
| Hypercholesterolaemia | 86% (30) |
Normally distributed variables are listed as mean ± standard deviation; non-normally distributed values are listed as median (interquartile range).
Focal impulse and rotor mapping/modulation of atrial fibrillation sources
Focal impulse and rotor mapping uses computational analyses of contact recordings (rather than virtual non-contact electrograms)14,24 to identify stable AF sources for FIRM ablation.2–4 Briefly, FIRM mapping records AF signals widely from both atria, analysed in near-real-time, vis-à-vis human atrial repolarization and conduction dynamics25–28 to construct AF propagation movies. Electrical rotors were defined as sequential activation around a centre of rotation emanating outwards to control local AF activation.7,8Focal impulses were defined by centrifugal activation from an origin. Importantly, rotors and focal impulses in FIRM were diagnosed as AF sources only if stable for tens of minutes (thousands of cycles) in multiple recordings. This excludes transient AF pivots and incomplete circuits determined in prior non-contact14,24 studies to be migratory waves. For illustration, single-cycle AF isochronal maps are presented although FIRM-guided ablation in our2 and external5,6 laboratories is guided by AF movies.
Focal impulse and rotor modulation ablation
Focal impulse and rotor modulation ablation was directed at stable AF rotors or focal impulses prior to pulmonary vein (PV) isolation or any other ablation, using the Thermocool catheter (Biosense-Webster) or the Blazer (Boston Scientific) in heart failure patients. The CONFIRM protocol2 permitted FIRM ablation at each rotor/focal origin for ≤10 min or AF termination, whichever came first, but was limited to ≤3 sources (and one map) due to slow early versions of mapping software. The acute FIRM endpoint was AF termination with non-reinducibility or, if not achieved, ≥10% AF cycle length prolongation (i.e. elimination of a secondary source)29 measured in the coronary sinus (where AF cycle length, CL, is 10–20 ms longer than the appendages).30 An online case video of FIRM ablation has been reported.4 In CONFIRM, all patients then received wide area circumferential ablation of both PV pairs, confirmation of PV isolation and, in persistent AF patients, a LA roof line.2
Electrocardiogram phase lock between electrocardiogram planes: quantifying atrial fibrillation spatial reproducibility
We developed an ECG algorithm to quantify F-wave spatial reproducibility (PL) in three pseudo-orthogonal ECG planes.
Vectorcardiography separates atrial macroreentry (‘flutter’) with repeatable loops from varying AF loops over time,15,16,31 yet is influenced by varying F-wave amplitude and shape that may reduce sensitivity for subtle variations in spatial phase between AF with more or less concurrent rotors or focal sources.
Phase lock was designed to quantify spatial synchronization between ECG leads for successive cycles, independent of variations in F-wave amplitude (i.e. scaling) or shape over time. Electrocardiogram leads I, aVF, and V1 were selected as in our prior work15–18 before ablation, digitized at 1 kHz frequency/16 bit resolution from our recorder (Bard), then median filtered (10 ms). QRST subtraction21,32 was achieved by computing mean complexes in bins for 2–3 distinct QRS shapes (Figure 1A), that were subtracted from each ECG. We found minimal differences whether subtracting QRS or QRST mean complexes.
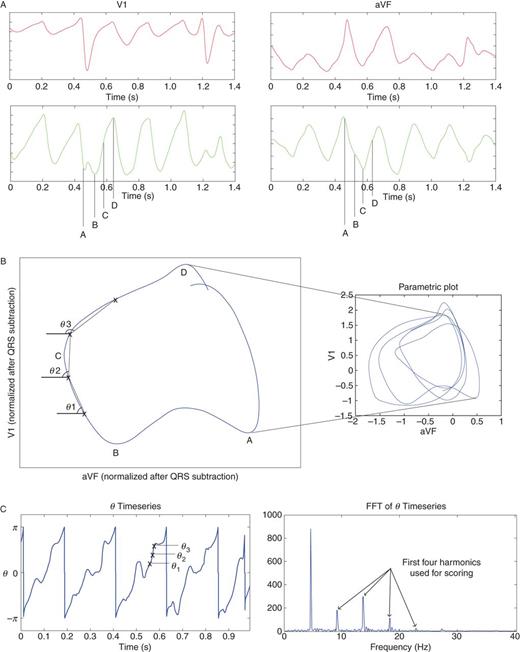
Electrocardiogram PL Method. (A) Electrocardiogram in orthogonal ECG leads V1 and aVF (lead I is also used). QRS subtracted ECG leads emphasize atrial activation. (B) Electrocardiogram spatial loop displayed from mutiple loops over AF cycles (right panel). Phase angles θ are calculated between times t, t + 1 for all time points t. (C) Timeseries of phase angle θ, showing periodicity that is highlighted in spectral plot. Electrocardiogram PL index is calculated from the area of the harmonics.
