-
PDF
- Split View
-
Views
-
Cite
Cite
Maite Izquierdo, Ricardo Ruiz-Granell, Angel Ferrero, Angel Martínez, JuanMiguel Sánchez-Gomez, Clara Bonanad, Beatriz Mascarell, Salvador Morell, Roberto García-Civera, Ablation or conservative management of electrical storm due to monomorphic ventricular tachycardia: differences in outcome, EP Europace, Volume 14, Issue 12, December 2012, Pages 1734–1739, https://doi.org/10.1093/europace/eus186
Close - Share Icon Share
Abstract
Electrical storm (ES) is a life-threatening condition that predicts bad prognosis. Treatment includes antiarrhythmic drugs (AAD) and catheter ablation (CA). The present study aims to retrospectively compare prognosis in terms of survival and ES recurrence in 52 consecutive patients experiencing a first ES episode.
Patients were admitted from 1995 to 2011 and treated for ES by conservative therapy (pharmacological, 29 patients) or by CA (23 patients), according to the physician's preference and time of occurrence, i.e. conservative treatments were more frequently administered during the first years of the study, as catheter ablation became more frequent as the years passed by. After a median follow-up of 28 months, no differences either in survival (32% vs. 29% P = 0.8) or in ES recurrence (38% in ablated vs. 57% in non-ablated patients, P = 0.29) were observed between groups. Low left ventricle ejection fraction (LVEF) was the only variable associated with ES recurrence in ablated patients. When including patients with LVEF > 25%, ES recurrence was significantly lower in ablated patients (24 months estimated risk of ES recurrence was 21% vs. 62% in ablated and non-ablated patients, respectively); however, no benefit in survival was observed.
Our data suggest that in most patients, especially those with an LVEF > 25%, catheter ablation following a first ES episode, decreases the risk of ES recurrence, without increasing survival.
Introduction
Electrical storm (ES) is a life-threatening condition with a poor prognosis.1–3 Electrical storm episodes most commonly present as multiple sustained episodes of ventricular tachycardia (VT) or incessant VT. In patients with implanted cardioverter defibrillators (ICD), ES usually leads to the delivery of multiple shocks. Treatment of such a condition includes antiarrhythmic drugs (AAD) and catheter ablation (CA). Many studies have demonstrated the effects and usefulness of AAD.4 Available experience on long-term outcomes in patients with ES treated with CA is limited. Nevertheless, results of a few published series are variable5–8 but no prospective series or registries compare ablation strategy and conservative pharmacological treatment in these patients.
The purpose of this study is to provide a comparison based on treatment strategy, i.e. ablation or conservative treatment, in different outcomes, such as mortality and ES recurrence in patients experiencing their first ES.
Methods
Patient population
We retrospectively reviewed consecutive patients admitted to our institution for their first episode of ES, from 1995 to 2011. Electrical storm was defined as the occurrence of three or more episodes of sustained VT, separated by 5 min, during a 24 h period or the presence of incessant VT (defined as persistent sustained VT or continuous episodes of VT separated by brief bouts of normal rhythm). Parameters recorded included baseline characteristics and outcomes including death from any cause and readmission for a new ES episode.
Treatment selection
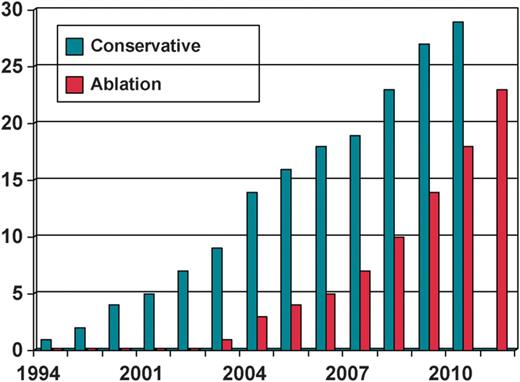
Patients' selection for VT ablation was based on physician's preference; however, conservative treatment with antiarrhythmic therapy was mainly chosen in patients admitted in the earliest years (when ablation technique was not as common as in the latest years of the study, when it became the first treatment choice) (Figure 1).
