-
PDF
- Split View
-
Views
-
Cite
Cite
Vern Hsen Tan, Jonathan Yap, Li-Fern Hsu,, Reginald Liew, Catheter ablation of ventricular fibrillation triggers and electrical storm, EP Europace, Volume 14, Issue 12, December 2012, Pages 1687–1695, https://doi.org/10.1093/europace/eus050
Close - Share Icon Share
Abstract
Ventricular fibrillation (VF) and electrical storm remain challenging conditions to manage despite the availability of various treatment modalities. Insertion of an implantable cardioverter defibrillator (ICD) remains the gold standard method for lowering the risk of sudden cardiac death in patients deemed to be at greatest risk of ventricular arrhythmias. However, ICDs do not alter the underlying substrate responsible for the arrhythmic events and a significant proportion of patients with ICDs may experience VF storm which may be life threatening and difficult to control with medication. Catheter ablation (CA) of the triggers or abnormal electrical substrate responsible for VF storm is an important treatment option in rare cases. In this article, we present an overview of the current theories underlying the mechanisms of VF and discuss how the technique of CA may be used to treat the triggers of VF and electrical storm. We review the literature on outcomes in patients who have undergone CA for VF in a variety of different settings, including those with structural heart disease and structurally normal hearts (e.g. patients with inherited arrhythmogenic diseases and idiopathic VF) and discuss the future directions in this field.
Introduction
Sudden cardiac death (SCD) is responsible for ∼300 000 deaths in the United States, with ventricular fibrillation (VF) accounting for up to one-third of all cases.1 Ventricular fibrillation can occur in patients with both structurally normal and abnormal hearts. The mortality benefit of implantable cardioverter defibrillators (ICDs) in patients at high risk of ventricular arrhythmias, such as those with depressed ventricular function following acute myocardial infarction (AMI), has been firmly established from a number of landmark prospective multi-centre trials and this has been the treatment of choice in the prevention of SCD.2–4 The use of ICDs comprises an important component of current international guidelines for device-based therapy for cardiac rhythm abnormalities.5 Although ICDs are effective in terminating life-threatening ventricular arrhythmias when they occur, they have no effect on the underlying substrate for the arrhythmias. Consequently, as many as 20% of patients with ICDs experience multiple VF episodes and electrical storm, resulting in significant distress and increased mortality.6,7
Advances in our understanding of the mechanisms responsible for VF and improvements in electrophysiological (EP) mapping techniques in recent years have made it possible for the use of catheter ablation (CA) strategies to be an effective option in the management of challenging cases. However, as VF is a heterogeneous condition involving a variety of mechanisms and triggers, CA is likely to be useful in only certain subtypes, where the triggers can be clearly identified and ablated. In this article, we provide an overview of the EP substrates and triggers that are currently believed to be responsible for VF and discuss the practicalities and limitations of using CA to treat this malignant arrhythmia.
Pathophysiology of ventricular fibrillation
Ventricular fibrillation can occur in people with electrically and structurally normal hearts in the presence of severe electrolyte abnormalities or can be artificially induced in the electrophysiology laboratory with the delivery of critically timed ventricular extra-stimuli during the vulnerable period of ventricular repolarization. However, most spontaneous episodes of VF occur in patients with electrophysiologically, structurally, and/or neurally abnormal hearts. These abnormalities may be congenital or acquired and may co-exist in the same patient. Recent advances have led to an improved understanding how these three factors work together to induce VF. A detailed discussion on all the potential mechanisms underlying VF and unstable ventricular arrhythmias is beyond the scope of this review. Instead, in this section we aim to provide a brief overview of some of the theories and mechanisms of VF that are particularly relevant to the use of CA as an effective treatment strategy.
