-
PDF
- Split View
-
Views
-
Cite
Cite
Antonio De Sisti, Joelci Tonet, Walid Amara, Denis Raguin, Philip Aouate, Fatima Gueffaf, Faouzi Touil, Françoise Hidden-Lucet, Correlations between long-term results after cryoablation for atrioventricular nodal reentry tachycardia and a residual jump associated or not with a single echo, EP Europace, Volume 14, Issue 2, February 2012, Pages 261–266, https://doi.org/10.1093/europace/eur297
Close - Share Icon Share
Abstract
While in radiofrequency ablation for atrioventricular nodal reentry tachycardia (AVNRT) a residual jump and a single echo do not seem to substantially modify long-term results, in cryoablation procedures their effects are still under evaluation. The purpose of this study was to evaluate if a residual jump associated or not with an isolated echo is correlated with outcome.
Inclusion criteria: acute successful slow pathway cryoablation for slow-fast AVNRT. Exclusion criteria: use of a 4 mm tip cryocatheter, no baseline elicitable jump or inducible AVNRT, and unwanted persistent first degree atrioventricular (AV) block at the end of the procedure. Cryoablation (−80°C × 4 min) was applied after successful cryomapping. Atrioventricular nodal reentry tachycardia inducibility was checked 30 min later on and off isoproterenol. Acute success was defined as AVNRT non-inducibility. Among 332 patients (pts) who had undergone cryoablation from May 2002 to March 2010 in our institutions, 245 of them fulfilled the entry criteria (173 women, mean age 41 ± 16 years, ineffective drugs 1.3 ± 1.1). A 7-Fr 6-mm tip cryocatheter (CryoCath®) was used in all cases. Baseline AV nodal effective refractory period (ERP) was 271 ± 55 ms, post-procedural ERP 331 ± 60 ms (P< 0.001), and the mean of the difference between baseline and post-procedural ERP 63 ± 38 ms. A/V ratio at successful site was 1 ± 0.4. Forty-four pts (18%) had a residual jump at the end of the procedure, and 14 of them had an associated single echo. Global cryoapplication time was 993 ± 797 s. During a follow-up of 40 ± 10 months, 43 pts (17.5%) had recurrences. At 12 months follow-up, actuarial rate of recurrence-free pts was 85% in the group without residual jump (201 pts), 63.3% with residual jump and no echo (30 pts), and 60.6% with residual jump associated with a single echo (P< 0.003 among groups). Univariate predictors of recurrences were persistence of a residual jump (P< 0.001) and total cryoapplication time (P< 0.02). In a multivariate model, only residual jump was independently correlated with recurrences (P< 0.01).
In patients undergoing AVNRT cryoablation, slow-pathway suppression is correlated with a better outcome. A single echo is associated with a recurrence risk similar to residual jump without echo. It may be suggested that pursuing a procedural endpoint up to slow pathway complete suppression may improve long-term success.
Introduction
Slow pathway ablation has become the treatment of choice for atrioventricular nodal reentry tachycardia (AVNRT). Currently, the immediate and long-term success rate of radiofrequency (RF) for AVNRT is high, but the occurrence of atrioventricular (AV)-block requiring permanent pacing remains clinically relevant (∼1%).1,2 In the last decade, cryoablation has been available as an alternative to RF for catheter ablation procedures. Current studies confirm that cryoablation for AVNRT is effective and safe,3–22 but the advantages of its use appear to be counteracted by a slightly lower efficacy when compared with RF.3–7,10,14,19,20,22 To improve results, cryocatheters using larger distal electrode tips have been used.8,11–17,23 In addition, it may be hypothesized that pursuing a procedural endpoint as slow pathway complete suppression may improve long-term success.7,11,13 The purpose of this study was to evaluate if a residual jump associated or not with an isolated echo is correlated with outcome.
Methods
This is a multicentre study involving three French centers: Pitié-Salpêtrière Hospital—Paris, Le Raincy-Montfermeil Hospital—Montfermeil, and Clinic of Europe—Amiens. All centres adhered to inclusion/exclusion criteria, procedural methods, and endpoints.
Inclusion criteria
Patients with successful cryoablation procedures for slow–fast AVNRT.
Exclusion criteria
Use of catheter-tip size other than 6 mm, lack of baseline elicitable jump, baseline AVNRT non-inducibility, previous ablation attempts, fast–slow and slow–slow AVNRT types and other associated arrhythmias, underlying heart disease, persisting first-degree AV-block at the end of the cryoprocedure, and need for beta-blocking agents.
