-
PDF
- Split View
-
Views
-
Cite
Cite
Jordana Kron, William Sauer, Joseph Schuller, Frank Bogun, Thomas Crawford, Sinan Sarsam, Lynda Rosenfeld, Teferi Y. Mitiku, Joshua M. Cooper, Davendra Mehta, Arnold J. Greenspon, Matthew Ortman, David B. Delurgio, Ravinder Valadri, Calambur Narasimhan, Nalla Swapna, Jagmeet P. Singh, Stephan Danik, Steven M. Markowitz, Adrian K. Almquist, Andrew D. Krahn, Luke G. Wolfe, Shawn Feinstein, Kenneth A. Ellenbogen, Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis, EP Europace, Volume 15, Issue 3, March 2013, Pages 347–354, https://doi.org/10.1093/europace/eus316
Close - Share Icon Share
Abstract
Implantable cardiac defibrillator (ICD) implantation is a class IIA recommendation for patients with cardiac sarcoidosis (CS). However, little is known about the efficacy and safety of ICDs in this population. The goal of this multicentre retrospective data review was to evaluate the efficacy and safety of ICDs in patients with CS.
Electrophysiologists at academic medical centres were asked to identify consecutive patients with CS and an ICD. Clinical information, ICD therapy history, and device complications were collected for each patient. Data were collected on 235 patients from 13 institutions, 64.7% male with mean age 55.6 ± 11.1. Over a mean follow-up of 4.2 ± 4.0 years, 85 of 234 (36.2%) patients received an appropriate ICD therapy (shocks and/or anti-tachycardia pacing) and 67 of 226 (29.7%) received an appropriate shock. Fifty-seven of 235 patients (24.3%) received a total of 222 inappropriate shocks. Forty-six adverse events occurred in 41 of 235 patients (17.4%). Patients who received appropriate ICD therapies were more likely to be male (73.8 vs. 59.6%, P = 0.0330), have a history of syncope (40.5 vs. 22.5%, P = 0.0044), lower left ventricular ejection fraction (38.1 ± 15.2 vs. 48.8 ± 14.7%, P ≤ 0.0001), ventricular pacing on baseline electrocardiogram (16.1 vs. 2.1%, P = 0.0002), and a secondary prevention indication (60.7 vs. 24.5%, P < 0.0001) compared with those who did not receive appropriate ICD therapies.
Patients with CS and ICDs are at high risk for ventricular arrhythmias. This population also has high rates of inappropriate shocks and device complications.
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown aetiology. Myocardial involvement occurs in at least 25% of patients with sarcoidosis1 and patients with cardiac sarcoidosis (CS) are at increased risk for sudden death from ventricular arrhythmias. Because cardiac involvement can be asymptomatic, ventricular tachycardia or sudden cardiac death may be the first presentation of CS.2,3 Implantable cardiac defibrillators (ICDs) have been used for primary and secondary prevention of sudden death in patients with CS,4–6 and small studies suggest a high incidence of ventricular tachyarrhythmias in these patients.7,8 Cardiac sarcoidosis is a class IIa (level of evidence C) recommendation for an ICD; however, there is little outcome data on the use of ICDs in this population.9 The purpose of this study was to evaluate the efficacy and safety of ICDs in patients with CS.
Methods
Study population and diagnosis of cardiac sarcoidosis
Clinical cardiac electrophysiologists at major medical centres in the United States, Canada, and India were asked to identify patients in their clinical practice with CS and an ICD. Deceased patients could be included but were not systematically identified. Inclusion criteria required patients to have CS based on: (i) biopsy-proven cardiac sarcoid, (ii) magnetic resonance imaging (MRI) findings suggestive of cardiac sarcoid, or (iii) biopsy-proven sarcoidosis in another organ and presumptive cardiac involvement based on conduction system disease involving the sinus node, AV node, or His-Purkinje system and/or ventricular arrhythmias. A questionnaire was completed for each patient by the electrophysiologist. The questionnaire included demographic information, history of extra-CS, medical therapy, electrocardiogram (ECG) information, diagnostic cardiac studies, ICD therapy history, and device complications. The clinical electrophysiologist at the centre made the determination of whether ICD therapy was appropriate or inappropriate.
This study was approved by the Institutional Review Board at Virginia Commonwealth University and the IRB at each participating centre as required. This study is registered at clinicaltrials.gov (NCT01013311).
Statistics
All statistical analyses were performed using SAS 9.3. All tests were two-tailed with a significance level of 0.05. Categorical data were analysed using Fisher's exact test due to the sparseness of variables in individual cells in some of the tables. Continuous variables were compared using the Student's t-test.
Results
Clinical characteristics of the study cohort
Data were collected on 235 patients from 13 institutions in the United States, Canada, and India. Demographic and clinical data are shown in Table 1. Ninety-nine patients from three centres were included in previous smaller CS series.7,8 The mean time from device implant to data collection was 4.2 ± 4.0 years (range: 1 day to 20.5 years). Pulmonary involvement, the most common extra-cardiac organ disease, occurred in 197 patients (83.8%). Other common sarcoid involvement included systemic disease manifesting as weight loss, fevers, or sweats, n = 24 (10.2%); skin disease, n = 24 (10.2%); and ophthalmic disease, n = 17 (7.2%). Neurologic, liver, and renal sarcoidosis were less common. Extra-cardiac involvement of sarcoidosis is summarized in Table 2. One hundred and three patients (43.8%) were on anti-arrhythmic drug therapy, most commonly sotalol, n = 58 (24.7%), followed by amiodarone, n = 45 (19.2%). More than half of the patients were on a beta-blocker, n = 150 (63.8%). Steroids were used as immunosuppressive therapy in 142 patients (60.4%) and methotrexate was used in 46 patients (19.6%). Anti-arrhythmic drug therapy and immunosuppressive therapy are shown in Table 3. Of 223 patients with ECGs available for analysis, 150 patients (67.3%) had an abnormal baseline ECG. Baseline ECG is defined as the first ECG performed as part of the work-up for diagnosis of CS. Right bundle branch block (RBBB) was the most common conduction abnormality, n = 63 (28.3%) followed by first-degree AV block, n = 34 (15.3%). Third-degree AV block was seen in 22 patients (9.9%), and 16 patients (7.2%) had ventricular pacing on ECG. The underlying rhythm in patients who had ventricular pacing was not evaluated.