We analysed each ECG for its 8 s duration. Parametric plots were generated from QRST-subtracted ECG leads pairwise in each of three pseudo-orthogonal planes (Figure 1B): XY (lead I vs. aVF), YZ (lead aVF vs. V1), ZX (lead V1 vs. I), normalized to root-mean-squared power, with time as the independent variable (Figure 1B). In each plane, a spatial angle θ was calculated between voltage in each axis on median-filtered QRST-subtracted ECG leads for time points t–t+ 1, repeated for all t. The resulting θ timeseries was plotted for each plane (Figure 1C). The periodicity of θ indicates phase (‘'synchronization’) in the two ECG leads defining each plane.
We generated a fast Fourier transform (FFT)19 of the θ timeseries, i.e. of parameterized spatial loops over time, that differs from prior spectral analyses of voltages20,21. For PL, the fundamental was selected as the frequency of maximum power for plausible rates (3 Hz ≤ f ≤ 12 Hz), and the first four harmonics (strict integer multiples of the fundamental) were identified (Figure 1C). Phase lock score was calculated as the ratio of total power of the first to fourth harmonics (width 1 Hz) to total spectral power (3–65 Hz), within a range of 0 (low repeatability) to ∼0.8 (highest repeatability). The mean PL score from the three plane plots was assigned as the score for that patient.
Comparative measures of electrocardiogram spectral regularity
We analysed spectral DF19 and organization for each ECG lead using described methods,20,33,34 for comparison with prior studies.15,16,18,22,23 Dominant frequency was identified as the frequency of highest power following FFT in the range of 3–12 Hz, and the resultant spectrum was quantified using the organisational index (OI)20: the ratio of area in the fundamental plus the first three harmonics to total spectral power area in the bandwidth from 2.5 Hz up to, but not including, the fifth harmonic.
Statistical analysis
The primary analyses of this work were to study the ability of ECG PL to separate patients who did or did not achieve (i) the acute endpoint (termination/non-inducibility + organization); and (ii) AF acute termination/non-inducibility alone. Continuous data are represented as mean ± standard deviation (SD). The t-test was used to compare continuous variables between groups. Paired continuous variables were compared using linear regression and the paired t-test. The χ2 test was applied to contingency tables. A probability of <5% was considered statistically significant.
Results
The clinical characteristics of our patients are summarized in Table 1.
Acute results of focal impulse and rotor modulation ablation
Stable sources were observed in 35 of 36 (97%) of patients, with non-significant trends for persistent AF patients to have more sources (2.4 ± 1.1 vs. 1.9 ± 0.7), and more rotors relative to focal sources than paroxysmal AF patients (Table 2).