Procedure
After giving informed consent, patients were investigated in the fasting state without sedation. Antiarrhythmic (AA) drugs were discontinued for at least five half-life periods. A standard electrophysiologic (EP) study was performed. Dual-AV nodal physiology was defined as a ≥50 ms increase in A2H2 in response to a 10 ms decrease during A1A2 stimulation.24 If sustained tachycardia could not be induced, isoproterenol was infused to facilitate induction. Slow pathway potentials in the Koch triangle were identified as the target site.24,25 A zone located anterior to the coronary sinus (CS), slightly below an ideal line delimiting the superior border of the CS ostium, with an A/V ratio of ∼1 was generally preferred as a target. Ablation was performed by using a 7-Fr 6-mm tip electrode cryocatheter (Freezor × Tra Cryocath®). Cryomapping was carried out first at a cryocatheter tip temperature of −30°C for a maximum duration of 30 s to test the EP effect on the target sites by using programmed stimulation which reproducibly demonstrated dual-nodal physiology or induced AVNRT. In the case of ineffective results or AV-block, cryomapping was terminated and then repeated at new target sites. Cryoablation (−75/80°C for 4/5 min) was initiated immediately following successful cryomapping, defined as abolition of the slow pathway or AVNRT non-inducibilty. If AVNRT was still inducible or AV-block occurred, cryoablation was stopped and cryomapping at new target sites was repeated. Electrophysiologic study was repeated during a waiting period of 30 min after cryoablation to check the effectiveness of the slow pathway ablation on and off isoproterenol infusion. Procedural success was defined as non-inducibility of AVNRT on and off isoproterenol administration. Residual slow pathway associated or not with a single echo was permitted at the discretion of the senior operating electrophysiologist. Baseline end post-procedural AV nodal effective refractory periods (ERPs) were evaluated before isoproterenol administration. Cryoablation procedures were performed by 10 different operators.
Post-ablation management and follow-up
After cryoablation all AA drugs were discontinued. During the follow-up, patients underwent trans-telephonic assessment of symptoms, rest ECG and 24-h Holter recording at 3 months and then every 6 months in our centres or by their referring physicians. Relapse was defined as recurrence of index arrhythmia-typical symptoms of tachycardia with sudden onset, tachycardia documented with ECG or 24-h Holter recording. The minimum observational period was set at 6 months.
Statistical analysis
Continuous variables were expressed as mean + SD. Comparison between and among groups used analysis of variance for continuous variables, and χ2 test for discrete variables. Correlation between long-term results in the follow-up and the different variables was performed using Cox's regression model for univariate and multivariate analysis. Following univariate analysis, factors with associated P value <0.10 were tested in multivariate analysis. A stepwise regression procedure was used to determine independent predictors. The P values for entry or removal of a variable from the regression model were 0.05 and 0.10, respectively. Actuarial graphs were constructed using the Kaplan–Meier method and differences were evaluated by log-rank test. A P value <0.05 was considered significant. Statistical analysis was performed using SPSS 19 for Windows.
Results
Among 332 patients who had undergone cryoablation for AVNRT from May 2002 to March 2010 in three different French centres, 245 of them fulfilled the entry criteria. The contribution of each participating centre to the study was: 281 patients from Pitié-Salpêtrière Hospital—Paris, 25 from Le Raincy-Montfermeil Hospital—Montfermeil, and 26 from Clinic of Europe—Amiens (Table 1).