Demographic and clinical variables are shown for the cardiac sarcoidosis cohort
| Demographics and clinical variable . | Patients . |
|---|---|
| Male | 152/235 (64.7%) |
| Age (n = 231) | 55.6 ± 11.1 (range 22–82) |
| Status alive | 193/209 (92.3%) |
| NYHA Class I | 89/203 (43.8%) |
| Class II | 68/203 (33.5%) |
| Class III | 42/203 (20.7%) |
| Class IV | 4/203 (2.0%) |
| Secondary prevention | 88/235 (37.5%) |
| Syncope | 68/235 (28.9%) |
| Family history of sarcoid | 14/187 (7.5%) |
| LVEF (%) (n = 233) | 45.0 ± 15.7 (range 5–74) |
| Abnormal cardiac MRI | 99/115 (86.1%) |
| Abnormal cardiac biopsy | 41/58 (70.7%) |
| Demographics and clinical variable . | Patients . |
|---|---|
| Male | 152/235 (64.7%) |
| Age (n = 231) | 55.6 ± 11.1 (range 22–82) |
| Status alive | 193/209 (92.3%) |
| NYHA Class I | 89/203 (43.8%) |
| Class II | 68/203 (33.5%) |
| Class III | 42/203 (20.7%) |
| Class IV | 4/203 (2.0%) |
| Secondary prevention | 88/235 (37.5%) |
| Syncope | 68/235 (28.9%) |
| Family history of sarcoid | 14/187 (7.5%) |
| LVEF (%) (n = 233) | 45.0 ± 15.7 (range 5–74) |
| Abnormal cardiac MRI | 99/115 (86.1%) |
| Abnormal cardiac biopsy | 41/58 (70.7%) |
Demographic and clinical variables are shown for the cardiac sarcoidosis cohort
| Demographics and clinical variable . | Patients . |
|---|---|
| Male | 152/235 (64.7%) |
| Age (n = 231) | 55.6 ± 11.1 (range 22–82) |
| Status alive | 193/209 (92.3%) |
| NYHA Class I | 89/203 (43.8%) |
| Class II | 68/203 (33.5%) |
| Class III | 42/203 (20.7%) |
| Class IV | 4/203 (2.0%) |
| Secondary prevention | 88/235 (37.5%) |
| Syncope | 68/235 (28.9%) |
| Family history of sarcoid | 14/187 (7.5%) |
| LVEF (%) (n = 233) | 45.0 ± 15.7 (range 5–74) |
| Abnormal cardiac MRI | 99/115 (86.1%) |
| Abnormal cardiac biopsy | 41/58 (70.7%) |
| Demographics and clinical variable . | Patients . |
|---|---|
| Male | 152/235 (64.7%) |
| Age (n = 231) | 55.6 ± 11.1 (range 22–82) |
| Status alive | 193/209 (92.3%) |
| NYHA Class I | 89/203 (43.8%) |
| Class II | 68/203 (33.5%) |
| Class III | 42/203 (20.7%) |
| Class IV | 4/203 (2.0%) |
| Secondary prevention | 88/235 (37.5%) |
| Syncope | 68/235 (28.9%) |
| Family history of sarcoid | 14/187 (7.5%) |
| LVEF (%) (n = 233) | 45.0 ± 15.7 (range 5–74) |
| Abnormal cardiac MRI | 99/115 (86.1%) |
| Abnormal cardiac biopsy | 41/58 (70.7%) |
| Organ system involvement of sarcoidosis . | Patients (n = 235) . |
|---|---|
| Pulmonary | 197 (83.8%) |
| Systemic | 24 (10.2%) |
| Skin | 24 (10.2%) |
| Ophthalmic | 17 (7.2%) |
| Neurologic | 15 (6.4%) |
| Liver | 15 (6.4%) |
| Renal | 9 (3.8%) |
| Spleen | 7 (2.9%) |
| Pericardium | 4 (1.7%) |
| Intestine | 2 (0.9%) |
| Stomach | 1 (0.4%) |
| Colon | 1 (0.4%) |
| Nose | 1 (0.4%) |
| Larynx | 1 (0.4%) |
| Testes | 1 (0.4%) |
| Pancreas | 1 (0.4%) |
| Muscle | 1 (0.4%) |
| Organ system involvement of sarcoidosis . | Patients (n = 235) . |
|---|---|
| Pulmonary | 197 (83.8%) |
| Systemic | 24 (10.2%) |
| Skin | 24 (10.2%) |
| Ophthalmic | 17 (7.2%) |
| Neurologic | 15 (6.4%) |
| Liver | 15 (6.4%) |
| Renal | 9 (3.8%) |
| Spleen | 7 (2.9%) |
| Pericardium | 4 (1.7%) |
| Intestine | 2 (0.9%) |
| Stomach | 1 (0.4%) |
| Colon | 1 (0.4%) |
| Nose | 1 (0.4%) |
| Larynx | 1 (0.4%) |
| Testes | 1 (0.4%) |
| Pancreas | 1 (0.4%) |
| Muscle | 1 (0.4%) |
| Organ system involvement of sarcoidosis . | Patients (n = 235) . |
|---|---|
| Pulmonary | 197 (83.8%) |
| Systemic | 24 (10.2%) |
| Skin | 24 (10.2%) |
| Ophthalmic | 17 (7.2%) |
| Neurologic | 15 (6.4%) |
| Liver | 15 (6.4%) |
| Renal | 9 (3.8%) |
| Spleen | 7 (2.9%) |
| Pericardium | 4 (1.7%) |
| Intestine | 2 (0.9%) |
| Stomach | 1 (0.4%) |
| Colon | 1 (0.4%) |
| Nose | 1 (0.4%) |
| Larynx | 1 (0.4%) |
| Testes | 1 (0.4%) |
| Pancreas | 1 (0.4%) |
| Muscle | 1 (0.4%) |
| Organ system involvement of sarcoidosis . | Patients (n = 235) . |
|---|---|
| Pulmonary | 197 (83.8%) |
| Systemic | 24 (10.2%) |
| Skin | 24 (10.2%) |
| Ophthalmic | 17 (7.2%) |
| Neurologic | 15 (6.4%) |
| Liver | 15 (6.4%) |
| Renal | 9 (3.8%) |
| Spleen | 7 (2.9%) |
| Pericardium | 4 (1.7%) |