| Characteristic . | Persistent AF . | Paroxysmal AF . | P . |
|---|---|---|---|
| Baseline AF cycle length (at coronary sinus), ms | 170 ± 21 | 189 ± 13 | 0.03 |
| Patients with detected sources | 28/29 (97%) | 7/7 (100%) | |
| Patients reaching acute endpoint | 24/29 (82.8%) | 7/7 (100%) | |
| No. of concurrent AF sources | 2.4 ± 1.1 | 1.9 ± 0.7 | 0.23 |
| No. of rotors, %a (LA/RA) | 74.1% (29/14) | 61.5% (6/2) | |
| No. of focal impulses, %a (LA/RA) | 25.9% (15/0) | 38.5% (5/0) | |
| FIRM time to AF termination, primary source/min | 4.5 ± 7.3 | 3.9 ± 4.1 | 0.86 |
| Total FIRM time, all sources/min | 17.5 ± 10.5 | 7.7 ± 4.2 | 0.29 |
| ECG analyses | |||
| XY phase lock | 0.12 ± 0.06 | 0.11 ± 0.02 | 0.60 |
| XZ phase lock | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.40 |
| YZ phase lock | 0.14 ± 0.06 | 0.12 ± 0.04 | 0.53 |
| X (ECG I) DF/Hz | 4.90 ± 1.04 | 4.85 ± 1.17 | 0.91 |
| X (ECG I) organization index | 0.39 ± 0.15 | 0.34 ± 0.06 | 0.35 |
| Y (ECG aVF) DF/Hz | 5.22 ± 0.88 | 5.42 ± 0.46 | 0.56 |
| Y (ECG aVF) organization index | 0.41 ± 0.21 | 0.33 ± 0.14 | 0.35 |
| Z (ECG V1) DF/Hz | 5.32 ± 1.16 | 5.20 ± 0.63 | 0.80 |
| Z (ECG V1) organization index | 0.42 ± 0.16 | 0.32 ± 0.18 | 0.17 |
| Characteristic . | Persistent AF . | Paroxysmal AF . | P . |
|---|---|---|---|
| Baseline AF cycle length (at coronary sinus), ms | 170 ± 21 | 189 ± 13 | 0.03 |
| Patients with detected sources | 28/29 (97%) | 7/7 (100%) | |
| Patients reaching acute endpoint | 24/29 (82.8%) | 7/7 (100%) | |
| No. of concurrent AF sources | 2.4 ± 1.1 | 1.9 ± 0.7 | 0.23 |
| No. of rotors, %a (LA/RA) | 74.1% (29/14) | 61.5% (6/2) | |
| No. of focal impulses, %a (LA/RA) | 25.9% (15/0) | 38.5% (5/0) | |
| FIRM time to AF termination, primary source/min | 4.5 ± 7.3 | 3.9 ± 4.1 | 0.86 |
| Total FIRM time, all sources/min | 17.5 ± 10.5 | 7.7 ± 4.2 | 0.29 |
| ECG analyses | |||
| XY phase lock | 0.12 ± 0.06 | 0.11 ± 0.02 | 0.60 |
| XZ phase lock | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.40 |
| YZ phase lock | 0.14 ± 0.06 | 0.12 ± 0.04 | 0.53 |
| X (ECG I) DF/Hz | 4.90 ± 1.04 | 4.85 ± 1.17 | 0.91 |
| X (ECG I) organization index | 0.39 ± 0.15 | 0.34 ± 0.06 | 0.35 |
| Y (ECG aVF) DF/Hz | 5.22 ± 0.88 | 5.42 ± 0.46 | 0.56 |
| Y (ECG aVF) organization index | 0.41 ± 0.21 | 0.33 ± 0.14 | 0.35 |
| Z (ECG V1) DF/Hz | 5.32 ± 1.16 | 5.20 ± 0.63 | 0.80 |
| Z (ECG V1) organization index | 0.42 ± 0.16 | 0.32 ± 0.18 | 0.17 |
aLA, left atrium; RA, right atrium.