| Patients (n) | 245 |
| Age (years) | 41 ± 16 |
| Sex (F/M) | 173/72 |
| Previous antiarrhythmic drugs (n) | 1.3 ± 1.1 |
| AVNRT cycle (ms) | 328 ± 60 |
| Operators (n) | 10 |
| Baseline AVNRT induction only on isoproterenol (pts) | 24 |
| Baseline AV nodal ERP (ms) | 271 ± 55 |
| Cryomapping test (n) | 6.4 ± 6.4 |
| Cryoablation application (n) | 6.1 ± 5.9 |
| Total cryoablation duration (s) | 993 ± 797 |
| A/V at last successful site (ratio) | 1 ± 0.4 |
| Freezing–thawing–freezing cycle (pts) | 102 |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 15 |
| Post-procedural AV nodal ERP (ms) | 331 ± 60 |
| ΔAV nodal ERP (ms) | 63 ± 38 |
| Procedural time (min) | 124 ± 45 |
| Fluoroscopy time (min) | 16 ± 13 |
| Post-procedural residual jump without echo (pts) | 30 |
| Post-procedural residual jump with echo (pts) | 14 |
| Follow-up (months) | 40 ± 10 |
| Recurrence (pts) | 43 |
| Cryo and RF Redo procedures (pts) | 20 |
| Patients (n) | 245 |
| Age (years) | 41 ± 16 |
| Sex (F/M) | 173/72 |
| Previous antiarrhythmic drugs (n) | 1.3 ± 1.1 |
| AVNRT cycle (ms) | 328 ± 60 |
| Operators (n) | 10 |
| Baseline AVNRT induction only on isoproterenol (pts) | 24 |
| Baseline AV nodal ERP (ms) | 271 ± 55 |
| Cryomapping test (n) | 6.4 ± 6.4 |
| Cryoablation application (n) | 6.1 ± 5.9 |
| Total cryoablation duration (s) | 993 ± 797 |
| A/V at last successful site (ratio) | 1 ± 0.4 |
| Freezing–thawing–freezing cycle (pts) | 102 |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 15 |
| Post-procedural AV nodal ERP (ms) | 331 ± 60 |
| ΔAV nodal ERP (ms) | 63 ± 38 |
| Procedural time (min) | 124 ± 45 |
| Fluoroscopy time (min) | 16 ± 13 |
| Post-procedural residual jump without echo (pts) | 30 |
| Post-procedural residual jump with echo (pts) | 14 |
| Follow-up (months) | 40 ± 10 |
| Recurrence (pts) | 43 |
| Cryo and RF Redo procedures (pts) | 20 |
AVNRT, atrioventricular nodal reentry tachycardia; AV, atrioventricular; ERP, effective refractory period; ΔERP, mean of the difference between baseline and post-procedural ERP; pts, patients.
| Patients (n) | 245 |
| Age (years) | 41 ± 16 |
| Sex (F/M) | 173/72 |
| Previous antiarrhythmic drugs (n) | 1.3 ± 1.1 |
| AVNRT cycle (ms) | 328 ± 60 |
| Operators (n) | 10 |
| Baseline AVNRT induction only on isoproterenol (pts) | 24 |
| Baseline AV nodal ERP (ms) | 271 ± 55 |
| Cryomapping test (n) | 6.4 ± 6.4 |
| Cryoablation application (n) | 6.1 ± 5.9 |
| Total cryoablation duration (s) | 993 ± 797 |
| A/V at last successful site (ratio) | 1 ± 0.4 |
| Freezing–thawing–freezing cycle (pts) | 102 |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 15 |
| Post-procedural AV nodal ERP (ms) | 331 ± 60 |
| ΔAV nodal ERP (ms) | 63 ± 38 |
| Procedural time (min) | 124 ± 45 |
| Fluoroscopy time (min) | 16 ± 13 |
| Post-procedural residual jump without echo (pts) | 30 |
| Post-procedural residual jump with echo (pts) | 14 |
| Follow-up (months) | 40 ± 10 |
| Recurrence (pts) | 43 |
| Cryo and RF Redo procedures (pts) | 20 |
| Patients (n) | 245 |
| Age (years) | 41 ± 16 |
| Sex (F/M) | 173/72 |
| Previous antiarrhythmic drugs (n) | 1.3 ± 1.1 |
| AVNRT cycle (ms) | 328 ± 60 |
| Operators (n) | 10 |
| Baseline AVNRT induction only on isoproterenol (pts) | 24 |
| Baseline AV nodal ERP (ms) | 271 ± 55 |
| Cryomapping test (n) | 6.4 ± 6.4 |
| Cryoablation application (n) | 6.1 ± 5.9 |
| Total cryoablation duration (s) | 993 ± 797 |
| A/V at last successful site (ratio) | 1 ± 0.4 |
| Freezing–thawing–freezing cycle (pts) | 102 |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 15 |
| Post-procedural AV nodal ERP (ms) | 331 ± 60 |
| ΔAV nodal ERP (ms) | 63 ± 38 |
| Procedural time (min) | 124 ± 45 |
| Fluoroscopy time (min) | 16 ± 13 |
| Post-procedural residual jump without echo (pts) | 30 |
| Post-procedural residual jump with echo (pts) | 14 |
| Follow-up (months) | 40 ± 10 |
| Recurrence (pts) | 43 |
| Cryo and RF Redo procedures (pts) | 20 |
AVNRT, atrioventricular nodal reentry tachycardia; AV, atrioventricular; ERP, effective refractory period; ΔERP, mean of the difference between baseline and post-procedural ERP; pts, patients.
Patient characteristics
Among the 245 selected patients there were 173 women and 72 men, mean age was 41 ± 16 years, number of previously ineffective AA drugs 1.3 ± 1.1, and body weight 67 ± 16 kg.
