-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Paulo Tomaz Barbosa, Manoel Otávio da Costa Rocha, Alexandre Barbosa de Oliveira, Federico Lombardi, Antonio Luiz Pinho Ribeiro, Efficacy and safety of implantable cardioverter-defibrillators in patients with Chagas disease, EP Europace, Volume 15, Issue 7, July 2013, Pages 957–962, https://doi.org/10.1093/europace/eut011
Close - Share Icon Share
Abstract
Implantable cardioverter-defibrillators (ICDs) are now a first-line option for prevention of sudden death in Chagas disease (ChD). However, efficacy and safety of ICD treatment in ChD remains controversial. The aim of our study was to compare clinical outcome after ICD implantation in ChD and non-ChD patients.
The study population consists of patients who received ICD implantation in a tertiary Reference Center for ChD in Brazil. The primary endpoint of the study was appropriate therapy (appropriate shocks or anti-tachycardia pacing); the secondary endpoint was the event-free survival defined as absence of death or appropriate therapy. Three hundred thirty-five patients were followed for the median time of 266 days. Sixty-five patients had ChD. Appropriate ICD therapy occurred in 32 (49.2%) ChD and in 19 (27.1%) non-ChD patients (P=0.005). Ventricular tachycardia occurred in 27 (42%) ChD and in 16 (23%) non-ChD (P = 0.01) patients. There was a statistically significant difference in event-free survival between the group of patients with and without ChD (P=0.004). The median event-free survival was 230 days (95% confidence interval, CI: 113–347) in patients with ChD and 549 days (95% CI: 412–687) in non-ChD patients. Chagas disease double the risk of the patient to have appropriate therapy (hazard ratio, HR = 2.2, 95% CI = 1.2–4.3, P = 0.02) and appropriate therapy or death (HR = 2.2, 95% CI = 1.2–4.2, P = 0.01) in multivariate analysis. There were 16 deaths (11.8%) with 8 deaths in each group and five inappropriate shocks (3.7%) with one in ChD patients (1.6%).
The higher frequency of appropriate ICD therapy and the shorter event-free survival in ChD patients are consistent with the presence of an arrhythmogenic substrate that characterizes this cardiomyopathy.
This is the largest study that compared the efficacy and safety of implantable cardioverter-defibrillator (ICD) therapy in Chagas disease (ChD) and non-ChD patients.
Our results suggest that ICD implantation is a safe and reliable procedure in ChD patients.
Our results provide evidence supporting the ICD implantation in ChD patients, suggest that ICD implantation is the standard therapy for secondary prevention in patients with ChD and the need for a randomized clinical trial is doubtful and, maybe, unethical.
Introduction
Chagas disease (ChD), caused by the protozoan Trypanosoma cruzi, discovered and described by the Brazilian physician Carlos Chagas, in 1909,1 remains a serious public health problem in the Americas, affecting about 10 million people Latin America and four million Brazilians.2 Sudden cardiac death (SCD) is a typical phenomenon in ChD since the first descriptions1 and it is the mechanism of death in more than 50% of ChD patients.3 The mechanism most frequently involved in sudden death in ChD is ventricular tachycardia (VT) degenerating into ventricular fibrillation (VF), or VF not proceeded by VT.4
The use of implantable cardioverter-defibrillator (ICD) has become a main therapeutic strategy for prevention of sudden death.5 Since malignant ventricular arrhythmias are more frequent in patients with ChD than in other forms of heart disease and SCD is frequently observed in ChD,6 this cardiomyopathy is now an emerging and attractive indication for ICD implantation. The ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities recommended ICD implantation in ChD patients for primary and secondary prevention of SCD.7 However, most of the data on which these recommendations were based were extrapolated from results of randomized studies conducted in other cardiac diseases, such as coronary artery disease or idiopathic dilated cardiomyopathy. For these reasons, it was suggested it would be necessary to conduct a randomized prospective trial to compare efficacy of amiodarone vs. ICD therapy in preventing SCD in Chagas' disease patients with malignant ventricular arrhythmias.8–10 Unfortunately, several factors, including economical ones, make the realization of such a study unlikely.
Thus, the benefits and safety of ICD therapy in patients with Chagas cardiomyopathy and ventricular arrhythmia remains controversial and has been evaluated only in a few observational studies6,11–15 that showed discordant results. The main objective of our study was to compare the clinical outcome of patients with and without ChD after ICD implantation.