Conservative management
Conservative management included admission to the intensive care unit or to a monitorized ward at hospital, ICD therapies switch-off, sedation with benzodiazepines, intravenous or oral beta-blocker therapy, and administration of AAD. Intravenous procainamide or amiodarone were the first-choice drugs in the acute phase. Oral amiodarone or sotalol when contraindicated was selected for chronic management. In patients not responding to amiodarone or sotalol, other AAD (procainamide, flecainide, propafenone, and mexiletine) or combinations were empirically selected.
All patients, including ablated patients, received pharmacological treatment to be stabilized, as a first step, previous ablation. Management of ischaemia was also indicated in all patients including coronary angiography and revascularization procedures if indicated.
Ablation procedure
The procedure was performed under local anaesthesia and conscious sedation. A quadripolar diagnostic catheter was introduced via the right femoral vein to the right ventricular apex. If the VT was not incessant, VT was induced with programmed stimulation and number of morphologies and ease of inducibility were recorded. The left ventricle was accessed retrogradely through the aortic valve or via a transseptal approach. Electroanatomical left ventricle maps were obtained using CARTO XP (Biosense Webster, Diamond Bar, CA, USA) or EnSite NavX (St Jude Medical, St Paul, MN, USA). Non-contact mapping was performed in two patients. Epicardial approach was not used in any case.
Ablation was performed using 3.5 mm saline-irrigated tip ablation catheters (Navistar Thermocool, Biosense Webster, or Celsius Thermocool, Biosense Webster). Simultaneous recordings of ventricular electrograms (bandpass filtered 30–500 Hz) and 12 lead surface electrocardiogram were stored digitally (Prucka Cardiolab, GE Medical Systems, Milwaukee, WI, USA). The procedure was performed under intravenous anticoagulation with sodium heparin (initial bolus of 50 IU/kg followed by a 1000 IU/h perfusion adjusted to maintain the partial time of tromboplastine activated above 250 s).
Isovoltage maps of the left ventricle were constructed whenever possible and areas with no electrograms were searched to localize a dense scar. The threshold voltage to consider an area as part of a scar was set around 1.5 mV trying to find isthmuses among scars. Regions with fragmented, abnormal electrograms and late potentials were annotated using colour tags. Points with QRS morphology during pace-mapping identical to that during documented VT were also annotated. When VT was present or induced and it was haemodynamically well tolerated, activation maps and entrainment-mapping techniques were performed trying to characterize the arrhythmia circuit. Radiofrequency energy was delivered in the power control mode through the irrigated tip catheters using a Stocker generator with power set to 25–40 W and irrigation set to 17–30 mL/min. Radiofrequency lesions were created either during VT or sinus rhythm in the regions identified or supposed to be critical for the sustenance of clinical or inducible VTs. Postprocedure inducibility was not systematically tested in all patients. However, induction or re-induction was found to be difficult in these patients often presenting with pleomorphism and multiple or bad tolerated tachycardias. In these cases, substrate-modifying, primarily potential-guided catheter ablation approach was performed.
All patients without previous ICD implant were implanted before discharge except for one patient with terminal prostate neoplasm and normal LVEF.
Follow-up
All patients were followed-up every 6 months. Recorded were mortality and further admissions due to ES recurrence.
Statistical analysis
Data are shown as number and percentage or as median and inter-quartile range (IQR). Comparisons of continuous variables were done using non-parametric tests (Mann–Whitney U test) and categorical variables were analysed using χ2 or the Fisher's exact test. Survival functions were estimated by Kaplan–Meier analysis and differences between strata were assessed by log-rank test. A P value <0.05 was considered significant. SPSS v19.0 statistical package was used for analysis.
Results
Baseline characteristics and follow-up
From January 1995 to September 2011, 52 patients experiencing a first episode of ES due to monomorphic VT were admitted at hospital. Patients' baseline characteristics are shown in Table 1. A total of 39 (75%) patients already had an ICD, of these, 5 (13%) patients had been implanted for primary prevention, and 34 (87%) patients for secondary prevention.