| Intestine | 2 (0.9%) |
| Stomach | 1 (0.4%) |
| Colon | 1 (0.4%) |
| Nose | 1 (0.4%) |
| Larynx | 1 (0.4%) |
| Testes | 1 (0.4%) |
| Pancreas | 1 (0.4%) |
| Muscle | 1 (0.4%) |
Medical therapy with anti-arrhythmic drugs, beta–blockers, and immunosuppressant drugs
| Medication . | Patients (n = 235) . |
|---|---|
| Any anti-arrhythmic | 103 (43.8%) |
| Sotalol | 58 (24.7%) |
| Amiodarone | 45 (19.2%) |
| Dofetilide | 16 (6.8%) |
| Propafenone | 7 (3.0%) |
| Flecainide | 5 (2.1%) |
| Quinidine | 5 (2.1%) |
| Disopyramide | 2 (0.9%) |
| Beta-blockers | 150 (63.8%) |
| Immunosuppressant therapy | |
| Steroids | 142 (60.4%) |
| Methotrexate | 46 (19.6%) |
| Azathioprine | 15 (6.4%) |
| Hydroxychloroquine | 15 (6.4%) |
| Medication . | Patients (n = 235) . |
|---|---|
| Any anti-arrhythmic | 103 (43.8%) |
| Sotalol | 58 (24.7%) |
| Amiodarone | 45 (19.2%) |
| Dofetilide | 16 (6.8%) |
| Propafenone | 7 (3.0%) |
| Flecainide | 5 (2.1%) |
| Quinidine | 5 (2.1%) |
| Disopyramide | 2 (0.9%) |
| Beta-blockers | 150 (63.8%) |
| Immunosuppressant therapy | |
| Steroids | 142 (60.4%) |
| Methotrexate | 46 (19.6%) |
| Azathioprine | 15 (6.4%) |
| Hydroxychloroquine | 15 (6.4%) |
Medical therapy with anti-arrhythmic drugs, beta–blockers, and immunosuppressant drugs
| Medication . | Patients (n = 235) . |
|---|---|
| Any anti-arrhythmic | 103 (43.8%) |
| Sotalol | 58 (24.7%) |
| Amiodarone | 45 (19.2%) |
| Dofetilide | 16 (6.8%) |
| Propafenone | 7 (3.0%) |
| Flecainide | 5 (2.1%) |
| Quinidine | 5 (2.1%) |
| Disopyramide | 2 (0.9%) |
| Beta-blockers | 150 (63.8%) |
| Immunosuppressant therapy | |
| Steroids | 142 (60.4%) |
| Methotrexate | 46 (19.6%) |
| Azathioprine | 15 (6.4%) |
| Hydroxychloroquine | 15 (6.4%) |
| Medication . | Patients (n = 235) . |
|---|---|
| Any anti-arrhythmic | 103 (43.8%) |
| Sotalol | 58 (24.7%) |
| Amiodarone | 45 (19.2%) |
| Dofetilide | 16 (6.8%) |
| Propafenone | 7 (3.0%) |
| Flecainide | 5 (2.1%) |
| Quinidine | 5 (2.1%) |
| Disopyramide | 2 (0.9%) |
| Beta-blockers | 150 (63.8%) |
| Immunosuppressant therapy | |
| Steroids | 142 (60.4%) |
| Methotrexate | 46 (19.6%) |
| Azathioprine | 15 (6.4%) |
| Hydroxychloroquine | 15 (6.4%) |
Primary vs. secondary prevention implantable cardiac defibrillator indication
In terms of indication for ICD implantation, 147 patients (62.6%) had their devices implanted for primary prevention while 88 patients (37.5%) were implanted for secondary prevention, including 7 for VF (3.0%), 63 for VT (26.8%), and 18 for syncope presumed to be due to an arrhythmia (7.7%).
Magnetic resonance imaging results
Of 115 patients who had an MRI, 99 (86.1%) were abnormal (Figure 1). Twenty-four patients (20.9%) had abnormalities, including delayed contrast enhancement or wall motion abnormalities, in the septum and other walls, 24 (20.9%) had septal abnormalities only, and 11 (9.6%) had abnormalities in other walls, but not the septum. Seventeen patients (14.8%) had delayed enhancement either diffusely or site not specified, and 23 (20.0%) had other abnormalities including LV dysfunction and chamber dilation. Patients with abnormal but non-diagnostic MRI findings such as LV dysfunction and chamber dilation had biopsy-proven cardiac or extra-CS or arrhythmias or ECG abnormalities to make the diagnosis of CS. Not all patients had an MRI due to physician preference, pre-existing cardiac device, or claustrophobia.
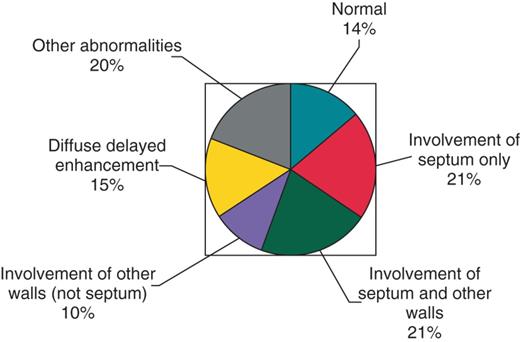
Magnetic resonance imaging findings for 115 patients who underwent magnetic resonance imaging.
Biopsy results
Of 56 patients who had cardiac tissue examined, 38 (67.9%) were abnormal. On endomyocardial biopsy, 31 patients had granulomas, 4 had fibrosis, and 1 had a lymphocytic infiltrate without granulomas. Two patients had sarcoid on pericardial biopsy, including 1 at the time of coronary artery bypass grafting, 1 had sarcoid diagnosed at the time of heart transplant, and 1 had sarcoid diagnosed at autopsy. In patients with abnormal but non-diagnostic cardiac biopsies, other criteria were met to make the diagnosis of CS.