| Characteristic . | Persistent AF . | Paroxysmal AF . | P . |
|---|---|---|---|
| Baseline AF cycle length (at coronary sinus), ms | 170 ± 21 | 189 ± 13 | 0.03 |
| Patients with detected sources | 28/29 (97%) | 7/7 (100%) | |
| Patients reaching acute endpoint | 24/29 (82.8%) | 7/7 (100%) | |
| No. of concurrent AF sources | 2.4 ± 1.1 | 1.9 ± 0.7 | 0.23 |
| No. of rotors, %a (LA/RA) | 74.1% (29/14) | 61.5% (6/2) | |
| No. of focal impulses, %a (LA/RA) | 25.9% (15/0) | 38.5% (5/0) | |
| FIRM time to AF termination, primary source/min | 4.5 ± 7.3 | 3.9 ± 4.1 | 0.86 |
| Total FIRM time, all sources/min | 17.5 ± 10.5 | 7.7 ± 4.2 | 0.29 |
| ECG analyses | |||
| XY phase lock | 0.12 ± 0.06 | 0.11 ± 0.02 | 0.60 |
| XZ phase lock | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.40 |
| YZ phase lock | 0.14 ± 0.06 | 0.12 ± 0.04 | 0.53 |
| X (ECG I) DF/Hz | 4.90 ± 1.04 | 4.85 ± 1.17 | 0.91 |
| X (ECG I) organization index | 0.39 ± 0.15 | 0.34 ± 0.06 | 0.35 |
| Y (ECG aVF) DF/Hz | 5.22 ± 0.88 | 5.42 ± 0.46 | 0.56 |
| Y (ECG aVF) organization index | 0.41 ± 0.21 | 0.33 ± 0.14 | 0.35 |
| Z (ECG V1) DF/Hz | 5.32 ± 1.16 | 5.20 ± 0.63 | 0.80 |
| Z (ECG V1) organization index | 0.42 ± 0.16 | 0.32 ± 0.18 | 0.17 |
| Characteristic . | Persistent AF . | Paroxysmal AF . | P . |
|---|---|---|---|
| Baseline AF cycle length (at coronary sinus), ms | 170 ± 21 | 189 ± 13 | 0.03 |
| Patients with detected sources | 28/29 (97%) | 7/7 (100%) | |
| Patients reaching acute endpoint | 24/29 (82.8%) | 7/7 (100%) | |
| No. of concurrent AF sources | 2.4 ± 1.1 | 1.9 ± 0.7 | 0.23 |
| No. of rotors, %a (LA/RA) | 74.1% (29/14) | 61.5% (6/2) | |
| No. of focal impulses, %a (LA/RA) | 25.9% (15/0) | 38.5% (5/0) | |
| FIRM time to AF termination, primary source/min | 4.5 ± 7.3 | 3.9 ± 4.1 | 0.86 |
| Total FIRM time, all sources/min | 17.5 ± 10.5 | 7.7 ± 4.2 | 0.29 |
| ECG analyses | |||
| XY phase lock | 0.12 ± 0.06 | 0.11 ± 0.02 | 0.60 |
| XZ phase lock | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.40 |
| YZ phase lock | 0.14 ± 0.06 | 0.12 ± 0.04 | 0.53 |
| X (ECG I) DF/Hz | 4.90 ± 1.04 | 4.85 ± 1.17 | 0.91 |
| X (ECG I) organization index | 0.39 ± 0.15 | 0.34 ± 0.06 | 0.35 |
| Y (ECG aVF) DF/Hz | 5.22 ± 0.88 | 5.42 ± 0.46 | 0.56 |
| Y (ECG aVF) organization index | 0.41 ± 0.21 | 0.33 ± 0.14 | 0.35 |
| Z (ECG V1) DF/Hz | 5.32 ± 1.16 | 5.20 ± 0.63 | 0.80 |
| Z (ECG V1) organization index | 0.42 ± 0.16 | 0.32 ± 0.18 | 0.17 |
aLA, left atrium; RA, right atrium.
Brief FIRM ablation alone achieved the acute endpoint in 31 of 36 patients (prior to PV isolation),2 comprising acute AF termination/non-inducibility in 20 patients and AF organization in 11 patients (AF CL prolongation of 33 ± 12 ms, 19 ± 8%; Table 2). Compared with patients with paroxysmal AF, those with persistent AF required a similar time for FIRM ablation at the primary source time to terminate AF (4.5 ± 7.3 vs. 3.9 ± 4.1 min; P = 0.86; Table 2), and longer overall FIRM time reflecting the greater number of sources.