| . | All patients . | Ablated patients . | Conservative treatment . | P . |
|---|---|---|---|---|
| LVEF (%) | 34 ± 10 | 32 | 35 | 0.26 |
| Age (years) | 70 (62–74) | 69 (62–74) | 70 (58–74) | 0.5 |
| Creatinine (mg/dl) | 1.1 (1–1.4) | 1.2 (0.9–1.4) | 1.1 (1–1.3) | 0.8 |
| LVEDD (mm) | 66 (58–71) | 60 (54–66) | 70 (63–76) | 0.01 |
| NYHA (mean) | 2 (2–2) | 2 (2–2) | 2 (2–2.5) | 0.4 |
| Previous VT (%) | 67.3 | 54 | 73 | 0.2 |
| Previous ICD (%) | 29 (75) | 86.2 | 60.9 | 0.054 |
| ARB (%) | 84.6 | 83 | 84 | 1 |
| Beta-blockers (%) | 51 | 47 | 52 | 1 |
| Diabetics (%) | 30.8 | 35 | 36 | 1 |
| Hypertension (%) | 69.2 | 47 | 52 | 1 |
| EPOC (%) | 21.2 | 16 | 28 | 0.4 |
| Previous SVT (%) | 28.8 | 34.5 | 21.7 | 0.24 |
| Previous AT (%) | 46.2 | 43.5 | 48.3 | 0.8 |
| AT post-ES (%) | 86.5 | 82.6 | 89.7 | 0.7 |
| Heart disease | ||||
| Ischaemic (%) | 19 (82.6) | 19 (65.5) | 0.6 | |
| NIDCM (%) | 3 (13) | 6 (20.7) | ||
| RVAD (%) | 0 | 1 (3.4) | ||
| Valvular (%) | 0 | 1 (3.4) | ||
| Mixed (%) | 1 (4.3) | 2 (6.9) | ||
| Median follow-up (months) | 28 (6–75) | 18 (3–48) | 41 (8–82) | 0.054 |
| . | All patients . | Ablated patients . | Conservative treatment . | P . |
|---|---|---|---|---|
| LVEF (%) | 34 ± 10 | 32 | 35 | 0.26 |
| Age (years) | 70 (62–74) | 69 (62–74) | 70 (58–74) | 0.5 |
| Creatinine (mg/dl) | 1.1 (1–1.4) | 1.2 (0.9–1.4) | 1.1 (1–1.3) | 0.8 |
| LVEDD (mm) | 66 (58–71) | 60 (54–66) | 70 (63–76) | 0.01 |
| NYHA (mean) | 2 (2–2) | 2 (2–2) | 2 (2–2.5) | 0.4 |
| Previous VT (%) | 67.3 | 54 | 73 | 0.2 |
| Previous ICD (%) | 29 (75) | 86.2 | 60.9 | 0.054 |
| ARB (%) | 84.6 | 83 | 84 | 1 |
| Beta-blockers (%) | 51 | 47 | 52 | 1 |
| Diabetics (%) | 30.8 | 35 | 36 | 1 |
| Hypertension (%) | 69.2 | 47 | 52 | 1 |
| EPOC (%) | 21.2 | 16 | 28 | 0.4 |
| Previous SVT (%) | 28.8 | 34.5 | 21.7 | 0.24 |
| Previous AT (%) | 46.2 | 43.5 | 48.3 | 0.8 |
| AT post-ES (%) | 86.5 | 82.6 | 89.7 | 0.7 |
| Heart disease | ||||
| Ischaemic (%) | 19 (82.6) | 19 (65.5) | 0.6 | |
| NIDCM (%) | 3 (13) | 6 (20.7) | ||
| RVAD (%) | 0 | 1 (3.4) | ||
| Valvular (%) | 0 | 1 (3.4) | ||
| Mixed (%) | 1 (4.3) | 2 (6.9) | ||
| Median follow-up (months) | 28 (6–75) | 18 (3–48) | 41 (8–82) | 0.054 |
Continuous variables are shown as median (interquartilic range).