Catheter ablation of arrhythmias
Thirty-five patients (14.9%) underwent VT ablation, including 20 patients who underwent one ablation and 15 who underwent two or more ablations. Four patients underwent ablation for atrial tachycardia, three for atrial flutter, and two underwent AV junction ablation.
Appropriate implantable cardiac defibrillator therapies
Over a mean follow-up of 4.2 (± 4.0) years, 85 of 234 (36.2%) patients received an appropriate ICD therapy [shocks and/or anti-tachycardia pacing (ATP)] (Figure 2) and 67 of 226 (29.7%) patients received an appropriate ICD shock. Patients in the appropriate therapy group (shocks and/or ATP) include patients in the appropriate shock group as well as those who received only ATP. The event rate for appropriate therapies ranged from 14 to 61% by institution. Forty-eight of 234 patients (20.5%) received five or more appropriate therapies and 29 of 226 patients (12.8%) received five or more appropriate shocks. Ten patients (4.3%) received 50 or more appropriate therapies and nine patients (3.5%) received 20 or more appropriate shocks. The event rate for appropriate therapies (shocks and/or ATP) was 8.6% per year and the event rate for appropriate shocks was 7.1% per year. Specific details about device programming, including rate cut-offs were not obtained. For eight patients, the total number of therapies was recorded, but whether the therapies were shocks or ATP was not known.
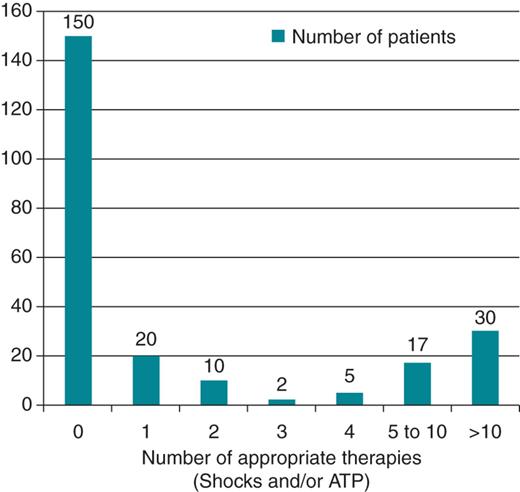
Frequency of appropriate therapies including shocks and/or anti-tachycardia pacing.
Inappropriate implantable cardiac defibrillator shocks
Fifty-seven of 235 patients (24.3%) received a total of 222 inappropriate shocks. The most common identified reasons were AF in 17 patients (29.8%), supraventricular tachycardia in 7 patients (12.3%), sinus tachycardia in 6 patients (10.5%), P/QRS/T wave oversensing in 5 patients (8.8%), and lead fracture in 4 patients (7.0%).
Adverse events
Forty-six adverse events occurred in 41 of 235 patients (17.4%) (Figure 3). The most common adverse event was lead dislodgement or fracture, n = 25 (10.64%). Seven infections occurred in six patients (2.6%), including two infections of epicardial systems. Of the six patients who had infectious complications, four were on steroids, including one patient on steroids and methotrexate, one was on hydroxychloroquine, and one was not on any immunosuppressive agents. Both patients who had epicardial system infections were on steroids. Four patients had advisory leads extracted (1.7%) and four patients had advisory pulse generators replaced (1.7%).
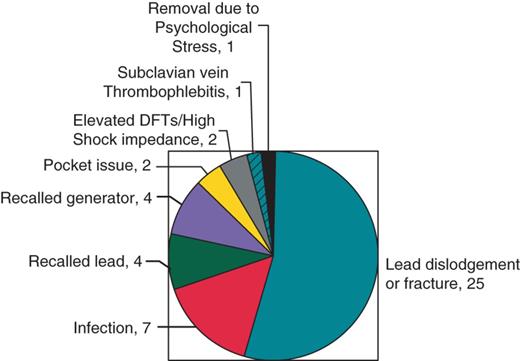
Adverse events related to implantable cardiac defibrillator s in cardiac sarcoidosis patients.
Predictors of implantable cardiac defibrillator therapies
Patients who received appropriate ICD therapies were more likely to be male (73.8 vs. 59.6%, P = 0.0330), more likely to have syncope (40.5 vs. 22.5%, P = 0.0044), and were younger (53.7 ± 12.0 vs. 56.6 ± 10.4 years, P = 0.0520) compared with those who did not receive appropriate ICD therapies (Table 4). The mean left ventricular ejection fraction (LVEF) for patients who received appropriate ICD therapies was lower compared with those who did not receive appropriate therapies (38.1 ± 15.2 vs. 48.8 ± 14.7, P < 0.0001). Patients who received appropriate ICD therapies were more likely to have had their device implanted for secondary prevention compared with those who did not receive an appropriate therapy (60.7 vs. 24.5%, P < 0.0001). First-, second-, and third-degree AV block and the presence of RBBB or left bundle branch block (LBBB) were not statistically different in patients who received appropriate therapies compared with patients who did not. Patients who received appropriate therapies were more likely to have ventricular pacing on baseline ECG than those who did not receive therapies (15.5 vs. 2.0%, P = 0.0002). Patients who received appropriate therapies were more likely to have third-degree AV block or ventricular pacing compared with those who did not (30.4 vs. 9.0%, P = 0.0001). Specific organ system involvement was not statistically different between the two groups.