Electrocardiogram phase lock reflects number of atrial fibrillation sources, not clinical presentation
Electrocardiogram PL was higher for patients in whom FIRM ablation achieved acute AF termination/non-inducibility vs. those in whom it did not (0.17 ± 0.06 vs. 0.13 ± 0.04; P = 0.04). The inter-plane SD of PL, also indicating spatial organization, trended higher for patients with FIRM termination than those without (0.036 vs. 0.024, P = 0.11). Conversely, ECG PL did not separate patients who were classified as persistent or paroxysmal AF per se (Table 2).
Figure 2 illustrates FIRM mapping, ablation, and ECG PL analysis in a 47-year-old man with persistent AF and frequent emergency visits due to rapid ventricular response (Figure 2A). FIRM mapping identified two concurrent AF sources: a RA rotor and LA focal source (Figure 2B). ECG and coronary sinus electrograms 1 h prior to, then spanning hyperacute AF termination by FIRM at the RA rotor after 5.5 min ablation. Atrial fibrillation was now non-inducible. The RA rotor was assigned as the primary source, but FIRM ablation was also applied at the LA focal impulse (secondary source). (Figure 2C) Repeatable ECG spatial loops in the XY (lead I vs. lead aVF), YZ (aVF vs. V1), and XZ (I vs. aVF) planes, with corresponding periodic θ time series and maximum PL = 0.36 (YZ plane) and mean PL = 0.25 (three planes). Electrocardiogram spectral DF was 4.64 ± 0.01 Hz, and OI was 0.36 ± 0.31.
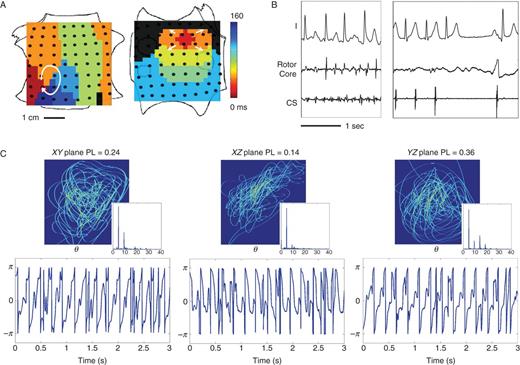
High ECG PL in persistent AF caused by two stable sources. (A) FIRM map (contact electrograms) shows RA rotor and LA focal source in AF; (B) ECG at baseline AF and during FIRM ablation, showing hyperacute termination of AF to sinus rhythm (5 min) with AF non-inducibility, before any other ablation. The RA rotor was primary. (C) Repeatable ECG spatial loops with periodic ECG phase θ timeseries and clearly delineated spectral harmonics. The maximum PL = 0.36 in YZ, or aVF/V1 (three-plane mean 0.25).
In contrast, Figure 3 illustrates low ECG PL in a 64-year-old man with persistent AF caused by four sources, with LA diameter 57 mm and left ventricular ejection fraction 55%. Persistent AF showed three rotors and one focal source on FIRM mapping (contact electrograms) (Figure 3A). Focal impulse and rotor modulation ablation at three sources did not terminate AF but prolonged CL. Less repeatable ECG spatial loops, with less periodic θ time series. Spectrograms indicate broadband spectra with PL maximum of 0.09 (in YZ-plane) and mean (three planes) of 0.08. The mean ECG DF was 5.98 ± 0.21 Hz, and mean OI was 0.29 ± 0.13 (Figure 3C).
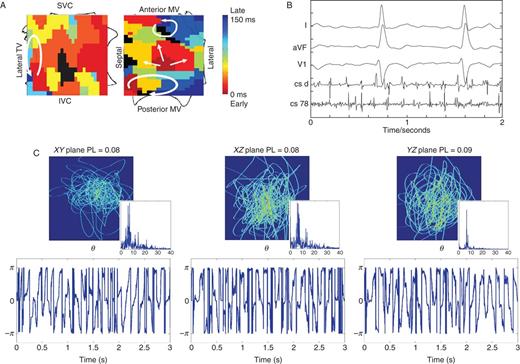
Low ECG PL in persistent AF caused by four stable sources, in which FIRM at three sources (per protocol) did not terminate AF. (A) FIRM map (contact electrograms) shows three rotors and one focal source. (B) Baseline AF. (C) Poorly reproducible ECG loops with aperiodic ECG phase θ timeseries and broadband spectra. Maximum PL = 0.086 in plane YZ (aVF/V1); three-plane mean = 0.083.