Methods
Patient population
The study population is a historic cohort of all patients with or without ChD that received ICD implantation for secondary prevention in a Reference Center at the University Hospital of the Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, from January 2007 until May 2009. In this period there were 141 implants, but five were excluded since the follow-up after defibrillator implantation was performed in another city and one due to loss of follow-up. The final study group thus consisted of 135 patients. Data were obtained retrospectively by chart review and recorded on a standardized questionnaire. Data from complimentary tests, as echocardiogram (ECG), Holter monitoring, and invasive electrophysiological test, were collected if available. We selected consecutive patients with a definite serological ChD status (≥2 different positive reactions to T. cruzi). Whole cohort underwent serological testing. All patients were referred for ICD implantation for secondary prevention, according to the guidelines to ICD implantation defined by Brazilian Ministry of Health,16 which includes the following situations: All patients are receiving chronic optimal medical therapy; if feasible, coronary revascularization was performed before ICD implantation in patients with ischaemic cardiomyopathy. Amiodarone was used mostly after the implantation, in order to reduce the frequency of shocks triggered by ventricular arrhythmia.
Resuscitated from cardiac arrest due to documented sustained VT or VF due to non-reversible, with left ventricular ejection fraction (LVEF) less than or equal to 35% or structural heart disease;
Ventricular tachycardia spontaneous, due to non-reversible cause, with LVEF less than or equal to 35% or structural heart disease;
Syncope of unknown origin, with inducibility of hemodynamically unstable or clinically relevant VT or VF, with LVEF less than or equal to 35% or structural heart disease.
Study protocol
The investigation complies with the principles outlined in the Declaration of Helsinki, and the Research Ethics Board of Universidade Federal de Minas Gerais approved the study protocol. Patients were systematically referred to the Pacemaker Clinic of the Hospital das Clinicas, Universidade Federal de Minas Gerais. The surgical techniques used for device implantation were similar to those previously reported.17 The follow-up was based on programmed control visits for evaluation of patients' clinical conditions and device interrogation as well as on hospital admissions for occurrence of device interventions or any acute cardiac illness. Patients' outcome was assessed at 3, 6, 12, 18, and 24 months after the implant, in the Pacemaker Clinic and, when necessary, by telephone interview and chart review. The events recorded and stored by the ICD were retrieved in the form of intracardiac electrogram, including the channel registration of marks, and analysed by three experienced cardiologists with expertise in cardiac arrhythmia.
The primary endpoint of the study was delivery of appropriate therapy, defined as the occurrence of appropriate shock or anti-tachycardia pacing, applied to a potentially lethal ventricular arrhythmia (VT or VF detected by the ICD).9,18 Device programming was standardized with three tachycardia detection zones: Zone 1—heart rate between 150 and 171 bpm; Zone 2—heart rate between 171 and 188 bpm, and Zone 3—heart rate above 188 bpm. In Zone 1, ATP was programmed to two sequences, in Zone 2, one ATP sequence followed by shock if ATP therapy had failed, and in Zone 3, only shock therapy was programmed. Anti-tachycardia pacing (ATP) was set according to a rate immediately superior of that of clinical or induced VT to interrupt the spontaneous detected arrhythmias.19 Shock energy was programmed according to the defibrillation threshold measured at the time of ICD implantation. The secondary endpoint was the event-free survival defined as absence of death and appropriate therapy. Inappropriate shock was defined as those triggered by a rapid ventricular rate due to supraventricular tachyarrhythmias, sinus tachycardia, or device malfunction.9 Sudden cardiac death was defined as death occurring within 1 h of symptoms or during sleep or unwitnessed in a previously medically stable patient.18
Statistical analysis
Continuous variables were expressed as mean±standard deviation or median and interquartil range (from Q1 to Q3) and qualitative variables were described as absolute number and frequency. Appropriate tests were applied for comparison of proportions (Fisher's exact test), means (t test), or medians (Kruskal–Wallis). In all tests we used P value below 0.05 to reject the null hypothesis. Survival curves were plotted using the Kaplan–Meier method and the rate of event-free survival and mortality were compared using the log-rank test (Mantel–Cox).
The contribution of each independent variables was evaluated using Cox regression multivariable models. The results were presented as hazard ratio (HR) with 95% confidence interval (CI). When variables were highly correlated (r ≥0.6), only those judged clinically important variable entered the multivariate model.
Results
Baseline features
The study population consisted of 96 men (71%) and 39 women (29%), aged between 9 and 88 years (median: 60 years). Of these, 65 (48%) patients had ChD, 22 (16.3%) ischaemic cardiomyopathy, 28 (20.7%) non-ischaemic dilated cardiomyopathy, 20 (15%) other cardiomyopathy. In this latter group, the aetiology was unknown but ChD presence was excluded. All were referred for ICD implantation for secondary prevention. Some variables such as New York Heart Association (NYHA) functional class (n=32), medication use (n=35), electrocardiographic parameters (n = 33), and LVEF by echocardiography (n=31), were missing in a few patients.
