-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroshi Furushima, Masaomi Chinushi, Kenichi Iijima, Kanae Hasegawa, Akinori Sato, Daisuke Izumi, Hiroshi Watanabe, Yoshifusa Aizawa, Is the coexistence of sustained ST-segment elevation and abnormal Q waves a risk factor for electrical storm in implanted cardioverter defibrillator patients with structural heart diseases?, EP Europace, Volume 14, Issue 5, May 2012, Pages 675–681, https://doi.org/10.1093/europace/eur386
Close - Share Icon Share
Abstract
The aim of this study was to determine whether or not the coexistence of sustained ST-segment elevation and abnormal Q waves (STe-Q) could be a risk factor for electrical storm (ES) in implanted cardioverter defibrillator (ICD) patients with structural heart diseases.
In all, 156 consecutive patients received ICD therapy for secondary prevention of sudden cardiac death and/or sustained ventricular tachyarrhythmias were included. Electrical storm was defined as ≥3 separate episodes of ventricular tachycardia (VT) and/or ventricular fibrillation (VF) terminated by ICD therapies within 24 h. During a mean follow-up of 1825 ± 1188 days, 42 (26.9%) patients experienced ES, of whom 12 had coronary artery disease, 15 had idiopathic dilated cardiomyopathy, 6 had hypertrophic cardiomyopathy, 4 had arrhythmogenic right ventricular cardiomyopathy, 4 had cardiac sarcoidosis, and 1 had valvular heart disease. Sustained ST-segment elevation and abnormal Q waves in ≥2 leads on the 12-lead electrocardiography was observed in 33 (21%) patients. On the Kaplan–Meier analysis, patients with STe-Q had a markedly higher risk of ES than those without STe-Q (P< 0.0001). The multivariate Cox proportional hazards regression model indicated that STe-Q and left ventricular ejection fraction (LVEF) (<30%) were independent risk factors associated with the recurrence of VT/VF (STe-Q: HR 1.962, 95% CI 1.24–3.12, P= 0.004; LVEF: HR 1.860, 95% CI 1.20–2.89, P= 0.006), and STe-Q was an independent risk factor associated with ES (HR 4.955, 95% CI 2.69–9.13, P< 0.0001).
Sustained ST-segment elevation and abnormal Q waves could be a risk factor of not only recurrent VT/VF but also ES in patients with structural heart diseases.
Introduction
Implanted cardioverter defibrillators (ICDs) prolong life when used for primary1,2 or secondary3 prophylaxis of sudden cardiac death in patients with various structural heart diseases (SHDs). In primary prevention, 21% of patients receive the benefit of appropriate ICD therapy within 5 years, as shown in the SCD-HeFT Trial,4 whereas in secondary prevention, benefits were seen in as many as 69–85% of patients within 3 years, as shown in the AVID Trial.5 However, some patients receive multiple shock therapies in a short period, which is referred to as an electrical storm (ES). Although the incidence of ES is 4% in patients with an ICD for primary prevention in the MADIT-II Trial,6 it increases to 11–29% over a 1- to 3-year follow-up period for secondary prevention.7–12 Multiple shocks produce profound psychological morbidity, resulting in a severely impaired quality of life.13 Moreover, there is evidence that ES is a factor related to increased mortality.10,11 Although previous studies suggested that age, left ventricular ejection fraction (LVEF),10 and advanced congestive heart failure [New York Heart Association (NYHA)]11 could be predictors of ES, there is still controversy.7,8,12 Recently, we reported that coexistence of sustained ST-segment elevation and abnormal Q waves (STe-Q) in the left precordial leads could be a risk factor for ES in ICD patients with hypertrophic cardiomyopathy (HCM).14 We suggested that STe-Q represents an extensive fibrous change of the ventricle and causes ES in patients with HCM. The aim of this study was to determine whether STe-Q could be a risk factor for ES in ICD patients with SHDs in a large cohort of consecutive patients who had received ICD therapy at a single institution.