For the diagnosis of persistent AF, Figure 4 shows that PL provided AUC 0.56, while spectral OI provided an AUC of 0.70 and DF an AUC of 0.50. Thus, OI better predicted clinical persistent AF but, as shown below, did not identify the number or the location of sources.
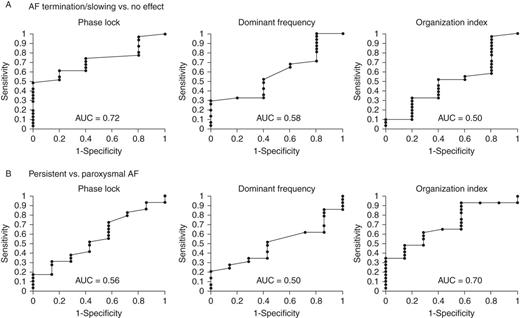
Receiver operating characteristic curves of ECG indices for (A) acute endpoint of AF termination/slowing by FIRM ablation, showing that ECG PL (mean of three planes) provides AUC = 0.72 with optimum cutpoint 0.09, while DF discriminates slightly less well (AUC = 0.58), and OI discriminates poorly (AUC = 0.50). (B) Clinical classification of paroxysmal AF.
Predictive accuracy of electrocardiogram indices for acute endpoints from focal impulse and rotor modulation
We constructed receiver operating characteristic (ROC) curves for the acute FIRM endpoint of AF termination/non-reinducibility or slowing, as well as AF termination/non-reinducibility alone.
For the acute endpoint in the CONFIRM trial, PL provided area under the curve (AUC) of 0.72 for mean PL (Figure 5A), and AUC of 0.68 for maximum PL (three planes). The optimum cutpoint of 0.09 provided specificity of 60%, sensitivity of 74%, and negative and positive predictive values (NPVs and PPVs) of 27 and 92%, respectively, for AF termination/slowing by FIRM. For comparison, spectral OI (optimal cutpoint 0.49) provided specificity 60%, sensitivity 52%, NPV 17%, and PPV 89% (ROC AUC = 0.50). Spectral DF (optimal cutpoint 5.89 Hz) provided specificity 60%, sensitivity 52%, NPV 17%, and PPV 89% (AUC = 0.58).
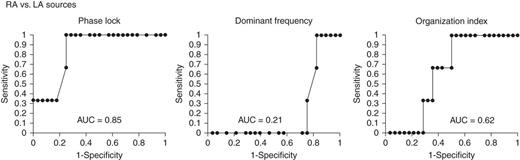
Electrocardiogram PL identifies RA from LA primary AF sources (where FIRM terminated or substantially organized AF). (A) Mean PL in any plane provided AUC = 0.85; by comparison, plane of mean, (B) DF, and (C) OI are less predictive.
For the endpoint of AF termination/non-reinducibility alone, mean PL provided AUC of 0.70 and AUC of 0.72 for maximum PL. The cutpoint of 0.15 provided specificity of 88%, sensitivity of 70%, NPV of 70%, and PPV of 88% for termination of AF by FIRM. Organizational index (maximum three planes for comparison) provided a slightly lower ROC with AUC of 0.67 and DF (maximum three planes) provided an AUC of 0.38.
Determination of right vs. left atrial primary atrial fibrillation sources
In each patient, the primary source was defined as that site where FIRM ablation caused acute AF termination/non-inducibility or AF organization (see Figure 2).
Primary LA sources were present in n = 28 patients, and primary RA sources in n = 3. Notwithstanding the small number of primary RA sources, PL was higher for RA primary sources than LA sources. The ROC curve for the ability of PL to predict the atrium harbouring the AF source produced an AUC of 0.85 (Figure 5). The cut-off of 0.1 to predict a RA source gave a sensitivity of 100%, a specificity of 75%, a PPV of 30%, and a NPV of 100%.