Most of the patients were in NYHA functional class I or II (76.74%). In the electrocardiogram, the median QRS duration was 120 ms (Q1=90 and Q3=130) and 10 patients (10%) had atrial fibrillation (AF). The Holter test was performed prior to ICD implantation in 32 patients. Of these, 17 (45%) had at least one episode of non-sustained ventricular tachycardia (NSVT), 4 (10%) had at least one VT, and 17 (45%) had NSVT or VT. Electrophysiological study (EPS) from 34 patients, performed before the procedure, showed that VT was induced in 28 (83%) patients, VF in 3 (9%), and 3 others (9%) there was no induction of malignant ventricular arrhythmia (VT or VF).
The median LVEF was 36%, ranging from 13% to 77%. Of the 65 patients with ChD, 20 patients (30%) had LVEF ≥ 45%. Of the 135 patients who received ICD in 69 (51.2%) the system was bicameral (dual chamber rate adaptive pacemaker), in 65 (48.1%) was unicameral system (rate modulated ventricular pacing), and 1 (0.7%) patient received the ICD and resynchronization system. Table 1 shows the comparison between the groups regarding baseline characteristics. The two groups differed on the use of amiodarone and β-blockers (P<0.001) and on the type of bundle branch block found in the baseline ECG (P=0.003).
Comparison of baseline characteristics: demographic, clinical, electrocardiographic, and echocardiographic between patients with and without Chagas undergoing ICD implantation, the HC-UFMG, between January 2007 and May 2009
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Age (years) | 59 (52–65) | 68 (45–73) | 0.89 |
| Male gender (n/%) | 44 (69.8%) | 52 (71.2%) | 0.86 |
| Use of β-blockers (n/%) | 27 (54%) | 41 (83.7%) | 0.001 |
| Use of amiodarone (n/%) | 46 (92%) | 30 (61.2%) | 0.001 |
| NYHA (n = 103) | |||
| I–II (n/%) | 40 (76.9%) | 36 (60.6%) | 0.83 |
| III–IV (n/%) | 12 (23%) | 15 (29.4%) | 0.43 |
| QRS duration (ms) | 120 (90–150) | 120 (87.5–127.5) | 0.07 |
| Atrial fibrillation (n/%) | 5 (9.3%) | 5 (10.6%) | 0.82 |
| RBBB (n/%) | 28 (53%) | 13 (23%) | 0.003 |
| LBBB (n/%) | 5 (9.4%) | 12 (25%) | 0.003 |
| LVEF (%) (n = 104) | 37 (30–50) | 32.5 (22.5–46.5) | 0.99 |
| Follow-up time in days | 270 (72–500) | 263 (60–450) | 0.82 |
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Age (years) | 59 (52–65) | 68 (45–73) | 0.89 |
| Male gender (n/%) | 44 (69.8%) | 52 (71.2%) | 0.86 |
| Use of β-blockers (n/%) | 27 (54%) | 41 (83.7%) | 0.001 |
| Use of amiodarone (n/%) | 46 (92%) | 30 (61.2%) | 0.001 |
| NYHA (n = 103) | |||
| I–II (n/%) | 40 (76.9%) | 36 (60.6%) | 0.83 |
| III–IV (n/%) | 12 (23%) | 15 (29.4%) | 0.43 |
| QRS duration (ms) | 120 (90–150) | 120 (87.5–127.5) | 0.07 |
| Atrial fibrillation (n/%) | 5 (9.3%) | 5 (10.6%) | 0.82 |
| RBBB (n/%) | 28 (53%) | 13 (23%) | 0.003 |
| LBBB (n/%) | 5 (9.4%) | 12 (25%) | 0.003 |
| LVEF (%) (n = 104) | 37 (30–50) | 32.5 (22.5–46.5) | 0.99 |
| Follow-up time in days | 270 (72–500) | 263 (60–450) | 0.82 |
Data are numbers (percentages) or medians (Q1–Q3).
HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais; NYHA, New York Heart Association; RBBB, right bundle-branch block; LBBB, left bundle-branch block; LVEF, left ventricular ejection fraction.