Procedural variables
The mean AVNRT cycle length was 328 ± 60 ms. At baseline, AVNRT was inducible in 221 patients off isoproterenol, while in the remaining 24 only on isoproterenol. The A/V ratio at successful site was 1 ± 0.4. The number of cryomapping tests was 6.4 ± 6.4 and cryoablation applications 6.1 ± 5.9 per patient. A freezing–thawing–freezing cycle was applied in 102 patients. Total cryoablation time was 993 ± 797 s. The procedure and fluoroscopy times were 124 ± 45 and 16 ± 13 min, respectively.
Transient inadvertent 2nd/3rd AV-block despite successful and uncomplicated cryomapping occurred at the last effective site in 15 patients. In these patients the last cryoapplication was interrupted after a short time of 65 ± 81 s. All patients with inadvertent AV-block had no residual slow pathway and no AVNRT induction at the end of the procedure. Atrioventricular conduction fully recovered in all patients (after a few seconds in 14, and 6 min in one) without changing the PR interval duration (142 ± 23 vs. 144 ± 24 ms; P= ns).
Electrophysiologic modifications after cryoablation
Baseline AV nodal ERP was 271 ± 55 ms, post-procedural ERP 331 ± 60 ms (P< 0.001), and the mean of the difference between baseline and post-procedural ERP (ΔERP) 63 ± 38 ms. Baseline Wenckebach nodal AV-block cycle length was 342 ± 65 ms, and post-procedural 404 ± 83 ms (P< 0.001). At the end of the procedure, a slow pathway was elicitable in 44 (18%) patients (of whom 14 were with an associated single echo).
Follow-up after the first cryoablation
No AA drug was prescribed at hospital discharge. During a follow-up of 40 ± 10 months there were 43 (17.5%) patients with recurrences. No patient was lost in follow-up. Among the 15 patients with transient inadvertent 2nd/3rd AV-block at the last site, two of them (13%) had recurrence during the follow-up.
Redo procedures were performed in 20 (8%) patients: 17 patients underwent a second cryoablation while three patients underwent a second RF ablation procedure, at the discretion of the referring electrophysiologist. In two patients with recurrences despite a second cryoablation, a subsequent RF procedure was performed.
Predictors of recurrence
Through univariate analysis, recurrences in the follow-up were correlated with persistence of a residual jump (P< 0.001) and total cryoablation time (P< 0.02). Through multivariate analysis, residual jump was independently correlated with recurrences (P< 0.01), while cryoablation time was not (Table 2).
| Variables . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 0.99 (0.97–1.01) | NS |
| Sex (M/F) | 0.76 (0.40–1.42) | NS |
| Previous antiarrhythmic drugs (n) | 1.10 (0.84–1.43) | NS |
| Operators (n) | 0.92 (0.74–1.13) | NS |
| Baseline AVNRT induction only on isoproterenol (pts) | 1.02 (0.36–2.87) | NS |
| Cryoablation application (n) | 1.04 (0.99–1.08) | NS |
| Total cryoablation duration (s) | 1.4 (0.8–1.1) | <0.02 |
| A/V at last successful site (ratio) | 0.54 (0.12–2.39) | NS |
| Freezing–thawing–freezing cycle (pts) | 0.70 (0.34–1.42) | NS |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 1.47 (0.35–6.10) | NS |
| ΔAV nodal ERP (ms) | 0.99 (0.98–1.01) | NS |
| Post-procedural residual jump (pts) | 0.36 (0.19–0.67) | <0.001 |
| Multivariate analysis | ||
| Total cryoablation duration (s) | 1.00 (1.00–1.01) | NS |
| Post-procedural residual jump (pts) | 0.40 (0.21–0.76) | <0.01 |
| Variables . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 0.99 (0.97–1.01) | NS |
| Sex (M/F) | 0.76 (0.40–1.42) | NS |
| Previous antiarrhythmic drugs (n) | 1.10 (0.84–1.43) | NS |
| Operators (n) | 0.92 (0.74–1.13) | NS |
| Baseline AVNRT induction only on isoproterenol (pts) | 1.02 (0.36–2.87) | NS |
| Cryoablation application (n) | 1.04 (0.99–1.08) | NS |
| Total cryoablation duration (s) | 1.4 (0.8–1.1) | <0.02 |
| A/V at last successful site (ratio) | 0.54 (0.12–2.39) | NS |
| Freezing–thawing–freezing cycle (pts) | 0.70 (0.34–1.42) | NS |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 1.47 (0.35–6.10) | NS |
| ΔAV nodal ERP (ms) | 0.99 (0.98–1.01) | NS |
| Post-procedural residual jump (pts) | 0.36 (0.19–0.67) | <0.001 |
| Multivariate analysis | ||
| Total cryoablation duration (s) | 1.00 (1.00–1.01) | NS |
| Post-procedural residual jump (pts) | 0.40 (0.21–0.76) | <0.01 |
HR, hazard ratio; CI, confidence interval; AVNRT, atrioventricular nodal reentry tachycardia; AV, atrioventricular; ERP, effective refractory period; Δ ERP, mean of the difference between baseline and post-procedural ERP; pts, patients.