Discussion
Phase lock is a novel ECG index that can identify patients whose AF is caused by fewer stable rotors or focal sources, and whether the primary source lay in RA or LA. A strength of this work is that the mechanistic role of AF sources was established in the CONFIRM trial, in which brief FIRM ablation alone prior to PV ablation terminated and rendered AF non-inducible or substantially organized AF in 86% of patients. Electrocardiogram PL indicates spatially synchronized AF activity, provided 74% sensitivity and 92% PPV for that endpoint. Electrocardiogram PL also identified patients whose AF sources lay in the RA. Electrocardiogram indices of spectral DF and OI had mixed efficacy for these endpoints. These results show the feasibility of bedside approaches to classify AF and provide prognostic information using patient-specific mechanisms for AF perpetuation and their response to therapy.
Electrocardiogram identification of stable sources vs. disorganized ‘bystanders’
These data show that spatial synchronization from stable AF sources2–4 influences the phase and ‘vector’ of atrial activation, despite surrounding ‘bystander’ disorganization, and can be detected from the ECG. Electrocardiogram PL was thus able to identify stable AF sources from a milieu of migratory wavelets.
Evidence that stable rotors or focal sources do indeed sustain AF comes from the recent CONFIRM trial,2 from independent laboratories performing FIRM ablation5,6 and from other intra-atrial contact mapping studies.11–13 In studies of FIRM,2,5,6 stable AF rotors and focal sources were seen in 97–100% of patients with paroxysmal as well as persistent AF, where FIRM ablation alone was able to acutely terminate AF and render it non-reinducible. The acute FIRM endpoint includes marked AF slowing, since AF slowing of 33 ± 12 ms (19 ± 8%)2 by ablation of <2 cm2 of atrial tissue4,10 indicates elimination of secondary AF sources as posited in computer models.29 Results from ECG PL were similar if examining only the endpoint of AF termination and non-reinducibility. The absence of AF termination, when encountered, was strongly associated with incomplete coverage of large atria by the largest current baskets (∼55–60 mm diameter) that may not map sources at all atrial locations.2
The mechanism of AF sustained by organized sources with bystander disorganization puts AF firmly into the existing continuum of arrhythmic organization. For instance, Rodriguez et al.35 reported that even extremely regular typical atrial flutter shows variable collision in the LA contralateral to the cavotricuspid isthmus, while Jais et al.36 and Narayan et al.17 showed that macroreentry at other locations shows marked cycle-to-cycle activation variability remote from the entrainable circuit.
Mechanisms for electrocardiogram phase lock
These data show that interatrial and ECG spatial synchronization assessed by PL falls with the number of concurrent AF sources. As seen in contact intra-atrial FIRM maps, the region of control (prior to fibrillatory breakdown) was larger for fewer AF sources, and smaller for multiple sources (Figures 2 and 3). Phase lock was especially high when solitary sources lay in the RA (Figure 2), since the RA and both appendages show high voltage that may overwhelm signals from elsewhere in the LA and make them more difficult to identify. Future studies should examine whether further refinements such as selective lead combinations may better separate RA from LA sources.
Comparison with prior electrocardiogram analyses of atrial fibrillation
Phase lock differs from prior spectral analyses of AF in that it measures spatial regularity,15,17 while OI analyses F-wave voltage over time19–21. Prior studies show that longer AF CL is more likely to terminate by conventional ablation,30,37 yet this may not in itself inform mechanism since short CL and fractionated electrograms may represent rapid drivers, wave collision, or noise.38 Our ECG analyses also differ from ECG imaging, that was reported to detect AF rotors in only a minority of patients (4 of 26) each with transient (single) rotations.14 Further work should determine if this reflects reported differences between virtual electrograms (from the inverse solution) and contact electrograms in AF,24,39 or other factors.