Methods
Study population
Among our cohort of 296 ICD patients, those who had SHD were eligible and we excluded patients who were undergoing primary prevention and full ventricular pacing as a result of sick sinus syndrome or atrioventricular block. Consecutively, 156 patients (118 men and 38 women, mean age 61 ± 12 years) received ICD therapy for secondary prevention of sudden cardiac death. All had one or more episodes of confirmed sustained ventricular tachyarrhythmias between 1992 and 2009 at the Niigata University Hospital. Coronary angiography and left ventriculography were performed in all patients. Patients with coronary artery disease (CAD) had a history of chest pain, serum enzyme elevation, and electrocardiography (ECG) findings compatible with acute or recent myocardial infarction. All subjects were found to have coronary occlusive lesions on coronary angiography. The diagnosis of HCM was based on the two-dimensional echocardiographic identification of a hypertrophied (wall thickness > 15 mm), non-dilated left ventricle in the absence of other cardiac or systemic disease capable of producing the magnitude of wall thickening evident.15 Arrhythmogenic right ventricular cardiomyopathy (ARVC) was confirmed using diagnostic criteria recommended by the Task Force of the European Society of Cardiology.16 The criteria for the diagnosis of cardiac sarcoidosis were based on the Handbook of the Diagnosis of Cardiac Sarcoidosis, prepared by the Specific Diffuse Pulmonary Disease Research Group, Sarcoidosis Division, organized by the Japanese Ministry of Health and Welfare.17 Patients with diffuse left ventricular dysfunction and enlargement of the left ventricle were defined as having idiopathic dilated cardiomyopathy (IDCM) when CAD, valvular disease (VHD), or any other cardiomyopathy was excluded. The LVEF was assessed on left ventriculography.
Implanted cardioverter defibrillator systems
All patients had multifunctional ICDs: single-chamber devices in 50 (32%) patients and dual-chamber devices in 106 (68%) patients. The following devices were implanted: Medtronic 7217, 7220, 7221, 7223, 7227, 7229, 7232, 7271, 7273, 7278, D164AWG, D164VWC, D234VRC, D234DRG, CPI/Guidant 1861, and St Jude Medical V-168, V-243, CD1207-36Q, CD2207-36Q.
The ICD was programmed according to the documented or induced arrhythmia with at least two detection zones. The lowest ventricular tachycardia (VT) detection zone was set at a cycle length of 422 ± 45 ms. In the VT zone, anti-tachycardia pacing including more than one burst pacing and/or one ramp pacing therapy followed by cardioversion was programmed. The maximum shocks were programmed in the ventricular fibrillation (VF) zone for rapid VT or VF.
A 12-lead electrocardiography and definitions
A 12-lead ECG was recorded before ICD implantation. The STe-Q was defined as sustained ST-segment elevation of more than 0.1 mV above the isoelectric line, and the Q wave was wider than 0.04 s;18 both were seen in ≥2 leads on ECG. Ventricular aneurysm was defined as a discrete and dyskinetic area of the ventricular wall with a broad neck on echocardiography or ventriculography.19
Definition of electrical storm
For the purpose of this analysis, ES was defined as ≥3 separate episodes of VT/VF terminated by ICD therapies, including anti-tachycardia pacing or shock, occurring over a single 24-h period.7–12
Follow-up
The patients visited the ICD outpatient clinic every 3–6 months, and additional visits were encouraged if the first shock, a cluster of shocks, or syncope occurred. During each visit, the device was examined to evaluate the number and type of episodes from the stored electrograms.
Statistics
Continuous variables are expressed as means ± standard deviation (SD). Continuous variables were compared with Student's t-test, and differences between categorical variables were tested by χ2 analysis. Survival curves were calculated according to the Kaplan–Meier actuarial method and compared using the log-rank test. The Cox proportional hazards regression model of multivariate analysis was used to detect the independent predictors of the recurrence of VT/VF and occurrence of ES. The covariates of multivariate analysis were age, sex, LVEF, and STe-Q. Statistical analysis was performed using the SPSS software package (version 18.0J, for Windows; Chicago, IL, USA).