| Variables . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 0.99 (0.97–1.01) | NS |
| Sex (M/F) | 0.76 (0.40–1.42) | NS |
| Previous antiarrhythmic drugs (n) | 1.10 (0.84–1.43) | NS |
| Operators (n) | 0.92 (0.74–1.13) | NS |
| Baseline AVNRT induction only on isoproterenol (pts) | 1.02 (0.36–2.87) | NS |
| Cryoablation application (n) | 1.04 (0.99–1.08) | NS |
| Total cryoablation duration (s) | 1.4 (0.8–1.1) | <0.02 |
| A/V at last successful site (ratio) | 0.54 (0.12–2.39) | NS |
| Freezing–thawing–freezing cycle (pts) | 0.70 (0.34–1.42) | NS |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 1.47 (0.35–6.10) | NS |
| ΔAV nodal ERP (ms) | 0.99 (0.98–1.01) | NS |
| Post-procedural residual jump (pts) | 0.36 (0.19–0.67) | <0.001 |
| Multivariate analysis | ||
| Total cryoablation duration (s) | 1.00 (1.00–1.01) | NS |
| Post-procedural residual jump (pts) | 0.40 (0.21–0.76) | <0.01 |
| Variables . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age (years) | 0.99 (0.97–1.01) | NS |
| Sex (M/F) | 0.76 (0.40–1.42) | NS |
| Previous antiarrhythmic drugs (n) | 1.10 (0.84–1.43) | NS |
| Operators (n) | 0.92 (0.74–1.13) | NS |
| Baseline AVNRT induction only on isoproterenol (pts) | 1.02 (0.36–2.87) | NS |
| Cryoablation application (n) | 1.04 (0.99–1.08) | NS |
| Total cryoablation duration (s) | 1.4 (0.8–1.1) | <0.02 |
| A/V at last successful site (ratio) | 0.54 (0.12–2.39) | NS |
| Freezing–thawing–freezing cycle (pts) | 0.70 (0.34–1.42) | NS |
| Inadvertent 2nd/3rd AV-block at last successful site (pts) | 1.47 (0.35–6.10) | NS |
| ΔAV nodal ERP (ms) | 0.99 (0.98–1.01) | NS |
| Post-procedural residual jump (pts) | 0.36 (0.19–0.67) | <0.001 |
| Multivariate analysis | ||
| Total cryoablation duration (s) | 1.00 (1.00–1.01) | NS |
| Post-procedural residual jump (pts) | 0.40 (0.21–0.76) | <0.01 |
HR, hazard ratio; CI, confidence interval; AVNRT, atrioventricular nodal reentry tachycardia; AV, atrioventricular; ERP, effective refractory period; Δ ERP, mean of the difference between baseline and post-procedural ERP; pts, patients.
Comparison between patients with and without recurrences in the follow-up depending on the presence or absence of post-procedural residual jump
Patients without a residual jump (n= 201; 82%)
No differences were found in any clinical and procedural variables between patients with and without recurrence, with the exception of a total cryoablation time that was more prolonged in patients with recurrences (1278 ± 888 vs. 898 ± 738 s; P< 0.02)
Patients with a residual jump (n= 44; 18%)
No differences were found in any clinical and procedural variables between patients with and without recurrence. Of note, total cryoablation time was similar between the two groups. However, Δ ERP was shorter in patients with recurrences, despite the fact that the difference did not reach significativity (35 ± 53 vs. 57 ± 39 ms; P= ns).