Technically, we designed to PL quantify harmonics and not their fundamental frequency, because harmonics are pronounced when sources are truly periodic. Conversely, even disorganized AF may show a pronounced DF, yet often with diminutive harmonics. Phase lock was designed to underemphasize such ‘disorganized’ AF. Accordingly, ECG PL indicated AF sources independently of the diagnosis of persistent or paroxysmal AF, while other indices provided better sensitivity for persistent AF but less efficacy for detecting sources.
Clinical implications
Electrocardiogram detection of proven AF mechanisms holds the promise of improving AF management. Classification of AF by PL was independent from presentation as paroxysmal or persistent AF (Figure 5), and may improve prognostication and therapeutic planning. This is particularly true since paroxysmal vs. persistent AF patients often overlap clinically, in that patients may transition from persistent to paroxysmal AF and vice versa,40 and after conventional ablation 40% of paroxysmal AF patients may recur while 40% of persistent AF patients may respond1 after one procedure. Electrocardiogram PL also offers the promise of identifying patients with primary RA AF sources (Figures 2 and 5). We and other investigators have encountered subgroups of patients in whom FIRM ablation in the RA alone hyperacutely terminated AF and rendered it non-inducible, and in whom ongoing studies will determine whether additional ablation is not required.
Limitations
The protocol for CONFIRM allowed FIRM ablation at only 1–3 AF sources,2 although some patients had 4 or more sources and greater success may have been observed if all had been targeted for FIRM. Moreover, not all sources were FIRM ablated due to proximity to the oesophagus, phrenic nerve, or other issues. In addition, the results of CONFIRM are new,2 although stable AF rotor and focal sources and the efficacy of FIRM ablation have now been validated by several independent laboratories in increasing populations (currently >150 patients).5,6 Moreover, the ECG indices developed in this study are complex and require further validation. Moreover, the reported clinical PPVs and NPVs are limited by the small population, high number of patients achieving the acute endpoint, and small number of RA source terminations. Validation of these indices in a larger population is thus required. For this study, we did not try to localize sources within each chamber using multiple planes to avoid the limitations of multiple comparisons. An important point is that while prior PV ablation may alter precise F-wave morphology in some patients, PL focuses only on cycle-to-cycle variations and is independent of F-wave shape. For instance, even if cavotricuspid isthmus flutter no longer shows classical sawtooth F-waves post-PV ablation,41 these ‘atypical’ F-waves will remain consistent from cycle to cycle. Conversely, AF shows cycle-to-cycle variations in F-waves distilled by our analyses to indicate numbers of sources. Finally, we measured AF CL in the coronary sinus as a stable position, acknowledging from our prior work that AF CL may be 10–15 ms longer here than in the appendages.30
Conclusions
The novel ECG metric of PL indicates intra-atrial spatial synchronization, varies inversely with the number of concurrent stable AF sources and can identify whether primary AF-sustaining sources lie in RA or LA. In this study, ECG PL suggests a novel approach for the bedside mechanistic classification of AF, independent of traditional labels of ‘persistent’ or ‘paroxysmal’ AF, and suggests applications to track disease progression, predict response to therapy, and assist with FIRM ablation planning.
Acknowledgements
We are grateful to the University of Cambridge, UK, for authorizing Dr A.J.'s elective in San Diego. We thank Kathleen Mills, BA, for coordinating this study, and Judith Hildreth, RN, Sherie Jaynes, RN, Elizabeth Greer, RN, Stephanie Yoakum, NP, Donna Cooper, RN, Kenneth Hopper CVT, and Anthony Moyeda, CVT for their clinical assistance. This work was supported in part by grants from the American Heart Association to DEK, and from the National Institutes of Health (HL83359, HL103800) to SMN.
Conflict of interest: This work was supported by grants to Dr S.M.N. from the NIH (HL70529, HL83359, HL83359-S1) and Doris Duke Charitable Foundation. Dr S.M.N. is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr S.M.N. holds equity in Topera, and reports having received honoraria from Medtronic, St Jude Medical, and Biotronik. Drs A.J. and D.E.K. report no conflicts.