ICD, implantable cardiac defibrillator; SVT, supra-ventricular tachycardia; LVEF, left ventricle ejection fraction; VT, ventricular tachycardia; EPOC, chronic obstructive bronchitis; ARB, angiotensin receptor blockers; AT, antyarrhythmic therapy; ES, electrical storm, NIDCM, non-ischaemic dilated cardiomiopathy; RVAD, right ventricle arrhythmogenic dysplasia.
| . | All patients . | Ablated patients . | Conservative treatment . | P . |
|---|---|---|---|---|
| LVEF (%) | 34 ± 10 | 32 | 35 | 0.26 |
| Age (years) | 70 (62–74) | 69 (62–74) | 70 (58–74) | 0.5 |
| Creatinine (mg/dl) | 1.1 (1–1.4) | 1.2 (0.9–1.4) | 1.1 (1–1.3) | 0.8 |
| LVEDD (mm) | 66 (58–71) | 60 (54–66) | 70 (63–76) | 0.01 |
| NYHA (mean) | 2 (2–2) | 2 (2–2) | 2 (2–2.5) | 0.4 |
| Previous VT (%) | 67.3 | 54 | 73 | 0.2 |
| Previous ICD (%) | 29 (75) | 86.2 | 60.9 | 0.054 |
| ARB (%) | 84.6 | 83 | 84 | 1 |
| Beta-blockers (%) | 51 | 47 | 52 | 1 |
| Diabetics (%) | 30.8 | 35 | 36 | 1 |
| Hypertension (%) | 69.2 | 47 | 52 | 1 |
| EPOC (%) | 21.2 | 16 | 28 | 0.4 |
| Previous SVT (%) | 28.8 | 34.5 | 21.7 | 0.24 |
| Previous AT (%) | 46.2 | 43.5 | 48.3 | 0.8 |
| AT post-ES (%) | 86.5 | 82.6 | 89.7 | 0.7 |
| Heart disease | ||||
| Ischaemic (%) | 19 (82.6) | 19 (65.5) | 0.6 | |
| NIDCM (%) | 3 (13) | 6 (20.7) | ||
| RVAD (%) | 0 | 1 (3.4) | ||
| Valvular (%) | 0 | 1 (3.4) | ||
| Mixed (%) | 1 (4.3) | 2 (6.9) | ||
| Median follow-up (months) | 28 (6–75) | 18 (3–48) | 41 (8–82) | 0.054 |
| . | All patients . | Ablated patients . | Conservative treatment . | P . |
|---|---|---|---|---|
| LVEF (%) | 34 ± 10 | 32 | 35 | 0.26 |
| Age (years) | 70 (62–74) | 69 (62–74) | 70 (58–74) | 0.5 |
| Creatinine (mg/dl) | 1.1 (1–1.4) | 1.2 (0.9–1.4) | 1.1 (1–1.3) | 0.8 |
| LVEDD (mm) | 66 (58–71) | 60 (54–66) | 70 (63–76) | 0.01 |
| NYHA (mean) | 2 (2–2) | 2 (2–2) | 2 (2–2.5) | 0.4 |
| Previous VT (%) | 67.3 | 54 | 73 | 0.2 |
| Previous ICD (%) | 29 (75) | 86.2 | 60.9 | 0.054 |
| ARB (%) | 84.6 | 83 | 84 | 1 |
| Beta-blockers (%) | 51 | 47 | 52 | 1 |
| Diabetics (%) | 30.8 | 35 | 36 | 1 |
| Hypertension (%) | 69.2 | 47 | 52 | 1 |
| EPOC (%) | 21.2 | 16 | 28 | 0.4 |
| Previous SVT (%) | 28.8 | 34.5 | 21.7 | 0.24 |
| Previous AT (%) | 46.2 | 43.5 | 48.3 | 0.8 |
| AT post-ES (%) | 86.5 | 82.6 | 89.7 | 0.7 |
| Heart disease | ||||
| Ischaemic (%) | 19 (82.6) | 19 (65.5) | 0.6 | |
| NIDCM (%) | 3 (13) | 6 (20.7) | ||
| RVAD (%) | 0 | 1 (3.4) | ||
| Valvular (%) | 0 | 1 (3.4) | ||
| Mixed (%) | 1 (4.3) | 2 (6.9) | ||
| Median follow-up (months) | 28 (6–75) | 18 (3–48) | 41 (8–82) | 0.054 |
Continuous variables are shown as median (interquartilic range).
ICD, implantable cardiac defibrillator; SVT, supra-ventricular tachycardia; LVEF, left ventricle ejection fraction; VT, ventricular tachycardia; EPOC, chronic obstructive bronchitis; ARB, angiotensin receptor blockers; AT, antyarrhythmic therapy; ES, electrical storm, NIDCM, non-ischaemic dilated cardiomiopathy; RVAD, right ventricle arrhythmogenic dysplasia.