Comparison among patients with no residual jump after cryoablation, persistence of jump but no echo, and jump associated with a single echo
Three groups of patients were compared: patients without residual jump (n= 201; 82%), patients with jump and no echo (n= 30; 12%), and those with residual jump associated with a single echo (n= 14; 6%). Total cryoablation time was shorter in patients without jump than in those with jump and no echo and jump with echo (926 ± 774 s vs. 1315 ± 947 s vs. 1281 ± 645 s; P< 0.02). Incidence of recurrences was lower in patients with no jump than in those with only jump or jump with echo [28 of 201 (14%) pts vs. 10 of 30 (33%) pts vs. 5 of 14 (36%) pts; P< 0.01)]. Incidence of cryoablation redo procedures was lower in the group without jump than in those with only jump or jump with echo, but the difference was not significant [12 of 201 (5.9%) pts vs. 3 of 30 (10%) pts vs. 2 of 14 (14.3%) pts; P= ns]. In contrast, incidence of RF redo procedures was significantly lower in patients with no jump than in those with only jump or jump with echo [1 of 201 (0.5%) pts vs. 2 of 30 (6.6%) pts vs. 2 of 14 (14.3%) pts; P< 0.02]. At 12-month follow-up, actuarial incidence of recurrence was 14.9% in patients with suppressed slow pathway, 36.7% in patients with jump and no echo, and 39.4% in patients with jump with a single echo (P< 0.003 among groups) (Table 3, Figure 1).
Comparison among patients with no residual jump after cryoablation, persistence of jump but no echo, and jump associated with a single echo
| Variables . | No jump . | Jump . | Jump . | P . |
|---|---|---|---|---|
| No echo . | No echo . | Echo . | χ2, ANOVA . | |
| Patients (n) | 201 | 30 | 14 | |
| Age (years) | 42 ± 16 | 39 ± 13 | 40 ± 19 | NS |
| Previous AA drugs (n) | 1.2 ± 1.0 | 1.3 ± 1.2 | 1.8 ± 1.5 | NS |
| A/V at last effective site (ratio) | 1 ± 0.3 | 1 ± 0.1 | 1.3 ± 0.7 | NS |
| Δ AV nodal ERP (ms) | 64 ± 35 | 51 ± 53 | 42 ± 29 | NS |
| Total cryoablation time (s) | 926 ± 774 | 1315 ± 947 | 1281 ± 645 | <0.02 |
| Recurrence (pts) | 28 (14%) | 10 (33%) | 5 (36%) | <0.01 |
| Cryoablation redo (pts) | 12 (5.9%) | 3 (10%) | 2 (14.3%) | NS |
| RF ablation redo (pts) | 1 (0.5%) | 2 (6.6%) | 2 (14.3%) | <0.01 |
| Variables . | No jump . | Jump . | Jump . | P . |
|---|---|---|---|---|
| No echo . | No echo . | Echo . | χ2, ANOVA . | |
| Patients (n) | 201 | 30 | 14 | |
| Age (years) | 42 ± 16 | 39 ± 13 | 40 ± 19 | NS |
| Previous AA drugs (n) | 1.2 ± 1.0 | 1.3 ± 1.2 | 1.8 ± 1.5 | NS |
| A/V at last effective site (ratio) | 1 ± 0.3 | 1 ± 0.1 | 1.3 ± 0.7 | NS |
| Δ AV nodal ERP (ms) | 64 ± 35 | 51 ± 53 | 42 ± 29 | NS |
| Total cryoablation time (s) | 926 ± 774 | 1315 ± 947 | 1281 ± 645 | <0.02 |
| Recurrence (pts) | 28 (14%) | 10 (33%) | 5 (36%) | <0.01 |
| Cryoablation redo (pts) | 12 (5.9%) | 3 (10%) | 2 (14.3%) | NS |
| RF ablation redo (pts) | 1 (0.5%) | 2 (6.6%) | 2 (14.3%) | <0.01 |
AA, antiarrhythmic; AV, atrioventricular; ΔERP, difference between baseline and post-procedural AV nodal effective refractory period; RF, radiofrequency; pts, patients.