Clinical characteristics and electrocardiogram findings of patients who received appropriate implantable cardiac defibrillator therapies, either shock or anti-tachycardia pacing, compared with patients who did not receive appropriate implantable cardiac defibrillator therapy
| Variable . | Appropriate therapies (n = 84) . | No appropriate therapies (n = 151) . | P value . |
|---|---|---|---|
| Male | 62 (73.8%) | 90 (59.6%) | 0.0330 |
| Syncope | 34 (40.5%) | 34 (22.5%) | 0.0044 |
| Age (years)a | 53.7 ± 12.0 | 56.6 ± 10.4 | 0.0520 |
| LVEFb | 38.1 ± 15.2 | 48.8 ± 14.7 | <0.0001 |
| Secondary prevention | 51 (60.7%) | 37 (24.5%) | <0.0001 |
| 1° AV block | 14 (16.7%) | 20 (13.3%) | 0.5621 |
| 2° AV block | 1 (1.2%) | 4 (2.7%) | 0.6573 |
| 3° AV block | 12 (14.3%) | 10 (6.6%) | 0.0633 |
| LBBB | 5 (6.0%) | 6 (4.0%) | 0.5286 |
| RBBB | 26 (31.0%) | 37 (24.5%) | 0.2872 |
| Atrial pacing | 6 (7.1%) | 6 (4.0%) | 0.3566 |
| Ventricular pacing | 13 (15.5%) | 3 (2.0%) | 0.0002 |
| Variable . | Appropriate therapies (n = 84) . | No appropriate therapies (n = 151) . | P value . |
|---|---|---|---|
| Male | 62 (73.8%) | 90 (59.6%) | 0.0330 |
| Syncope | 34 (40.5%) | 34 (22.5%) | 0.0044 |
| Age (years)a | 53.7 ± 12.0 | 56.6 ± 10.4 | 0.0520 |
| LVEFb | 38.1 ± 15.2 | 48.8 ± 14.7 | <0.0001 |
| Secondary prevention | 51 (60.7%) | 37 (24.5%) | <0.0001 |
| 1° AV block | 14 (16.7%) | 20 (13.3%) | 0.5621 |
| 2° AV block | 1 (1.2%) | 4 (2.7%) | 0.6573 |
| 3° AV block | 12 (14.3%) | 10 (6.6%) | 0.0633 |
| LBBB | 5 (6.0%) | 6 (4.0%) | 0.5286 |
| RBBB | 26 (31.0%) | 37 (24.5%) | 0.2872 |
| Atrial pacing | 6 (7.1%) | 6 (4.0%) | 0.3566 |
| Ventricular pacing | 13 (15.5%) | 3 (2.0%) | 0.0002 |
aN = 81 for appropriate therapies and N = 150 for no appropriate therapies for age.
bN = 83 for appropriate therapies and N = 150 for no appropriate therapies for LVEF.
Clinical characteristics and electrocardiogram findings of patients who received appropriate implantable cardiac defibrillator therapies, either shock or anti-tachycardia pacing, compared with patients who did not receive appropriate implantable cardiac defibrillator therapy
| Variable . | Appropriate therapies (n = 84) . | No appropriate therapies (n = 151) . | P value . |
|---|---|---|---|
| Male | 62 (73.8%) | 90 (59.6%) | 0.0330 |
| Syncope | 34 (40.5%) | 34 (22.5%) | 0.0044 |
| Age (years)a | 53.7 ± 12.0 | 56.6 ± 10.4 | 0.0520 |
| LVEFb | 38.1 ± 15.2 | 48.8 ± 14.7 | <0.0001 |
| Secondary prevention | 51 (60.7%) | 37 (24.5%) | <0.0001 |
| 1° AV block | 14 (16.7%) | 20 (13.3%) | 0.5621 |
| 2° AV block | 1 (1.2%) | 4 (2.7%) | 0.6573 |
| 3° AV block | 12 (14.3%) | 10 (6.6%) | 0.0633 |
| LBBB | 5 (6.0%) | 6 (4.0%) | 0.5286 |
| RBBB | 26 (31.0%) | 37 (24.5%) | 0.2872 |
| Atrial pacing | 6 (7.1%) | 6 (4.0%) | 0.3566 |
| Ventricular pacing | 13 (15.5%) | 3 (2.0%) | 0.0002 |
| Variable . | Appropriate therapies (n = 84) . | No appropriate therapies (n = 151) . | P value . |
|---|---|---|---|
| Male | 62 (73.8%) | 90 (59.6%) | 0.0330 |
| Syncope | 34 (40.5%) | 34 (22.5%) | 0.0044 |
| Age (years)a | 53.7 ± 12.0 | 56.6 ± 10.4 | 0.0520 |
| LVEFb | 38.1 ± 15.2 | 48.8 ± 14.7 | <0.0001 |
| Secondary prevention | 51 (60.7%) | 37 (24.5%) | <0.0001 |
| 1° AV block | 14 (16.7%) | 20 (13.3%) | 0.5621 |
| 2° AV block | 1 (1.2%) | 4 (2.7%) | 0.6573 |
| 3° AV block | 12 (14.3%) | 10 (6.6%) | 0.0633 |
| LBBB | 5 (6.0%) | 6 (4.0%) | 0.5286 |
| RBBB | 26 (31.0%) | 37 (24.5%) | 0.2872 |
| Atrial pacing | 6 (7.1%) | 6 (4.0%) | 0.3566 |
| Ventricular pacing | 13 (15.5%) | 3 (2.0%) | 0.0002 |
aN = 81 for appropriate therapies and N = 150 for no appropriate therapies for age.
bN = 83 for appropriate therapies and N = 150 for no appropriate therapies for LVEF.
Predictors of multiple implantable cardiac defibrillator therapies
Patients who received ≥5 appropriate ICD therapies were more likely to have syncope (46.8 vs. 24.5%, P = 0.0038), were younger (mean age 51.8 ± 12.5 vs. 56.5 ± 10.5 years, P = 0.0108), and were more likely to have ventricular pacing on baseline ECG (21.3 vs. 3.2%, P = 0.0001) compared with those who received <5 appropriate therapies (Table 5). The mean LVEF for patients who received ≥5 appropriate ICD therapies was lower compared with those who received <5 appropriate therapies (35.9 ± 15.5 vs. 47.3 ± 15.0%, P < 0.0001). Patients who received ≥5 appropriate ICD therapies were more likely to have had their device implanted for secondary prevention compared with those who received <5 appropriate therapies (62.1 vs. 29.8%, P < 0.0001). The groups were not significantly different in terms of gender, the presence of AV block, or the presence of LBBB or RBBB on ECG. Patients who received ≥5 appropriate therapies were more likely to have third-degree AV block or ventricular pacing compared with those who received <5 therapies (33.3 vs. 12.4%, P = 0.0015).