Comparison of baseline characteristics: demographic, clinical, electrocardiographic, and echocardiographic between patients with and without Chagas undergoing ICD implantation, the HC-UFMG, between January 2007 and May 2009
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Age (years) | 59 (52–65) | 68 (45–73) | 0.89 |
| Male gender (n/%) | 44 (69.8%) | 52 (71.2%) | 0.86 |
| Use of β-blockers (n/%) | 27 (54%) | 41 (83.7%) | 0.001 |
| Use of amiodarone (n/%) | 46 (92%) | 30 (61.2%) | 0.001 |
| NYHA (n = 103) | |||
| I–II (n/%) | 40 (76.9%) | 36 (60.6%) | 0.83 |
| III–IV (n/%) | 12 (23%) | 15 (29.4%) | 0.43 |
| QRS duration (ms) | 120 (90–150) | 120 (87.5–127.5) | 0.07 |
| Atrial fibrillation (n/%) | 5 (9.3%) | 5 (10.6%) | 0.82 |
| RBBB (n/%) | 28 (53%) | 13 (23%) | 0.003 |
| LBBB (n/%) | 5 (9.4%) | 12 (25%) | 0.003 |
| LVEF (%) (n = 104) | 37 (30–50) | 32.5 (22.5–46.5) | 0.99 |
| Follow-up time in days | 270 (72–500) | 263 (60–450) | 0.82 |
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Age (years) | 59 (52–65) | 68 (45–73) | 0.89 |
| Male gender (n/%) | 44 (69.8%) | 52 (71.2%) | 0.86 |
| Use of β-blockers (n/%) | 27 (54%) | 41 (83.7%) | 0.001 |
| Use of amiodarone (n/%) | 46 (92%) | 30 (61.2%) | 0.001 |
| NYHA (n = 103) | |||
| I–II (n/%) | 40 (76.9%) | 36 (60.6%) | 0.83 |
| III–IV (n/%) | 12 (23%) | 15 (29.4%) | 0.43 |
| QRS duration (ms) | 120 (90–150) | 120 (87.5–127.5) | 0.07 |
| Atrial fibrillation (n/%) | 5 (9.3%) | 5 (10.6%) | 0.82 |
| RBBB (n/%) | 28 (53%) | 13 (23%) | 0.003 |
| LBBB (n/%) | 5 (9.4%) | 12 (25%) | 0.003 |
| LVEF (%) (n = 104) | 37 (30–50) | 32.5 (22.5–46.5) | 0.99 |
| Follow-up time in days | 270 (72–500) | 263 (60–450) | 0.82 |
Data are numbers (percentages) or medians (Q1–Q3).
HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais; NYHA, New York Heart Association; RBBB, right bundle-branch block; LBBB, left bundle-branch block; LVEF, left ventricular ejection fraction.
Follow-up
The median follow-up of patients was 266 days (Q1=72, Q3=466). One hundred and thirty-five patients we entered in study. Of the 133 patients who had data on the type of prevention, 121 (91%) were recovered from sudden death, 7 (5%) had experienced at least one episode of spontaneous VT, and five (4%) underwent ICD implantation, because they presented with VT-induced syncope in the electrophysiologic study. Perioperative complications were observed in six patients (4.4%); pneumothorax occurred in three cases, two presented hemothorax, and one patient died in the immediate post-implantation. During follow-up there was only one heart transplantation (a ChD patient). This patient was censored. One patient developed infective endocarditis in the defibrillator electrode cable and was treated with exchange of the system and antibiotics, with therapeutic success.
There was no statistically significant difference between groups regarding time to first appropriate therapy, mortality, occurrence of VF, rate of inappropriate shocks and complications (Table 2).
Comparison between patients with and without ChD on the characteristics of treatment with implantable cardioverter-defibrillator, undergoing ICD implantation, the HC-UFMG, between January 2007 and May 2009
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Appropriate therapy (n/%) | 32 (49.2%) | 19 (27.1%) | 0.005 |
| Ventricular tachycardia (n/%) | 27 (42%) | 16 (23%) | 0.01 |
| Number of appropriate therapy | 6 (1–35) | 1.5 (0–2.75) | 0.01 |
| Number of shocks | 4 (2–11) | 1.5 (0.25–3.75) | 0.03 |
| Number of antitachycardia pacing | 12 (5–54) | 3 (2–4.75) | 0.004 |
| Number of ventricular tachycardia | 843 | 66 | 0.004 |
| Number of ventricular fibrillation | 63 | 8 | 0.03 |
| The time to first a appropriate therapy (days) | 120 (93–246) | 93 (54.5–259.5) | 0.51 |
| Complications (n/%) | 3 (4.7%) | 3 (4.2%) | 0.89 |
| Inappropriate shock (n/%) | 1 (1.6%) | 4 (5.5%) | 0.23 |
| Deaths (n/%) | 8 (12.3%) | 8 (11.4%) | 0.82 |
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Appropriate therapy (n/%) | 32 (49.2%) | 19 (27.1%) | 0.005 |
| Ventricular tachycardia (n/%) | 27 (42%) | 16 (23%) | 0.01 |
| Number of appropriate therapy | 6 (1–35) | 1.5 (0–2.75) | 0.01 |
| Number of shocks | 4 (2–11) | 1.5 (0.25–3.75) | 0.03 |
| Number of antitachycardia pacing | 12 (5–54) | 3 (2–4.75) | 0.004 |
| Number of ventricular tachycardia | 843 | 66 | 0.004 |
| Number of ventricular fibrillation | 63 | 8 | 0.03 |
| The time to first a appropriate therapy (days) | 120 (93–246) | 93 (54.5–259.5) | 0.51 |
| Complications (n/%) | 3 (4.7%) | 3 (4.2%) | 0.89 |
| Inappropriate shock (n/%) | 1 (1.6%) | 4 (5.5%) | 0.23 |
| Deaths (n/%) | 8 (12.3%) | 8 (11.4%) | 0.82 |
Data are numbers (percentages) or medians (Q1–Q3).
HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais.
Comparison between patients with and without ChD on the characteristics of treatment with implantable cardioverter-defibrillator, undergoing ICD implantation, the HC-UFMG, between January 2007 and May 2009
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Appropriate therapy (n/%) | 32 (49.2%) | 19 (27.1%) | 0.005 |
| Ventricular tachycardia (n/%) | 27 (42%) | 16 (23%) | 0.01 |
| Number of appropriate therapy | 6 (1–35) | 1.5 (0–2.75) | 0.01 |
| Number of shocks | 4 (2–11) | 1.5 (0.25–3.75) | 0.03 |
| Number of antitachycardia pacing | 12 (5–54) | 3 (2–4.75) | 0.004 |
| Number of ventricular tachycardia | 843 | 66 | 0.004 |
| Number of ventricular fibrillation | 63 | 8 | 0.03 |
| The time to first a appropriate therapy (days) | 120 (93–246) | 93 (54.5–259.5) | 0.51 |
| Complications (n/%) | 3 (4.7%) | 3 (4.2%) | 0.89 |
| Inappropriate shock (n/%) | 1 (1.6%) | 4 (5.5%) | 0.23 |
| Deaths (n/%) | 8 (12.3%) | 8 (11.4%) | 0.82 |
| . | Chagas disease, n = 65 . | Non-Chagas disease, n = 70 . | P value . |
|---|---|---|---|
| Appropriate therapy (n/%) | 32 (49.2%) | 19 (27.1%) | 0.005 |
| Ventricular tachycardia (n/%) | 27 (42%) | 16 (23%) | 0.01 |
| Number of appropriate therapy | 6 (1–35) | 1.5 (0–2.75) | 0.01 |
| Number of shocks | 4 (2–11) | 1.5 (0.25–3.75) | 0.03 |
| Number of antitachycardia pacing | 12 (5–54) | 3 (2–4.75) | 0.004 |
| Number of ventricular tachycardia | 843 | 66 | 0.004 |
| Number of ventricular fibrillation | 63 | 8 | 0.03 |
| The time to first a appropriate therapy (days) | 120 (93–246) | 93 (54.5–259.5) | 0.51 |
| Complications (n/%) | 3 (4.7%) | 3 (4.2%) | 0.89 |
| Inappropriate shock (n/%) | 1 (1.6%) | 4 (5.5%) | 0.23 |
| Deaths (n/%) | 8 (12.3%) | 8 (11.4%) | 0.82 |
Data are numbers (percentages) or medians (Q1–Q3).
HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais.
In comparison to non-ChD patients, ChD patients showed higher percentages of appropriate therapy: appropriate shocks and antitachycardia pacing; higher percentage of VT, greater number of episodes of VT and VF. The median time to first appropriate therapy was 120 days (Q1 = 93, Q3 = 246) in patients with ChD and 93 days (Q1 = 54, Q3 = 259) in non-ChD patients (P = 0.55). Ventricular tachycardia occurred in 27 (42%) ChD and in 16 (23%) non-ChD patients (P = 0.01) with 843 VT episodes in ChD and in 66 VT episodes in non-ChD patients (P = 0.004). Patients with ChD had 13 VT episodes detected per patient, while patients with non-ChD had 0.9 VT episodes per patient. Regarding of VF episodes, VF occurred in nine (13%) ChD and in four (6%) non-ChD patients (P = 0.06) with 63 VF episodes in ChD and in 8 VF episodes in non-ChDpatients (P = 0.03).
During the follow-up period, 16 (11.8%) patients died, eight in each group. In the non-ChD group, two patients had SCD, two (25%) died for pump failure, one for a non-cardiac cause whereas in three subjects the cause of death was unknown. In ChD patients, two had SCD, two died for congestive heart failure, and four for a non-cardiac cause.
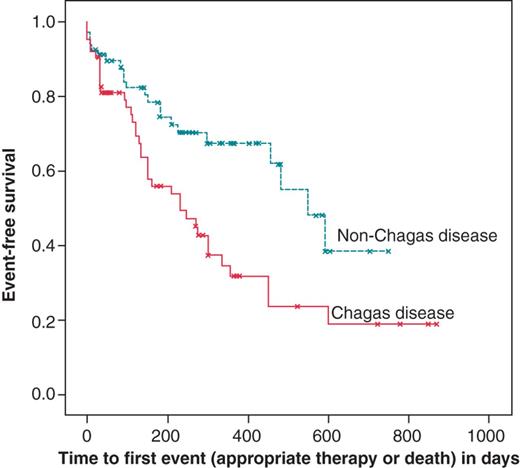
There was a statistically significant difference in event-free survival between the group of patients with and without ChD (P=0.004). The median event-free survival was 230 days (95% CI: 113–347) in patients with ChD and 549 days (95% CI: 412–687) in non-ChD patients. Figure 1 displays the curve of event-free survival of patients with and without ChD.