Twenty-nine patients were conservatively managed. Two of them died during the admission because of cardiogenic shock. The rest of non-ablated patients were stabilized with conservative management and the final AAD regimen was as follows: amiodarone (13 patients), sotalol (6 patients), flecainide (3 patients), procainamide (1 patient), combination of two AAD (2 patients) and no AAD (2 patients).
A total of 23 patients (44%) underwent CA during the admission.
During a median follow-up of 28 months (IQR 6–75 months), 22 patients died (42%); causes of death were cardiac for 10 (45.5%) patients, non-cardiac for 9 (41%) patients and unknown in 3 patients. Non-cardiac deaths were due to malignancies (four patients), sepsis (two patients) and subdural bleeding, cirrhosis and COPD in the remaining three patients.
During follow-up, 26 patients (50%) had at least one re-admission because of an ES.
At baseline, no differences in age, New York Heart Association class, medical treatment, or LVEF were observed between groups; however, left ventricular end-diastolic diameter (LVEDD) was significantly higher in non-ablated patients compared with those who underwent CA. Mean follow-up was longer in non-ablated patients.
Ablation procedure
Two CA procedures out of 23 were performed using the Ensite Array system; CA was guided by activation maps in eight patients and in four of them substrate modification guided by isovoltage maps was also performed. Substrate modification combined with pace-mapping was performed in 13 patients only. Post-ablation inducibility of VT was tested in 16 patients and 9 of them remained non-inducible for any type of VT, clinical or not.
Outcome in ablated patients
Electrical storm recurrence
In the ablation group, eight (34.8%) patients had an ES recurrence during follow-up. LVEF was significantly lower in patients that recurred (25% vs. 35% in those that did not recur; P = 0.023). Underlying cardiac disease was not associated to ES recurrence (Table 2).
| . | ES recurrence . | No new ES . | P . |
|---|---|---|---|
| LVEF (%) | 25 (22.5–32.5) | 35 (30–40) | 0.023 |
| Age (years) | 72 (47–77) | 68 (62–73) | 0.7 |
| Creatinin (mg/dl) | 1.1 (0.9–1.3) | 1.2 (1–1.7) | 0.2 |
| LVEDD (mm) | 63 (60–66) | 56 (51–68) | 0.2 |
| NYHA | 2 (1.3–2.8) | 2 (2–2) | 0.8 |
| BB (%) | 62 | 60 | 1 |
| ARB (%) | 100 | 73 | 0.68 |
| EPOC (%) | 13 | 37 | 0.29 |
| Diabetics (%) | 25 | 33 | 1 |
| Previous SVT (%) | 25 | 20 | 1 |
| . | ES recurrence . | No new ES . | P . |
|---|---|---|---|
| LVEF (%) | 25 (22.5–32.5) | 35 (30–40) | 0.023 |
| Age (years) | 72 (47–77) | 68 (62–73) | 0.7 |
| Creatinin (mg/dl) | 1.1 (0.9–1.3) | 1.2 (1–1.7) | 0.2 |
| LVEDD (mm) | 63 (60–66) | 56 (51–68) | 0.2 |
| NYHA | 2 (1.3–2.8) | 2 (2–2) | 0.8 |
| BB (%) | 62 | 60 | 1 |
| ARB (%) | 100 | 73 | 0.68 |
| EPOC (%) | 13 | 37 | 0.29 |
| Diabetics (%) | 25 | 33 | 1 |
| Previous SVT (%) | 25 | 20 | 1 |
Continuous variables are shown as median (interquartilic range).
LVEDD, left ventricle end diastolic diameter; BB, beta-blocker; ARB, angiotensin receptor blockers; EPOC, chronic obstructive bronchitis.