Comparison among patients with no residual jump after cryoablation, persistence of jump but no echo, and jump associated with a single echo
| Variables . | No jump . | Jump . | Jump . | P . |
|---|---|---|---|---|
| No echo . | No echo . | Echo . | χ2, ANOVA . | |
| Patients (n) | 201 | 30 | 14 | |
| Age (years) | 42 ± 16 | 39 ± 13 | 40 ± 19 | NS |
| Previous AA drugs (n) | 1.2 ± 1.0 | 1.3 ± 1.2 | 1.8 ± 1.5 | NS |
| A/V at last effective site (ratio) | 1 ± 0.3 | 1 ± 0.1 | 1.3 ± 0.7 | NS |
| Δ AV nodal ERP (ms) | 64 ± 35 | 51 ± 53 | 42 ± 29 | NS |
| Total cryoablation time (s) | 926 ± 774 | 1315 ± 947 | 1281 ± 645 | <0.02 |
| Recurrence (pts) | 28 (14%) | 10 (33%) | 5 (36%) | <0.01 |
| Cryoablation redo (pts) | 12 (5.9%) | 3 (10%) | 2 (14.3%) | NS |
| RF ablation redo (pts) | 1 (0.5%) | 2 (6.6%) | 2 (14.3%) | <0.01 |
| Variables . | No jump . | Jump . | Jump . | P . |
|---|---|---|---|---|
| No echo . | No echo . | Echo . | χ2, ANOVA . | |
| Patients (n) | 201 | 30 | 14 | |
| Age (years) | 42 ± 16 | 39 ± 13 | 40 ± 19 | NS |
| Previous AA drugs (n) | 1.2 ± 1.0 | 1.3 ± 1.2 | 1.8 ± 1.5 | NS |
| A/V at last effective site (ratio) | 1 ± 0.3 | 1 ± 0.1 | 1.3 ± 0.7 | NS |
| Δ AV nodal ERP (ms) | 64 ± 35 | 51 ± 53 | 42 ± 29 | NS |
| Total cryoablation time (s) | 926 ± 774 | 1315 ± 947 | 1281 ± 645 | <0.02 |
| Recurrence (pts) | 28 (14%) | 10 (33%) | 5 (36%) | <0.01 |
| Cryoablation redo (pts) | 12 (5.9%) | 3 (10%) | 2 (14.3%) | NS |
| RF ablation redo (pts) | 1 (0.5%) | 2 (6.6%) | 2 (14.3%) | <0.01 |
AA, antiarrhythmic; AV, atrioventricular; ΔERP, difference between baseline and post-procedural AV nodal effective refractory period; RF, radiofrequency; pts, patients.
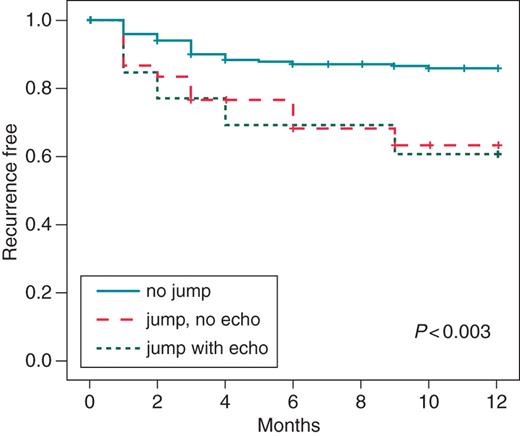
Comparison among patients with no residual jump after cryoablation, persistence of jump but no echo, and jump associated with a single echo.
Discussion
Main findings
In a selected population of patients undergoing cryoablation for slow-fast AVNRT, slow pathway suppression was correlated with a better long-term outcome. Persistence of a single echo in patients with residual jump did not add an adjunctive risk.
Immediate and long-term results of cryoablation for atrioventricular nodal reentry tachycardia—data from the literature
Cryoablation for AVNRT has been the object of numerous studies in the last decade. Pooled data from 20 published studies including 2351 patients3–22 show that the initial success rate for AVNRT cryoablation was 95% (range 85–99%), not so far from acute success described in RF series.1,2 Overall, the recurrence rate was 11% (range 2–19.7%) higher than RF catheter ablation in which a recurrence rate of between 3 and 5% has been reported.1,2 Additionally, some studies showed that slow pathway cryoablation for AVNRT was globally associated with a higher recurrence rate when compared with RF.3–7,10,14,19,20,22 Speculations to explain this discrepancy include comparatively smaller lesions, in part due to cryocatheter adherence to underlying endocardium, that eliminates a sliding effect along a larger area as under RF ablation.