Electrophysiological abnormalities
Ventricular fibrillation can occur in humans and experimental animals with structurally normal hearts in the presence of underlying EP abnormalities. These may take the form of inherited cardiac ion channel abnormalities that predispose to an increased risk of VF8 or changes in other proteins that affect cardiac electrical stability, such as connexins, peroxisome proliferator-activated receptor-γ, and calcium-related signalling proteins.9–11 Under physiological conditions, sarcoplasmic reticulum (SR) calcium release is primarily triggered by electrical activation (so-called calcium-induced calcium release following normal cellular depolarization). In pathological states, such as inherited mutations of the type 2 ryanodine receptor or acquired heart failure, there is increased spontaneous SR calcium release which leads to electrical instability and an increased predisposition to VF.12,13 In view of the diverse and widespread EP abnormalities (inherited and acquired) that are involved in initiating VF in these cardiac conditions, it can be appreciated that CA has no routine role to play in the management of these types of VF. A more suitable strategy would be to target the underlying EP substrate, such as intracellular calcium overload within the SR.14,15 Such strategies are still at an early stage and in the realms of pre-clinical evaluation. However, as will be discussed later, CA may have a role in rare cases where electrical triggers of VF can be mapped and identified.
Structural abnormalities
In animal studies, abnormal automaticity from triggers within the His–Purkinje system has been shown to contribute to the initiation of VF.16 Ablation of the subendocardium hastens spontaneous termination of VF and alters VF activation sequences, suggesting that Purkinje fibres are also important in the maintenance of VF.17 Apart from the His–Purkinje system, myocardial fibrosis also acts as an important substrate for the initiation and maintenance of VF. In Langendorff-perfused human hearts, areas of increased fibrosis appear to serve as the anchor for re-entrant excitation.18 Differentiation of fibroblasts into myofibroblasts in pathological states such as ischaemic cardiomyopathy may result in myocyte–fibroblast electrical coupling via gap junctions, leading to an increased tendency to the development of malignant arrhythmias.19,20
Neural abnormalities
Neural remodelling, characterized by heterogeneous cardiac nerve sprouting and sympathetic hyperinnervation in the infarcted myocardium,21,22 may predispose to spontaneous VF. The importance of neural remodelling in ventricular arrhythmia is supported by the efficacy of β-blocker therapy in reducing sudden death after myocardial infarction.23,24 β-Blockers also seem to be effective in reducing sudden death in other high-risk patients, such as those on haemodialysis25 and patients with long QT syndrome (LQTS) types 1 and 2.26 In addition, a recent study in which rats were subjected to cardiac-specific bilateral stellate ganglionectomy showed that the procedure shortened action potential duration (APD), flattened the APD restitution curve, and altered the dynamics of VF.27 Thus, clinical and experimental data exist linking neural abnormalities with a predisposition to VF, although the contribution of these abnormalities relative to the other abnormalities (structural and EP) is not fully understood and is likely to vary between different patients, depending on their co-existent cardiovascular morbidities and underlying cardiac condition.
Mechanisms of ventricular fibrillation
Key theories which may be relevant with regard to the use of CA as a therapeutic strategy for VF include the focal source hypothesis which postulates that a single, rapidly firing focus is the main driver of VF and the multiple wavelet hypotheses, in which anatomic differences and differences in tissue refractoriness result in the formation of new wavelets or rotors.28,29 Studies with animal models lend support to both hypotheses, demonstrating the presence of rapidly firing foci (initiated by early or delayed after-depolarization), micro-re-entry or abnormal automaticity.30–32 Data from humans with VF are also consistent with a number of mechanisms, in part related to the heterogeneous mix of cardiac diseases with distinct aetiologies in which VF may occur. For example, a recent study using contrast-enhanced magnetic resonance imaging in patients with previous myocardial infarction and left ventricular dysfunction suggested that infarct tissue heterogeneity around the scar border region may be the underlying substrate responsible for subsequent VF episodes.33 This is consistent with earlier observations using non-contact mapping that pacing-induced VF in the infarcted human heart is initiated by the development of functional lines of block which result in areas of increased tissue refractoriness.34 In contrast, in patients with Brugada syndrome, the underlying substrate may be related to abnormal areas of delayed depolarization (rather than transmural repolarization) in the right ventricular outflow tract (RVOT) region which results in conduction slowing.35,36 These abnormal areas may give rise to pathological premature ventricular complexes (PVCs) which may trigger VF episodes.37 Much of the early experience involving EP studies and CA of VF in humans involved patients with idiopathic VF, in which triggers of the arrhythmia were also found to be related to PVCs, although their origin appeared to be from the Purkinje system.38,39 These pioneering reports provided the basis and theory behind the current clinical use of CA to treat recurrent VF.