Clinical characteristics and electrocardiogram findings of patients who received ≥5 appropriate implantable cardiac defibrillator therapies, either shock or anti-tachycardia pacing, compared with patients who received <5 appropriate implantable cardiac defibrillator therapies
| Variable . | Appropriate therapies ≥5 (n = 47) . | Appropriate therapies <5 (n = 188) . | P value . |
|---|---|---|---|
| Male | 33 (70.2%) | 119 (63.3%) | 0.3997 |
| Syncope | 22 (46.8%) | 46 (24.5%) | 0.0038 |
| Age (years)a | 51.8 ± 12.5 | 56.5 ± 10.5 | 0.0108 |
| LVEFb | 35.9 ± 15.5 | 47.3 ± 15.0 | <0.0001 |
| Secondary prevention | 32 (62.1%) | 56 (29.8%) | <0.0001 |
| 1° AV block | 10 (21.3%) | 24 (12.8%) | 0.1636 |
| 2° AV block | 0 (0%) | 5 (2.7%) | 0.5860 |
| 3° AV block | 6 (12.8%) | 16 (8.5%) | 0.4015 |
| LBBB | 2 (4.3%) | 9 (4.8%) | 1.0000 |
| RBBB | 15 (31.9%) | 48 (25.5%) | 0.3651 |
| Atrial pacing | 4 (8.5%) | 8 (4.3%) | 0.2646 |
| Ventricular pacing | 10 (21.3%) | 6 (3.2%) | 0.0001 |
| Variable . | Appropriate therapies ≥5 (n = 47) . | Appropriate therapies <5 (n = 188) . | P value . |
|---|---|---|---|
| Male | 33 (70.2%) | 119 (63.3%) | 0.3997 |
| Syncope | 22 (46.8%) | 46 (24.5%) | 0.0038 |
| Age (years)a | 51.8 ± 12.5 | 56.5 ± 10.5 | 0.0108 |
| LVEFb | 35.9 ± 15.5 | 47.3 ± 15.0 | <0.0001 |
| Secondary prevention | 32 (62.1%) | 56 (29.8%) | <0.0001 |
| 1° AV block | 10 (21.3%) | 24 (12.8%) | 0.1636 |
| 2° AV block | 0 (0%) | 5 (2.7%) | 0.5860 |
| 3° AV block | 6 (12.8%) | 16 (8.5%) | 0.4015 |
| LBBB | 2 (4.3%) | 9 (4.8%) | 1.0000 |
| RBBB | 15 (31.9%) | 48 (25.5%) | 0.3651 |
| Atrial pacing | 4 (8.5%) | 8 (4.3%) | 0.2646 |
| Ventricular pacing | 10 (21.3%) | 6 (3.2%) | 0.0001 |
aN = 45 for ≥5 appropriate therapies and N = 186 for <5 appropriate therapies for age.
bN = 47 for ≥5 appropriate therapies and N = 186 for <5 appropriate therapies for LVEF.
Clinical characteristics and electrocardiogram findings of patients who received ≥5 appropriate implantable cardiac defibrillator therapies, either shock or anti-tachycardia pacing, compared with patients who received <5 appropriate implantable cardiac defibrillator therapies
| Variable . | Appropriate therapies ≥5 (n = 47) . | Appropriate therapies <5 (n = 188) . | P value . |
|---|---|---|---|
| Male | 33 (70.2%) | 119 (63.3%) | 0.3997 |
| Syncope | 22 (46.8%) | 46 (24.5%) | 0.0038 |
| Age (years)a | 51.8 ± 12.5 | 56.5 ± 10.5 | 0.0108 |
| LVEFb | 35.9 ± 15.5 | 47.3 ± 15.0 | <0.0001 |
| Secondary prevention | 32 (62.1%) | 56 (29.8%) | <0.0001 |
| 1° AV block | 10 (21.3%) | 24 (12.8%) | 0.1636 |
| 2° AV block | 0 (0%) | 5 (2.7%) | 0.5860 |
| 3° AV block | 6 (12.8%) | 16 (8.5%) | 0.4015 |
| LBBB | 2 (4.3%) | 9 (4.8%) | 1.0000 |
| RBBB | 15 (31.9%) | 48 (25.5%) | 0.3651 |
| Atrial pacing | 4 (8.5%) | 8 (4.3%) | 0.2646 |
| Ventricular pacing | 10 (21.3%) | 6 (3.2%) | 0.0001 |
| Variable . | Appropriate therapies ≥5 (n = 47) . | Appropriate therapies <5 (n = 188) . | P value . |
|---|---|---|---|
| Male | 33 (70.2%) | 119 (63.3%) | 0.3997 |
| Syncope | 22 (46.8%) | 46 (24.5%) | 0.0038 |
| Age (years)a | 51.8 ± 12.5 | 56.5 ± 10.5 | 0.0108 |
| LVEFb | 35.9 ± 15.5 | 47.3 ± 15.0 | <0.0001 |
| Secondary prevention | 32 (62.1%) | 56 (29.8%) | <0.0001 |
| 1° AV block | 10 (21.3%) | 24 (12.8%) | 0.1636 |
| 2° AV block | 0 (0%) | 5 (2.7%) | 0.5860 |
| 3° AV block | 6 (12.8%) | 16 (8.5%) | 0.4015 |
| LBBB | 2 (4.3%) | 9 (4.8%) | 1.0000 |
| RBBB | 15 (31.9%) | 48 (25.5%) | 0.3651 |
| Atrial pacing | 4 (8.5%) | 8 (4.3%) | 0.2646 |
| Ventricular pacing | 10 (21.3%) | 6 (3.2%) | 0.0001 |
aN = 45 for ≥5 appropriate therapies and N = 186 for <5 appropriate therapies for age.
bN = 47 for ≥5 appropriate therapies and N = 186 for <5 appropriate therapies for LVEF.
Discussion
Study results
In this multicentre retrospective cohort study of 235 subjects, we found that patients with CS and ICDs were at high risk for ventricular arrhythmias, with 36% of patients receiving an appropriate ICD therapy and 30% of patients receiving an appropriate shock over a mean follow-up of 4.2 years. Our study population also had high rates of inappropriate shocks (24%) and device complications (18%). Patients receiving appropriate therapies were more likely to be male, have a history of syncope, have a lower LVEF, a secondary prevention ICD indication, and have ventricular pacing on baseline ECG. To our knowledge, this is the largest study evaluating ICD efficacy and safety in patients with CS.