Predictors of events
In univariate analysis, none of the candidates variables, including age, sex, drugs, LVEF (as a continuous variable or dichotomized as < or ≥35%), NYHA functional class, AF, QRS width, 24 h Holter and EPS parameters and the number of shocks, were associated with the occurrence of appropriate therapy and with the event-free survival (appropriate therapy or death). The only variable associated with these outcomes was the diagnosis of ChD.
The HR for appropriate therapy and event-free survival were, respectively, 2.2 (95% CI 1.2–4.3; P=0.02) and 2.1 (95%CI 1.3–3.6, P=0.005). Table 3 shows patients characteristics according to the occurrence of the combined outcome in univariate analysis.
Characteristics of 135 patients undergoing ICD implantation by the occurrence of the combined outcome (appropriate therapy or death), HC-UFMG, from January 2007 to May 2009, in univariate analysis
| . | Appropriate therapy or death, n = 62 . | Without appropriate therapy or death, n = 73 . | P value . | Hazard ratio (CI 95%) . |
|---|---|---|---|---|
| Age (years) | 61.5 (51.5–66) | 59 (49.75–68.25) | 0.87 | 0.99 (0.9–1.0) |
| Male gender (n/%) | 44 (45.8%) | 52 (54.2%) | 0.93 | 1.4 (0.8–2.4) |
| Chagas disease (n/%) | 39 (62.9%) | 26 (35.3%) | 0.02 | 2.2 (1.2–4.3) |
| NYHA III–IV (n/%) | 13 (48.1%) | 14 (51.9%) | 0.71 | 1.0 (0.5–1.9) |
| Beta-blockers (n/%) | 32 (47.1%) | 36 (52.9%) | 0.86 | 1.1 (0.6–2.1) |
| Amiodarone (n/%) | 37 (48.7%) | 39 (51.3%) | 0.42 | 1.2 (0.6–2.5) |
| Atrial fibrillation (n/%) | 4 (40%) | 6 (60%) | 0.66 | 0.8 (0.2–2.5) |
| QRS (ms) | 120 (92.5–130) | 120 (90–135) | 0.75 | 1.0 (0.9–1.0) |
| LVEF (%) | 35 (28.25–46.1) | 36 (30–51) | 0.31 | 0.9 (0.9–1.0) |
| LVEF < 35% (n/%) | 25 (49%) | 26 (51%) | 0.57 | 1.0 (0.6–1.8) |
| Follow-up time in days | 276 (113–529) | 259 (57–373) | 0.39 |
| . | Appropriate therapy or death, n = 62 . | Without appropriate therapy or death, n = 73 . | P value . | Hazard ratio (CI 95%) . |
|---|---|---|---|---|
| Age (years) | 61.5 (51.5–66) | 59 (49.75–68.25) | 0.87 | 0.99 (0.9–1.0) |
| Male gender (n/%) | 44 (45.8%) | 52 (54.2%) | 0.93 | 1.4 (0.8–2.4) |
| Chagas disease (n/%) | 39 (62.9%) | 26 (35.3%) | 0.02 | 2.2 (1.2–4.3) |
| NYHA III–IV (n/%) | 13 (48.1%) | 14 (51.9%) | 0.71 | 1.0 (0.5–1.9) |
| Beta-blockers (n/%) | 32 (47.1%) | 36 (52.9%) | 0.86 | 1.1 (0.6–2.1) |
| Amiodarone (n/%) | 37 (48.7%) | 39 (51.3%) | 0.42 | 1.2 (0.6–2.5) |
| Atrial fibrillation (n/%) | 4 (40%) | 6 (60%) | 0.66 | 0.8 (0.2–2.5) |
| QRS (ms) | 120 (92.5–130) | 120 (90–135) | 0.75 | 1.0 (0.9–1.0) |
| LVEF (%) | 35 (28.25–46.1) | 36 (30–51) | 0.31 | 0.9 (0.9–1.0) |
| LVEF < 35% (n/%) | 25 (49%) | 26 (51%) | 0.57 | 1.0 (0.6–1.8) |
| Follow-up time in days | 276 (113–529) | 259 (57–373) | 0.39 |
Data are numbers (percentages) or medians (Q1–Q3).
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais.