Results
The baseline characteristics of the 156 consecutive patients with SHD are outlined in Table 1. All patients received ICD therapy for secondary prevention. At the time of implantation, patients' mean age was 61 ± 13 years and they had a mean LVEF of 41 ± 14%. The underlying heart diseases were as follows: CAD in 57 patients, IDCM in 42, HCM in 28, ARVC in 13, cardiac sarcoidosis in 8, congenital heart disease (CHD) in 6, and VHD in 2. Their medications included β-blockers in 99 (63%) patients, amiodarone in 20 (13%), dl-sotalol in 40 (26%), and angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) in 104 (67%).
| Clinical characteristics . | . |
|---|---|
| Age (years) | 61 ± 13 |
| Sex (male, n) (%) | 118 (75.6) |
| LVEF, n (%) | 41 ± 14 |
| Underlying heart disease, n (%) | |
| CAD | 57 (37) |
| IDCM | 42 (27) |
| HCM | 28 (18) |
| ARVC | 13 (8) |
| Sarcoidosis | 8 (5) |
| CHD/VHD | 8 (5) |
| Medication, n (%) | |
| β-Blockade | 99 (63) |
| Amiodarone | 20 (13) |
| Sotalol | 40 (26) |
| ACEI/ARB | 104 (67) |
| Electrical storm, n (%) | 42 (26.9) |
| Clinical characteristics . | . |
|---|---|
| Age (years) | 61 ± 13 |
| Sex (male, n) (%) | 118 (75.6) |
| LVEF, n (%) | 41 ± 14 |
| Underlying heart disease, n (%) | |
| CAD | 57 (37) |
| IDCM | 42 (27) |
| HCM | 28 (18) |
| ARVC | 13 (8) |
| Sarcoidosis | 8 (5) |
| CHD/VHD | 8 (5) |
| Medication, n (%) | |
| β-Blockade | 99 (63) |
| Amiodarone | 20 (13) |
| Sotalol | 40 (26) |
| ACEI/ARB | 104 (67) |
| Electrical storm, n (%) | 42 (26.9) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CHD, congenital heart disease; HCM, hypertrophic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LVEF, left ventricular ejection fraction; VHD, valvular heart disease.
| Clinical characteristics . | . |
|---|---|
| Age (years) | 61 ± 13 |
| Sex (male, n) (%) | 118 (75.6) |
| LVEF, n (%) | 41 ± 14 |
| Underlying heart disease, n (%) | |
| CAD | 57 (37) |
| IDCM | 42 (27) |
| HCM | 28 (18) |
| ARVC | 13 (8) |
| Sarcoidosis | 8 (5) |
| CHD/VHD | 8 (5) |
| Medication, n (%) | |
| β-Blockade | 99 (63) |
| Amiodarone | 20 (13) |
| Sotalol | 40 (26) |
| ACEI/ARB | 104 (67) |
| Electrical storm, n (%) | 42 (26.9) |
| Clinical characteristics . | . |
|---|---|
| Age (years) | 61 ± 13 |
| Sex (male, n) (%) | 118 (75.6) |
| LVEF, n (%) | 41 ± 14 |
| Underlying heart disease, n (%) | |
| CAD | 57 (37) |
| IDCM | 42 (27) |
| HCM | 28 (18) |
| ARVC | 13 (8) |
| Sarcoidosis | 8 (5) |
| CHD/VHD | 8 (5) |
| Medication, n (%) | |
| β-Blockade | 99 (63) |
| Amiodarone | 20 (13) |
| Sotalol | 40 (26) |
| ACEI/ARB | 104 (67) |
| Electrical storm, n (%) | 42 (26.9) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CHD, congenital heart disease; HCM, hypertrophic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LVEF, left ventricular ejection fraction; VHD, valvular heart disease.