Prior investigations evaluating cardiac sarcoidosis and arrhythmias
No prospective randomized trial has evaluated the mortality benefit of ICDs in CS patients; however, several smaller trials suggest a benefit. In 32 patients with CS who underwent programmed ventricular stimulation, 12 patients with spontaneous or inducible sustained ventricular arrhythmias underwent ICD insertion.10 Five of six patients (83%) with spontaneous sustained ventricular arrhythmias and four of six patients (67%) without spontaneous but with inducible sustained ventricular arrhythmias received appropriate ICD therapy. No patient with an ICD died of a primary arrhythmic event, while 2 of 20 patients (10%) with neither spontaneous nor inducible sustained arrhythmias had sustained ventricular arrhythmias or sudden death. In a single-centre study of 45 patients with CS and ICDs, Betensky et al.7 found that 37.8% of patients received an appropriate ICD therapy over a median follow-up of 2.0 years. In that study, longer ICD follow-up, depressed LVEF, and compete heart block were associated with appropriate ICD therapy. In a cohort study of 112 CS patients, 36 (32.1%) received an appropriate therapy over a mean follow-up of 29.2 months.8 Left ventricular ejection fraction <55%, right ventricular dysfunction, and symptomatic heart failure were associated with appropriate ICD therapies.
Our findings suggest that male gender, history of syncope, lower LVEF, and ventricular pacing on baseline ECG may identify higher risk patients. Syncope in patients with CS can be due to bradyarrhythmias or tachyarrhythmias. Necropsy findings suggest that CS causing sudden death is characterized by extensive active granulomas with a predilection for the subepicardium and ventricular septum.2 Syncope may be a clinical marker for either ventricular arrhythmias or for bradyarrhythmias due to more extensive involvement of the septum. Ventricular pacing on baseline ECG may correlate with high-degree AV block and be a clinical surrogate for more extensive granulomatous involvement of the septum. Other baseline ECG findings including first-, second-, and third-degree AV block did not occur more frequently in patients with appropriate therapies; however, the combination of either third-degree AV block or ventricular pacing was seen more commonly in patients with appropriate therapies.
Predictors of appropriate implantable cardiac defibrillator therapy
We found that patients who received an appropriate ICD therapy had a lower mean LVEF (38.1%) compared with those who did not receive an appropriate therapy (48.8%). While reduced LVEF has been associated with appropriate therapies in some smaller studies,7,8 the American College of Cardiology/ American Heart Association/Heart Rhythm Society Guidelines for device-based therapies do not specify reduced LVEF as a criterion for ICD implantation in patients with CS.9 Our findings suggest that CS patients with reduced left ventricular function may be at higher risk for ventricular arrhythmias. In patients with non-ischaemic cardiomyopathy, a cut-off of LVEF ≤35% is typically used as an indication for primary prevention ICD. While our cohort included patient with ICDs implanted for both primary and secondary indications, most patients receiving appropriate therapies had an LVEF >35%, suggesting that CS patients with mild or moderately reduced LVEF may be at risk for ventricular arrhythmias, unlike other non-ischaemic cardiomyopathy patients.
Medical therapy for prevention of arrhythmias in cardiac sarcoidosis
The efficacy of medical therapy with corticosteroids or anti-arrhythmic drugs on ventricular arrhythmias has not been prospectively studied in large numbers of CS patients. Corticosteroids have been a mainstay of treatment of CS and, indeed, 60% of the patients in this study were treated with steroids. Banba et al.11 used gallium-67 citrate (Ga) scintigraphy to investigate the relationship between disease activity and arrhythmic events in CS and the effect of corticosteroid therapy. Complete AV block developed mainly during the active phase of the disease and resolved in five of nine patients who received early treatment with corticosteroids. In contrast, sustained VT was not closely linked with disease activity and developed primarily in the advanced stage of the disease. In addition, ventricular arrhythmias did not improve after corticosteroid treatment. These findings suggest that the ability of corticosteroids to suppress ventricular arrhythmias in CS may be limited, although one study showed that corticosteroid therapy may effectively treat ventricular arrhythmias in the early stage of CS.12 Other immunosuppressive agents including methotrexate and azathioprine have not been systematically studied in cardiac CS.
In terms of antiarrhythmic medications, we found that sotalol was used more commonly than amiodarone, likely due to the young age of the patients in this cohort and the high incidence of pulmonary sarcoidosis. In one study of seven sarcoidosis patients with sustained VT, two patients had sudden cardiac death and four had recurrence of VT despite anti-arrhythmic drug therapy.13 Corticosteroids did not prevent spontaneous sustained VT. In the four patients who received an ICD, all received appropriate shocks despite anti-arrhythmic drug therapy. Randomized, prospective trials of anti-arrhythmic drugs are needed to determine the most appropriate therapy for ventricular arrhythmias in CS patients.
Adverse events in patients with cardiac sarcoidosis receiving an implantable cardiac defibrillator
In our study, 24% of patients received an inappropriate shock, most commonly for supraventricular arrhythmias in 52.6%. Betenksy et al.7 found that inappropriate shocks occurred in 13.3% of patients, most often for supraventricular tachyarrhythmias in 78%, while Schuller et al.8 found that inappropriate therapies occurred in only 11.6% of CS ICD patients in their cohort study. Cardiac sarcoidosis is associated with supraventricular arrhythmias, including atrial fibrillation, atrial flutter, and atrial tachycardia.14 Cardiac sarcoidosis patients are relatively young and often systemically ill, increasing the risk of inappropriate shocks from sinus tachycardia. Implantable cardiac defibrillator ventricular arrhythmia therapy detections should be programmed to minimize inappropriate shocks, utilizing SVT discrimination algorithms, increased detection times, and high detection rates when appropriate. Therapy with beta-blockers or other AV nodal blocking agents may reduce inappropriate shocks as well.