Characteristics of 135 patients undergoing ICD implantation by the occurrence of the combined outcome (appropriate therapy or death), HC-UFMG, from January 2007 to May 2009, in univariate analysis
| . | Appropriate therapy or death, n = 62 . | Without appropriate therapy or death, n = 73 . | P value . | Hazard ratio (CI 95%) . |
|---|---|---|---|---|
| Age (years) | 61.5 (51.5–66) | 59 (49.75–68.25) | 0.87 | 0.99 (0.9–1.0) |
| Male gender (n/%) | 44 (45.8%) | 52 (54.2%) | 0.93 | 1.4 (0.8–2.4) |
| Chagas disease (n/%) | 39 (62.9%) | 26 (35.3%) | 0.02 | 2.2 (1.2–4.3) |
| NYHA III–IV (n/%) | 13 (48.1%) | 14 (51.9%) | 0.71 | 1.0 (0.5–1.9) |
| Beta-blockers (n/%) | 32 (47.1%) | 36 (52.9%) | 0.86 | 1.1 (0.6–2.1) |
| Amiodarone (n/%) | 37 (48.7%) | 39 (51.3%) | 0.42 | 1.2 (0.6–2.5) |
| Atrial fibrillation (n/%) | 4 (40%) | 6 (60%) | 0.66 | 0.8 (0.2–2.5) |
| QRS (ms) | 120 (92.5–130) | 120 (90–135) | 0.75 | 1.0 (0.9–1.0) |
| LVEF (%) | 35 (28.25–46.1) | 36 (30–51) | 0.31 | 0.9 (0.9–1.0) |
| LVEF < 35% (n/%) | 25 (49%) | 26 (51%) | 0.57 | 1.0 (0.6–1.8) |
| Follow-up time in days | 276 (113–529) | 259 (57–373) | 0.39 |
| . | Appropriate therapy or death, n = 62 . | Without appropriate therapy or death, n = 73 . | P value . | Hazard ratio (CI 95%) . |
|---|---|---|---|---|
| Age (years) | 61.5 (51.5–66) | 59 (49.75–68.25) | 0.87 | 0.99 (0.9–1.0) |
| Male gender (n/%) | 44 (45.8%) | 52 (54.2%) | 0.93 | 1.4 (0.8–2.4) |
| Chagas disease (n/%) | 39 (62.9%) | 26 (35.3%) | 0.02 | 2.2 (1.2–4.3) |
| NYHA III–IV (n/%) | 13 (48.1%) | 14 (51.9%) | 0.71 | 1.0 (0.5–1.9) |
| Beta-blockers (n/%) | 32 (47.1%) | 36 (52.9%) | 0.86 | 1.1 (0.6–2.1) |
| Amiodarone (n/%) | 37 (48.7%) | 39 (51.3%) | 0.42 | 1.2 (0.6–2.5) |
| Atrial fibrillation (n/%) | 4 (40%) | 6 (60%) | 0.66 | 0.8 (0.2–2.5) |
| QRS (ms) | 120 (92.5–130) | 120 (90–135) | 0.75 | 1.0 (0.9–1.0) |
| LVEF (%) | 35 (28.25–46.1) | 36 (30–51) | 0.31 | 0.9 (0.9–1.0) |
| LVEF < 35% (n/%) | 25 (49%) | 26 (51%) | 0.57 | 1.0 (0.6–1.8) |
| Follow-up time in days | 276 (113–529) | 259 (57–373) | 0.39 |
Data are numbers (percentages) or medians (Q1–Q3).
LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; HC-UFMG, Hospital das Clínicas, Universidade Federal de Minas Gerais.
In multivariate analysis, four variables entered the full model: age, male gender, LVEF, and ChD were considered potential predictors of appropriate therapy combined outcome or death. Only ChD remained with prognostic significance after multivariate analysis, using the backward stepwise method. Chagas disease doubles the risk of the patient to have appropriate therapy (HR=2.2, 95% CI = 1.2–4.3, P=0.02) and appropriate therapy or death (HR=2.2, 95% CI=1.2–4.2, P=0.01).
Discussion
This observational study reports the clinical impact of ICD therapy in patients with Chagas' disease treated for secondary prevention of sudden death, comparing them with patients with non-ChD. The main finding of this study was the high frequency of appropriate ICD therapy during a relatively short period of follow-up, which was significantly higher than that observed in non-ChD patients.
This result reinforces the concept that malignant ventricular arrhythmias are a main feature of ChD with important physiopathological and clinical implications.