Electrical storm
During a mean follow-up of 1825 ± 1188 days (median: 1563 days, range: 19–9840 days), 42 (26.9%) patients experienced at least one ES episode (Table 1). All episodes consisted of monomorphic VT, and the VT morphology was a right bundle branch block pattern in 34 patients and a left bundle branch block pattern in 8 patients. According to the underlying disease, 12 patients (21%) with CAD, 15 (36%) with IDCM, 6 (21%) with HCM, 4 (31%) with ARVC, 4 (50%) with cardiac sarcoidosis, and 1 (50%) with VHD developed ES (Figure 1). There were no significant differences in LVEF (39 ± 12 vs. 43 ± 14%), age (60 ± 11 vs. 61 ± 13 years), treatment with β-blockers (55 vs. 67%), amiodarone (14 vs. 12%), dl-sotalol (33 vs. 23%), and ACEIs/ARBs (64 vs. 68%) between patients with and without ES.
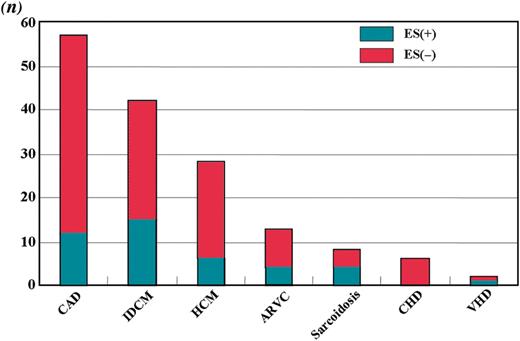
The number of patients and the proportion with electrical storm by underlying disease.
Coexistence of sustained ST-segment elevation and abnormal Q waves
In this study, STe-Q was seen in 33 (21%) patients. The baseline characteristics of the patients with and without STe-Q are shown in Table 2. There were no significant differences in age and sex. In underlying heart disease, 18 patients (55%) with CAD, 8 (24%) with IDCM, and 7 (21%) with HCM had STe-Q. No patients with ARVC, cardiac sarcoidosis, CHD, or VHD had STe-Q. The LVEF was significantly lower in patients with STe-Q than in those without STe-Q overall (35 ± 9 vs. 43 ± 15%, P< 0.05). Representative patients with STe-Q having ES with CAD, IDCM, and HCM are shown in Figure 2. There were no significant differences in medications (β-blockers, amiodarone, dl-sotalol, and ACEIs/ARBs) between patients with and without STe-Q. Electrical storm occurred significantly more frequently in patients with than in those without STe-Q, as analysed with the χ2 test (64 vs. 17%, P< 0.0001). Sustained ST-segment elevation and abnormal Q waves was observed more frequently in leads V4–V6 than in the other leads. Furthermore, the occurrence of ES was higher in patients with STe-Q in V4–V6 than in those with STe-Q in the other leads.