Possible strategies for further benefit of cryoablation for AVNRT
Further benefit may potentially be expected from cryocatheters with larger distal electrode tips. Several studies indicated that 6 mm-tip catheters are associated with a better outcome than 4 mm-tip catheters,8,11–13,26 and one study conducted with an 8 mm tip showed better acute and long-term results, higher than 6 mm-tip catheters.21 At the same time, it may be hypothesized that pursuing a more aggressive procedural endpoint, such as complete slow pathway abolition, may improve long-term success. With RF energy, persistence of dual-AV nodal physiology post-ablation with no more than a single echo beat is commonly considered as an acceptable endpoint.23,27,28 In a recent meta-analysis, Stern et al.29 found that if isoproterenol was systematically used after RF ablation to assess the ability of a residual slow pathway to generate AV nodal echo beats or sustained AVNRT, no statistically significant difference in recurrence rate was found between patients with complete suppression of the slow pathway and those with either a residual jump and/or single echo beat. However, in studies in which isoproterenol was not systematically used after RF ablation, complete slow pathway suppression was associated with a better outcome. In studies in which cryoablation for AVNRT was performed, AVNRT inducibility on isoproterenol at the end of the procedure was systematically performed in all patients in the vast majority of studies and limited to patients with a residual slow pathway in only a few studies. In a series of patients treated using cryoenergy, Sandilands et al.13 found that complete slow pathway suppression was associated with long-term success. Immediate success was considered as complete slow pathway block or AVNRT non-inducibility on isoproterenol in the presence of a single atrial echo. Recurrence rates were greater where slow pathway suppression was not achieved [8 of 12 (66.7%), compared with complete slow pathway block 11 of 129 (8.5%, P < 0.0001)]. Recurrence was significantly more likely if atrial echo beats were still present after cryoablation [12 of 130 (9.2%) patients with no recurrence vs. 7 of 19 (36.8%) patients with recurrence (P< 0.0001)]. Other authors found a non-significant trend in terms of recurrence in the case of persistence of dual-AV nodal physiology.11 In the present study, conducted in a highly selected population of patients with baseline elicitable jump and inducible slow-fast AVNRT successfully ablated using a 6 mm cryocatheter, persistence of a residual slow pathway was associated with a higher incidence of recurrence, but the presence of an associated single echo did not add an adjunctive risk. We can speculate that a longer cryoapplication time in patients with a residual jump could indicate more difficult procedures but might also result in a more stable lesion effect with a more pronounced slow pathway conduction modification. This fact might explain why long-term results were similar between patients with a residual jump without a single echo and those with an associated single echo.
Slow pathway conduction modification
There are scant data concerning slow pathway modification effects and outcome between RF and cryoenergy. In the series of Gupta et al.,7 a single echo beat could be induced in 20 of 71 (28%) patients in the RF group and 19 of 71 (27%) patients in the cryoablation group. Of these, one (5%) and seven (36.8%) patients, respectively, had documented arrhythmia recurrence (P< 0.05). In this study isoproterenol was not uniformly used after ablation.
However, not all patients with a residual slow pathway associated or not with a single echo after ablation had recurrence. Recently, Posan et al.30 demonstrated that in patients treated with RF with persistent slow pathway conduction, but no recurrent AVNRT, slow pathway ERP increased, while the difference between the fast and slow pathway ERP was reduced, decreasing the AVNRT induction window,9 The authors argue that these changes prevent the induction of AVNRT because the slow pathway can no longer sustain persistent 1:1 A/V conduction. In our study, the Δ AV nodal ERP was noticeably prolonged in patients with no slow pathway conduction. However, it was also prolonged in patients with a residual slow pathway associated or not with a single echo, indicating a slow pathway modification despite no slow pathway suppression.
Transient 2nd/3rd atrioventricular-block at last site
We found that in patients with 2nd/3rd AV-block at the last effective site and AVNRT non-inducibility, the incidence of recurrence was relatively low, despite a very short cryoapplication time at the last effective site. Of note, no patients had a residual slow pathway at the end of the procedure. In a previous study9 we speculated that a relatively low recurrence rate may be explained by an effective cryolesion involving the compact AV node and the site of connection with the slow pathway (whatever its anatomical and functional substrate), in this case more fragile or limited in size.
Clinical implications
Our data suggest that patients with residual jump associated or not with a residual echo are prone to a high recurrence rate, but so are patients with more difficult procedures. On the basis of our results, a procedure up to complete elimination of slow pathway conduction appears clearly better. However, a very aggressive endpoint can be potentially dangerous for AV node conduction integrity, even if no AV-block requiring a pacemaker after AVNRT cryoablation has been reported in the literature until now. Another theoretical strategy would be to continue the procedure in the case of residual slow pathway after cryoablation despite AVNRT non-inducibility and to limit the endpoint to nodal AV ERP prolongation with reduction of the slow pathway ‘window’ in more resistant cases. In the present study, we found that in the sub-group of patients with a residual slow pathway, the AV nodal Δ ERP was shorter in patients with recurrences when compared with those without recurrences, although the difference did not reach significance. However, there are no randomized controlled trials comparing acute and long-term effects and complication rates in patients receiving slow pathway ablation vs. slow pathway modification in either RF or cryoablation procedures.
Conclusions
In patients undergoing AVNRT cryoablation, slow-pathway suppression is correlated with a better outcome. A single echo is associated with a recurrence risk similar to residual jump without echo. It may be suggested that pursuing a procedural endpoint up to slow pathway complete suppression may improve long-term success.
Conflict of interest: none declared.