| . | ES recurrence . | No new ES . | P . |
|---|---|---|---|
| LVEF (%) | 25 (22.5–32.5) | 35 (30–40) | 0.023 |
| Age (years) | 72 (47–77) | 68 (62–73) | 0.7 |
| Creatinin (mg/dl) | 1.1 (0.9–1.3) | 1.2 (1–1.7) | 0.2 |
| LVEDD (mm) | 63 (60–66) | 56 (51–68) | 0.2 |
| NYHA | 2 (1.3–2.8) | 2 (2–2) | 0.8 |
| BB (%) | 62 | 60 | 1 |
| ARB (%) | 100 | 73 | 0.68 |
| EPOC (%) | 13 | 37 | 0.29 |
| Diabetics (%) | 25 | 33 | 1 |
| Previous SVT (%) | 25 | 20 | 1 |
| . | ES recurrence . | No new ES . | P . |
|---|---|---|---|
| LVEF (%) | 25 (22.5–32.5) | 35 (30–40) | 0.023 |
| Age (years) | 72 (47–77) | 68 (62–73) | 0.7 |
| Creatinin (mg/dl) | 1.1 (0.9–1.3) | 1.2 (1–1.7) | 0.2 |
| LVEDD (mm) | 63 (60–66) | 56 (51–68) | 0.2 |
| NYHA | 2 (1.3–2.8) | 2 (2–2) | 0.8 |
| BB (%) | 62 | 60 | 1 |
| ARB (%) | 100 | 73 | 0.68 |
| EPOC (%) | 13 | 37 | 0.29 |
| Diabetics (%) | 25 | 33 | 1 |
| Previous SVT (%) | 25 | 20 | 1 |
Continuous variables are shown as median (interquartilic range).
LVEDD, left ventricle end diastolic diameter; BB, beta-blocker; ARB, angiotensin receptor blockers; EPOC, chronic obstructive bronchitis.
Prescriptions
Ten patients (43.5%) were already on AAD before ablation. After ES ablation, 19 patients (82.6%) were prescribed AAD. Prescription or not of AAD after ablation was not associated to ES recurrence. (In the group of patients with non-ES recurrence, 80% were on AAD post-ablation vs. 87.5% of patients in the group of recurrence, P = 1.)
Other variables
Table 2 shows other variables that were analysed. We did not find any variable associated with survival.
Survival in ablated vs. non-ablated patients
During follow-up, nine non-ablated patients (51.7%) died vs. six patients (30.4%) in the ablated patients group. Survival curves did not differ between ablated and non-ablated patients. The estimated cumulative survival at 24 months was 72% vs. 63% for non-ablated and ablated patients, respectively (log rank, P = 0.8). No differences in ES recurrence were found between groups. The estimated cumulative ES-free survival at 24 months was 40% vs. 57% in ablated and non-ablated patients, respectively (log rank, P = 0.29) (Figure 2A).
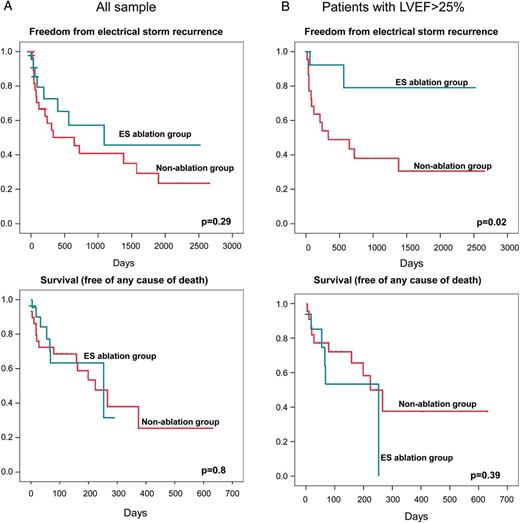
Kaplan–Meier event-free survival (superior panel: ES recurrence; inferior panel: death) estimates comparing CA vs. non-CA group: (A) total sample and (B) patients with LVEF > 25%.
Catheter ablation reduced the rate of readmission because of a new ES only in patients with LVEF > 25%. The 24 months estimated risk of ES recurrence was 21% vs. 62% in ablated and non-ablated patients respectively, with LVEF > 25% (log rank, P = 0.02). However, in this group of relatively preserved LVEF (>25%) no differences were found in the estimated mortality: 47% vs. 23% in ablated and non-ablated patients, respectively, (log rank, P = 0.39) (Figure 2B).
Discussion
This study retrospectively evaluated clinical outcomes of patients experiencing a first ES, comparing two treatments, i.e. VT ablation and pharmacological treatment. Our data showed that catheter ablation seems to reduce new episodes of ES, mostly in patients with LVEF > 25%. However, mortality for any cause remained high in both groups.