Comparison of baseline characteristics between patients with and without coexistence ST-elevation and abnormal Q wave
| Clinical characteristics . | Patients with STe-Q (n = 33) . | Patients without STe-Q (n = 123) . | P value . |
|---|---|---|---|
| Age (years) | 63 ± 10 | 60 ± 13 | NS |
| Sex (male, n) (%) | 27 (81.8) | 91 (74.0) | NS |
| LVEF, n (%) | 35 ± 9 | 43 ± 15 | 0.0022 |
| Underlying heart disease, n (%) | |||
| CAD | 18 (55) | 39 (32) | |
| IDCM | 8 (24) | 34 (28) | |
| HCM | 7 (21) | 21 (17) | |
| ARVC | 0 (0) | 13 (11) | |
| Sarcoidosis | 0 (0) | 8 (6) | |
| CHD/VHD | 0 (0) | 8 (6) | |
| Medication, n (%) | |||
| β-Blockade | 21 (64) | 78 (63) | NS |
| Amiodarone | 6 (18) | 14 (11) | NS |
| Sotalol | 8 (24) | 32 (26) | NS |
| ACEI/ARB | 23 (70) | 81 (66) | NS |
| Electrical storm, n (%) | 21 (64) | 21 (17) | <0.0001 |
| Clinical characteristics . | Patients with STe-Q (n = 33) . | Patients without STe-Q (n = 123) . | P value . |
|---|---|---|---|
| Age (years) | 63 ± 10 | 60 ± 13 | NS |
| Sex (male, n) (%) | 27 (81.8) | 91 (74.0) | NS |
| LVEF, n (%) | 35 ± 9 | 43 ± 15 | 0.0022 |
| Underlying heart disease, n (%) | |||
| CAD | 18 (55) | 39 (32) | |
| IDCM | 8 (24) | 34 (28) | |
| HCM | 7 (21) | 21 (17) | |
| ARVC | 0 (0) | 13 (11) | |
| Sarcoidosis | 0 (0) | 8 (6) | |
| CHD/VHD | 0 (0) | 8 (6) | |
| Medication, n (%) | |||
| β-Blockade | 21 (64) | 78 (63) | NS |
| Amiodarone | 6 (18) | 14 (11) | NS |
| Sotalol | 8 (24) | 32 (26) | NS |
| ACEI/ARB | 23 (70) | 81 (66) | NS |
| Electrical storm, n (%) | 21 (64) | 21 (17) | <0.0001 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CHD, congenital heart disease; HCM, hypertrophic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LVEF, left ventricular ejection fraction; STe-Q, coexistence of sustained ST-segment elevation and abnormal Q wave; VHD, valvular heart disease.
Comparison of baseline characteristics between patients with and without coexistence ST-elevation and abnormal Q wave
| Clinical characteristics . | Patients with STe-Q (n = 33) . | Patients without STe-Q (n = 123) . | P value . |
|---|---|---|---|
| Age (years) | 63 ± 10 | 60 ± 13 | NS |
| Sex (male, n) (%) | 27 (81.8) | 91 (74.0) | NS |
| LVEF, n (%) | 35 ± 9 | 43 ± 15 | 0.0022 |
| Underlying heart disease, n (%) | |||
| CAD | 18 (55) | 39 (32) | |
| IDCM | 8 (24) | 34 (28) | |
| HCM | 7 (21) | 21 (17) | |
| ARVC | 0 (0) | 13 (11) | |
| Sarcoidosis | 0 (0) | 8 (6) | |
| CHD/VHD | 0 (0) | 8 (6) | |
| Medication, n (%) | |||
| β-Blockade | 21 (64) | 78 (63) | NS |
| Amiodarone | 6 (18) | 14 (11) | NS |
| Sotalol | 8 (24) | 32 (26) | NS |
| ACEI/ARB | 23 (70) | 81 (66) | NS |
| Electrical storm, n (%) | 21 (64) | 21 (17) | <0.0001 |
| Clinical characteristics . | Patients with STe-Q (n = 33) . | Patients without STe-Q (n = 123) . | P value . |
|---|---|---|---|
| Age (years) | 63 ± 10 | 60 ± 13 | NS |
| Sex (male, n) (%) | 27 (81.8) | 91 (74.0) | NS |
| LVEF, n (%) | 35 ± 9 | 43 ± 15 | 0.0022 |
| Underlying heart disease, n (%) | |||
| CAD | 18 (55) | 39 (32) | |
| IDCM | 8 (24) | 34 (28) | |
| HCM | 7 (21) | 21 (17) | |
| ARVC | 0 (0) | 13 (11) | |
| Sarcoidosis | 0 (0) | 8 (6) | |
| CHD/VHD | 0 (0) | 8 (6) | |
| Medication, n (%) | |||
| β-Blockade | 21 (64) | 78 (63) | NS |
| Amiodarone | 6 (18) | 14 (11) | NS |
| Sotalol | 8 (24) | 32 (26) | NS |
| ACEI/ARB | 23 (70) | 81 (66) | NS |
| Electrical storm, n (%) | 21 (64) | 21 (17) | <0.0001 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CAD, coronary artery disease; CHD, congenital heart disease; HCM, hypertrophic cardiomyopathy; IDCM, idiopathic dilated cardiomyopathy; LVEF, left ventricular ejection fraction; STe-Q, coexistence of sustained ST-segment elevation and abnormal Q wave; VHD, valvular heart disease.