Adverse events occurred in nearly 18% of our population, most commonly lead dislodgement or fracture. To our knowledge, no previous study has evaluated the incidence of ICD complications in CS patients. The high rates of lead dislodgement and fracture may be due in part to the relatively young age of CS patients who may be more physically active than older ICD patients. The timing of our study also corresponded with peak implantation of Medtronic Sprint Fidelis leads, which have a high failure rate of approaching 17% at 5 years.15 Risk of Sprint Fidelis defibrillator lead failure is significantly higher in patients <62.5 years, and the majority of our patients were in this age category.16 The high adverse event rates may also reflect referral bias as all of the centres in the study are tertiary referral centres, and CS patients may have been referred for management of lead complication or device infection. One patient in this study, previously reported in a case report by Schuller et al.,17 had fluctuations in ventricular sensing due to loss of R-wave voltage resulting from inflammation related to CS. Implantable cardiac defibrillator lead parameter electrical stability over time was not specifically examined in our study, but fluctuating sensing parameters are another potential source of device complication in CS patients and will require further study.
Potential limitations
The potential limitations of observational research must be considered in our study. The role of ICD for primary prevention of sudden death in patients with CS remains unproven. Although a high rate of appropriate ICD therapy is observed in this population, it is unknown whether a mortality benefit would be seen in a randomized trial evaluating this issue. Still, we believe that the ICD is likely a life-saving intervention and therefore advocate screening programmes to identify these patients. In the absence of a randomized trial, we must rely on expert consensus and observational research to guide clinical practice. We cannot define any single characteristic for risk stratification of sudden death in order to refine the population of CS patients most at risk. Although we have noted some characteristics associated with a higher rate of appropriate ICD therapy, the unpredictable progression of CS makes it difficult to recommend any specific sub-group who may not require an ICD for primary prevention of sudden death. Recently, the role of the electrophysiology study has been introduced as a potential method for risk stratification18; however, we did not collect these data to determine its role for predicting ventricular arrhythmias in our population.
Ninety-nine patients from three centres included in the present study have been previously included in other CS series.7,8 However, given the rarity of CS and the lack of data guiding clinical decision making, we felt it was important to include these patients in the present analysis, as the size of the cohort is a strength of the study. The current study remains the largest analysis of CS patients with ICDs to date.
The diagnosis of CS used in our study was broadly defined to include patients with an abnormal MRI result and those patients with advanced conduction system disease. It is possible that a misclassification bias is introduced with our inclusion criteria. However, this bias would be towards a reduced number of appropriate therapies and does not affect our results demonstrating the high risk of primary and recurrent ventricular arrhythmias observed in our population.
The subjects included in this study had CS and an ICD, and the findings may not apply more broadly to CS patients. Most of the centres involved in the present study have active pulmonary and CS programmes. This may not be the case in lower volume centres. Still, we believe that our findings suggest a role for the ICD for prevention of sudden death in patients with CS.
Conclusions
Patients with CS are at increased risk for sudden death from ventricular arrhythmias. In this multicentre retrospective cohort study of 235 patients, we found that patients with CS and ICDs were at high risk for ventricular arrhythmias, with 36% of patients receiving an appropriate ICD therapy over a mean follow-up of 4.2 years. Patients also had high rates of inappropriate shocks and device-related complications. Patients receiving appropriate therapies were more likely to be male, have a history of syncope, have a lower LVEF, have had the ICD implanted for secondary prevention, and have ventricular pacing on baseline ECG. However, most patients with appropriate therapies had an LVEF >35%. Prospective, multicentre studies are needed to further evaluate ventricular arrhythmias in CS patients, and particularly the roles of anti-arrhythmic therapy, immunosuppressive therapy, and ablation in reducing life-threatening arrhythmias.
Conflict of interest: J.K. has reported that she has no relationships relevant to the contents of this paper to disclose. W.S. has reported that he received education grants for EP fellowship and consulting fees from Medtronic Inc., St. Jude Medical, and Boston Scientific Corp. J.S. has reported that he has no relationships relevant to the contents of this paper to disclose. F.B. has reported that he has no relationships relevant to the contents of this paper to disclose. T.C. has received grant support from Cardiovascular Center at the University of Michigan for development of cardiac sarcoidosis registry. S.S. has reported that he has no relationships relevant to the contents of this paper to disclose. L.R. has reported that she has received fellowship support from Medtronic Inc., Boston Scientific, and St. Jude Medical. T.Y.M. has reported that he has no relationships relevant to the contents of this paper to disclose. J.M.C. has received honoraria from Medtronic Inc., Boston Scientific, Biotronik, St. Jude Medical, and Spectranetics. D.M. has reported that he has no relationships relevant to the contents of this paper to disclose. A.J.G. has received honoraria from Medtronic Inc., Boston Scientific, and St. Jude Medical. M.O. has received speaking fees from St. Jude Medical and Boston Scientific. D.B.D. has reported that he has no relationships relevant to the contents of this paper to disclose. R.V. has reported that he has no relationships relevant to the contents of this paper to disclose. C.N. has reported that he has no relationships relevant to the contents of this paper to disclose. N.S. has reported that she has no relationships relevant to the contents of this paper to disclose. J.P.S. has reported that he received research grants from St. Jude Medical, Medtronic Inc., Boston Scientific Corp., and Biotronik; consultant fees from Boston Scientific Corp., Biotronik, St. Jude Medical, Medtronic Inc., CardioInsight Inc., Thoratec Inc., and Biosense Webster; honoraria from Medtronic Inc., Biotronik, Guidant Corp., St. Jude Medical, and Sorin Group. S.D. has reported consultant fees from St. Jude Medical, Boston Scientific. S.M.M. has received honoraria from Medtronic Inc., St. Jude Medical, Biotronik, and Sanofi-Aventis. A.K.A. has reported that he has no relationships relevant to the contents of this paper to disclose. A.D.K. has reported that he has no relationships relevant to the contents of this paper to disclose. L.G.W. has reported that he has no relationships relevant to the contents of this paper to disclose. S.F. has reported that he has no relationships relevant to the contents of this paper to disclose. K.A.E. has received honoraria from Medtronic Inc., Boston Scientific, and Cameron Medical; speaking fees from Medtronic Inc., Boston Scientific, St. Jude Medical, Biotronik, and Sanofi; research grants from Medtronic Inc., Boston Scientific, Biosense Web, and Sanofi; fellowship support from Medtronic Inc., Boston Scientific, and Biosense Web.