Impact on mortality
Independently of other variables as LVEF, ES is recognized as an important marker of death, especially cardiac death.1,5 The mortality after an ES is about 35% per year1,2 and after an initial ES there is a high incidence of subsequent ES.3,5–8 This fact leads to hypothesize that ES directly affects patient prognosis and even that preventing ES recurrence by cardiac ablation may exert a beneficial role in the prevention of cardiac death.5 Carbucicchio et al. studied the treatment effect on mortality and ES reurrence in a group of 95 patients with ES, after one to three ablation procedures. After a median follow-up of 22 months, 92% of patients were free of ES. Non-inducibility of VT, post-ablation was a predictor of ES recurrence and of cardiac and total mortality.5 Our study suggests different conclusions. Mortality remained high (42% in 28 months of median follow-up) in both. Also, no differences in all-cause mortality were found between patients with or without recurrence of ES groups. These different results between the present study and the one reported by Carbucicchio et al. may come from different reasons:
In Carbucicchio et al. study, 27% of patients died (non-cardiac deaths). In contrast, non-cardiac causes accounted for 40% of mortality in our series. This could indicate more co-morbidity among our patients. Furthermore, the mortality in our sample was higher than that of Carbucicchio's series (42% in a median follow-up of 28 months vs. 16% in a median of 22 months). Unfortunately, no statistical analysis could be performed regarding cardiac death as a specific target because of the small sample size.5
We did not stratify patients depending on post-ablation VT inducibility, and all ablated patients were included in the same prognosis group. To this respect, the value of post-ablation VT inducibility as a predictor of death and VT recurrence is controversial.6,9 Kozeluhova et al.6 have recently published a study of 50 patients with ES that were ablated. Non-inducibility of VT at the end of the procedure was not found predictive of the recurrence or risk of death.
Impact on electrical storm recurrence
It has been thoroughly demonstrated that VT ablation reduces the number of subsequent VT episodes in ICD patients with one or multiple previous episodes. There is a lack of studies addressing the role of ablation in the outcome of ES in comparison with a pharmacological strategy. In the present study, no differences in ES recurrence were found between ablated and pharmacologically treated patients.
In the study by Carbucicchio et al., the estimated cumulative ES-free survival was 92% in 22 months, which is surprising compared with the 57% of ES-free survival in our group of ablated patients in Kaplan–Meier analysis.5 Unlike Carbucicchio et al., who tested post-ablation VT inducibility and performed two or three procedures on a significant number of patients before achieving complete acute success, we did not systematically test VT inducibility and only performed one procedure in all patients. Moreover, in our series, an acute ES occurring after the first ablation was considered a recurrence. Nevertheless, the benefit of multiple ablation procedures is controversial. In a recent study, Kosmidou et al.,10 in a series of patients undergoing catheter ablation for post-infarction VT, concluded that failure of an initial ablation procedure does not preclude subsequent successful ablation. The discrepancy showed in ES ablation results, in the available series, is probably linked to differences not only in the baseline characteristics of the sample but also in the ablation procedure end-points, and, probably, in the definition of ES recurrence.5–8
Furthermore, LVEF is known to predict ICD therapies, a first ES episode and ES recurrence, in ablated6,10 and non-ablated patients.3,7,11,12 In the present study, ablation significantly reduced ES recurrence only in patients with LVEF > 25.Thus, our study supports the idea that ES ablation is less effective in patients with very severe dysfunction of LV. However no effects of CA on survival were observed.
Study limitations
This study was a retrospective analysis of long-term outcomes comparing patients with their first ES that underwent ablation and patients only medically treated, and not a prospective randomized one. However, selection of the treatment depended, in most of the patients, on the period presenting the ES, since in the first years ablation was not a common procedure. In fact, baseline characteristics were similar in both groups and follow-up was longer in non-ablated patients. Methodology of ablation procedure (inducibility not systematically tested, no repetition of procedures, and no epicardial approach) could have led to greater number of ES recurrences.
Conclusions
It is unclear as to whether ES plays an inciting, contributing, or bystander role in the observed excess mortality. Our data suggest that in most patients, especially those with higher LVEF, the CA option decreases the risk of ES recurrence without increasing survival. ES may mark an advanced stage of the disease and intensive treatment, including VT ablation, should be performed earlier.
Conflict of interest: none declared.