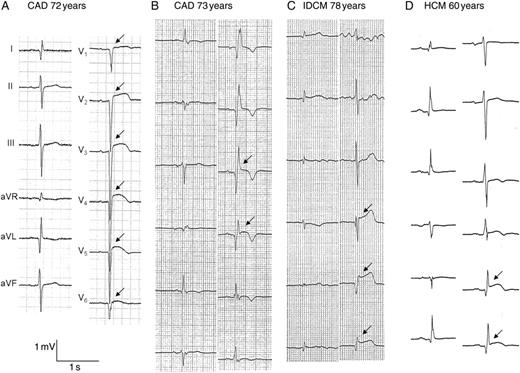
Representative electrocardiographs of patients having electrical storm. (A, B) Electrocardiographs of patients with CAD. (A) Sustained ST-segment elevation and abnormal Q waves is seen in V1–V6 (arrow), and this patient had a broad anteroseptal old myocardial infarction and diffusely severe hypokinetic wall motion of the left ventricle. (B) Sustained ST-segment elevation and abnormal Q waves is seen in V3 and V4 (arrow), and this patient had an apical aneurysm. (C) Sustained ST-segment elevation and abnormal Q waves is seen in V4–V6 (arrow), and this patient had idiopathic dilated cardiomyopathy and diffusely hypokinetic wall motion of the left ventricle. (D) Sustained ST-segment elevation and abnormal Q waves is seen in V5 and V6, and this patient had hypertrophic cardiomyopathy with an apical aneurysm.
The Kaplan–Meier curves of freedom from recurrence of VT/VF between patients with and without STe-Q are illustrated in Figure 3. The recurrence rate of VT/VF was higher in patients with than in those without STe-Q (P< 0.01). The Kaplan–Meier curves of freedom from ES events between patients with and without STe-Q are shown in Figure 4. Patients with STe-Q had a markedly higher risk of ES than those without STe-Q (P< 0.0001).
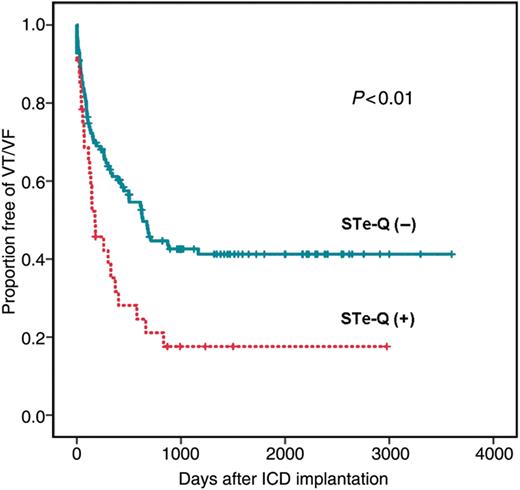
The cumulative probability of freedom from VT/VF recurrence with and without coexistence of sustained ST-segment elevation and abnormal Q waves. Recurrent VT/VF occurs significantly more frequently in patients with than in those without sustained ST-segment elevation and abnormal Q waves (P< 0.01).
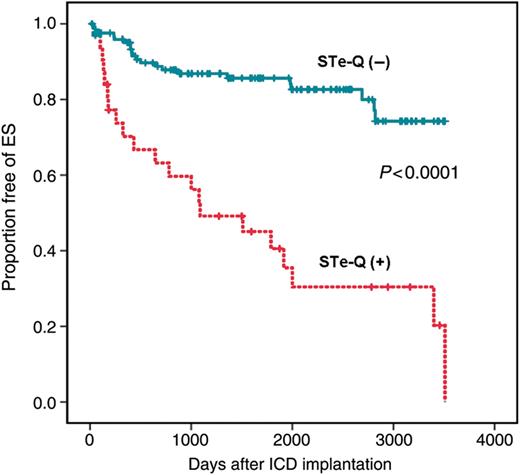
The cumulative probability of freedom from electrical storm in patients with and without coexistence of ST elevation and abnormal Q waves. Patients with coexistence of ST elevation and abnormal Q waves had a significantly higher risk of electrical storm occurrence than those without coexistence of ST elevation and abnormal Q waves (P< 0.0001).