To our knowledge, this is the largest study that compared the efficacy and safety of ICD therapy in ChD and non-ChD patients;6,11,12,15 however, there are series of ICD recipients in ChD larger than the described in this article, although without a comparison group (in Dubner et al.'s,11 study, 201 patients and in Muratore et al.'s9 study, 89 patients). It is worth recalling that controversial results have been reported in previous studies. For example, in relation to ICD interventions, a higher frequency of ICD therapy in ChD patients in comparison to non-ChD ones was reported by two studies6,12 and but not by other two studies.11,15 The small number of ChD patients enrolled in these studies may explain the variety of results due to inadequate statistical power to detect differences between groups. Our study, in which 65 ChD patients were followed for the median time of 270 days, is suggestive that events are commoner in ChD patients than in patients with ischaemic or other non-ischaemic cardiomyopathy. Indeed, previous studies showed that 84–100% of ChD patients received at least one appropriate therapy during a follow-up period that varied from 180 to 660 days.4,6,12 Nonetheless, we could not find a higher frequency of arrhythmic events in the first months after implantation of the ICD in ChD patients, as reported by Rabinovich et al.12 These authors observed that 55% of ChD patients received the first shock in the first month after implantation, compared with only 14% of patients with coronary artery disease.12 In our study, carried out in ChD patients in which the ICD was implanted for secondary prevention, the average time to first appropriate therapy was 120 days, with no difference between patients with and without ChD.
The high prevalence of appropriate therapy triggered by the ICD secondary to malignant arrhythmia observed in ChD patients in our study and in others4,6,9,13,19 could indeed reflect the pro-arrhythmic role of two main characteristics of this cardiomyopathy. First, the alterations of the electrophysiological substrate, where multiple re-entry circuits involving areas of fibrosis or aneurysm of the left ventricle may predispose the patient to a high incidence of sudden death, which could be prevented by appropriate ICD therapy. Second, the presence of progressive alterations in autonomic control mechanisms that may further alter cardiac electrical properties.3
The high frequency of shocks in patients with ChD during this relatively short follow-up indicates the presence of significant arrhythmic burden in patients with ChD.3,20 We previously reported that ChD patients with implanted pacemakers presented more frequent ventricular arrhythmia21 and lower scores of a quality of life when compared with pacemaker patients without ChD.22,23
The low occurrence of perioperative (4.7%) and late complications (one case of infective endocarditis), in ChD patients coupled with the low rate of inappropriate shocks (1.6%), confirms that ICD implantation is a safe and reliable procedure in ChD patients.9,14,15 The percentage of inappropriate shock was also lower than the frequency reported in another Brazilian report, by Fonseca et al.,15 which was 9.7%.
In our sample of patients in which the ICD was implanted as secondary prevention of sudden death, diagnosis of ChD was the only independent predictor of appropriate therapy or death (P=0.01). At variance with previous reports,24–26 the traditional parameters such as age, male sex, ventricular dysfunction, severe ventricular dysfunction (LVEF < 35%), and NYHA functional class were not predictive of the combined endpoint: death or appropriate therapy.
Indeed, since Carlos Chagas first studies, it is known that sudden death can be the first clinical manifestation in ChD, a finding reinforced by recent studies showing that sudden death can occur in ChD patients with preserved LV function.27,28
Of particular interest, in our opinion, was the finding that the diagnosis of ChD doubles the risk of the patient to receive appropriate therapy or to die. One interpretation of these data is that ChD patients have a more severe prognosis than patients with other cardiopathies,27,29 and ICD therapy has significant efficacy in ChD patients. Indeed, these data suggest that ICD implantation is the standard therapy for secondary prevention in patients with ChD; the need for a randomized clinical trial is doubtful and, maybe, unethical. However, it should be noted that appropriate therapy is a surrogate endpoint and that appropriate shocks could not be considered an outcome equivalent to that aborted SCD. Indeed, several episodes of VT and some of VF stored in ICD memory and treated by the device could end spontaneously and do not necessarily result in sudden death cardiac.30
Some limitations of this study must be outlined: this was an observational and retrospective study, reporting the experience of a single reference centre. Some variables such as NYHA functional class, medication use, dose of drugs, electrocardiographic parameters, and LVEF by echocardiography, were missing in a few patients. The mean follow-up was relatively short, thus limiting the possibility of detecting the incidence of appropriate and inappropriate therapies as well as late complications during a longer follow-up period. However, the follow-up time can be considered sufficient, considering the short median time to first appropriate therapy observed in both groups. Moreover, the control group is unmatched to the ChD group.
In conclusion, we found that ChD increased by 2.2 times the chance of patients receiving an ICD appropriate therapy. The higher incidence of arrhythmic events in ChD patients is consistent with the concept of ChD is an arrhythmogenic disease. The difference in event-free survival between ChD and non-ChD patients, coupled with the fact that ChD was the only independent predictor of combined outcome death or appropriate therapy in this sample, indicates the importance of ICD implantation in ChD patients.
Conflict of interest: There are no conflicts of interest and the study complies with current ethical considerations.