The multivariate Cox proportional hazards regression model indicated that, adjusted by other variables, STe-Q and LVEF (<30%) were independent risk factors associated with the recurrence of VT/VF (STe-Q: HR 1.962, 95% CI 1.24–3.12, P= 0.004; LVEF: HR 1.860, 95% CI 1.20–2.89, P= 0.006), and STe-Q was an independent risk factor associated with ES (HR 4.955, 95% CI 2.69–9.13, P< 0.0001).
Relation to ventricular aneurysm
Of the 33 patients with STe-Q, 17 patients (51%) had left ventricular aneurysms on left ventriculography, and the remaining 16 patients (8 with CAD, 5 with IDCM, and 3 with HCM) did not have definitive ventricular aneurysms. In 4 of the 8 patients with CAD without an aneurysm, STe-Q was located in the inferior leads, and in the remaining 4 patients, STe-Q was located in the precordial leads and left ventricular wall motion was diffusely hypokinetic. In all five IDCM patients without aneurysms, STe-Q was located in the precordial leads and left ventricular wall motion was diffusely hypokinetic. In all three HCM patients without aneurysm, STe-Q was located in the left precordial leads and left ventricular wall motion in the anteroseptal or apical area was hypokinetic. In contrast, four patients (one with CAD, one with IDCM, one with cardiac sarcoidosis, and one with ARVC) did not have STe-Q, although they had ventricular aneurysms. In the patient with CAD, an aneurysm was located in the inferior wall of the left ventricle. The patient with IDCM had an aneurysm of the lateral wall of the left ventricle. The patient with cardiac sarcoidosis had multiple small aneurysms of the left ventricle and ES. The patient with ARVC had a right ventricular aneurysm and ES.
Discussion
Main findings
To the best of our knowledge, this study is the first to assess the relationship between ECG findings (STe-Q) and the occurrence of ES. The presence of STe-Q could be a risk factor of not only the recurrent VT/VF but also ES in patients with SHDs.
Risks of electrical storm
In previous studies that focused on ES in ICD recipients, the incidence of ES was variously described as 11–29% during 1–3 years of follow-up in secondary prevention.7–12 The incidence (26.9%) of ES in this study was relatively higher than in previous reports, and this might be due to the longer follow-up period. Several studies have attempted to identify risk factors for ES. Identifiable causes such as congestive heart failure (31%) and electrolyte disorders (20%) were reported in a previous study.20 However, in the majority of events, no triggers were found. Some previous studies10,11 reported that lower ejection fraction or advanced heart failure was a risk factor, but others did not.7,8,12
In this study, LVEF tended to be lower in patients with ES than in those without ES, but the difference was not significant. This discrepant result is because of the different proportions of SHDs, drug treatments, and follow-up periods.
Several studies have assessed the relationship between ECG findings and ES. Although Takigawa et al.21 compared QRS width and QT interval between patients with and without ES, no differences were found. Fragmented QRS22 and QRS scoring23, which is thought to represent myocardial scars, were shown to predict the occurrence of VT/VF. Recently, we reported that STe-Q in the left precordial leads could be a risk factor for ES in ICD patients with HCM.14 These patients had an apical aneurysm with mid-ventricular obstruction or apical abnormal wall motion with asymmetric hypertrophy. We suggested that STe-Q represents a severe or large area of fibrous change of the ventricle, and it might cause ES in patients with HCM. In this study, it was shown that, in patients with various SHDs including CAD and IDCM, STe-Q can be a risk factor for ES as well as for recurrent VT/VF. The LVEF was significantly lower in patients with than in those without STe-Q. However, in the multivariate Cox proportional hazards regression model, adjusted by other variables, STe-Q was an independent risk factor associated with ES and the recurrence of VT/VF.
Pathogenesis of the coexistence of ST-segment elevation and abnormal Q waves
Sustained ST-segment elevation and abnormal Q waves were the main features of post-myocardial infarction aneurysm,24 whereas these rarely occurred in patients with IDCM with left ventricular aneurysms.25,26 Patients with HCM with apical aneurysms might show such ECG findings.14 Previous studies showed that the tissue of the ventricular aneurysm was significantly non-homogeneous with viable, normal myocytes, fibrotic tissue, and/or hypertrophic myocytes.27 This may well represent an arrhythmogenic substrate due to local conduction delay and dispersion of excitability and refractoriness. The presence of abnormal Q waves and sustained ST-segment elevation might reflect the large fibrotic tissue and the extensive arrhythmogenic substrate with re-entrant circuits.
In this study, the incidence of STe-Q was higher in the left precordial leads (V4–V6) than in other leads, and it was associated with ES. This suggests that the extensive aneurysm around the apex was relatively arrhythmogenic in patients with CAD, IDCM, and HCM. In contrast, in patients with ARVC or cardiac sarcoidosis, STe-Q was never seen in any ECG leads, despite the presence of aneurysms and the occurrence of ES; this may be because, in patients with ARVC or cardiac sarcoidosis, the aneurysm was located in the right ventricle or tended to be smaller.
Clinical implication
In this study, we reported that patients with STe-Q had a risk of ES that was often associated with an aneurysm. Recently, it was reported that empiric ablation techniques for substrate modification and prevention of VT/VF could reduce the ICD therapy.28,29 It suggests that empiric ablation might be one of the options to prevent ES in patients with STe-Q, especially when haemodynamically stable VT is reproducibly induced. On the other hand, surgery for the repair of a left ventricle aneurysm is indicated in cases of congestive heart failure, malignant ventricular arrhythmia, or recurrent embolization from the left ventricle.30 The efficacy of aneurysmectomy seems to be controversial. Although a high rate of recurrence of VT after the surgery has been previously reported,31 several other studies have reported satisfactory long-term results of anti-arrhythmic surgery in patients with left ventricle aneurysm after operation.32,33 The surgery for left ventricle aneurysm to prevent ES seems useful, but further study is needed to clarify this issue.
Limitations
The major limitation of this study is the small number of patients. However, according to the Kaplan–Meier curves, the difference in the occurrence of ES widened greatly from an early time. This suggests that STe-Q is strongly predictive of ES occurrence. Secondly, we did not investigate patients undergoing treatment for primary prevention. It might be important to assess whether STe-Q is a risk factor of ES similar to lower LVEF or whether we overestimated the value of STe-Q for predicting ES.
Finally, in basic treatment of the present patients, β-blockers were used in 63% of the patients, amiodarone in 13% of the patients, and dl-sotalol in 26% of the patients. We used dl-sotalol because it reduced the recurrence of VT and all-cause mortality in some patients with SHD34. Most patients using dl-sotalol did not have β-blockers and the proportion of β-blockers was relatively low.
Conclusion
STe-Q could be a risk factor of not only recurrent VT/VF but also ES in patients with SHDs.
Conflict of interest: none declared.
References
- hypertrophic cardiomyopathy
- electrocardiogram
- left ventricular ejection fraction
- coronary arteriosclerosis
- tachycardia, ventricular
- arrhythmogenic right ventricular dysplasia
- survival analysis
- st segment elevation
- electrocardiogram q waves test
- primary idiopathic dilated cardiomyopathy
- heart diseases
- follow-up
- secondary prevention
- defibrillators
- cardiac sarcoidosis
- cardiac electrical storm