Ventricular fibrillation ablation in non-structural heart disease
Idiopathic ventricular fibrillation
Idiopathic VF, occurring in the absence of structural heart disease or surface electrocardiographic abnormalities, accounts for 5–10% of survivors of out-of-hospital cardiac arrest.5 In many cases, the initiating events appear to be related to potentials arising from His–Purkinje fibres.38–40 Insertion of an ICD is usually recommended in patients with idiopathic VF who have survived an out-of-hospital cardiac arrest as the cause is unknown and it is possible the life-threatening ventricular arrhythmia may occur again. In rare situations in which patients experience frequent ICD shocks or present with electrical storm and the triggering PVCs can be mapped, CA may be necessary. The largest report to date on out-of-hospital cardiac arrests in patients with idiopathic VF involved a series of 206 patients across 22 centres worldwide.40 The investigators found that early repolarization, which was previously considered a benign electrocardiographic feature, was significantly more frequent in patients who had been resuscitated from VF compared with a control group (31% vs. 5%). Of the patients with idiopathic VF, eight underwent EP study and mapping of the triggering PVCs. The origin of ectopy that initiated the VF episodes was mapped to sites concordant with the localization of the repolarization abnormalities. In total, 26 different PVCs were mapped either to the ventricular myocardium or Purkinje tissue. Catheter ablation was successful in eliminating ventricular ectopy in five of the eight patients. Most published series on VF ablation have reported immediate success rates ranging from 81 to 100%.38,39,41 During short-term follow-up of 24–32 months, VF recurrence rates vary from 0 to 11%.38,39 In the study by Knecht et al.,41 the longest series reported to date (median follow-up period of 63 months), 18% experienced recurrence of VF at a median of 24 months. Catheter ablation of triggering PVCs therefore does not currently appear to be a cure for VF or a substitute for an ICD, although it has a useful role to play in controlling electrical storm.
Long QT syndrome
Ventricular fibrillation may occur in patients with acquired or inherited LQTS. Prolongation of ventricular repolarization increases the vulnerable period for ventricular arrhythmias and allows for premature depolarization to occur. β-Blockers have been shown to be effective in reducing the risk of life-threatening events in patients with inherited LQTS especially LQT1, although patients may still experience ventricular arrhythmias and SCD especially if they have a history of syncope.26 In cases of acquired LQTS, withdrawal of the offending QT-prolonging drug may be effective and all that is required, although such individuals may also have a genetic predisposition to QT prolongation which increases their risk of future events.42 In addition, VF may be related to other triggers in patients with acquired LQTS, such as stress cardiomyopathy.43
Catheter ablation of culprit PVCs that trigger VF episodes may sometimes be necessary in patients with LQTS and recurrent VF that do not respond to conventional treatment. To date, there have been only two reports of CA being used in LQTS patients—both have involved cases of congenital LQTS.44,45 Haissaguerre et al.44 described VF ablation in four patients with LQTS—the triggering PVC was monomorphic in two patients and polymorphic in the other two. The initiating PVC arose from the distal Purkinje fibres in three of the patients and from the RVOT in the other. The patients were followed up for a mean period of 24 months with no recurrences of VF (an ICD was inserted in two of the patients).
Figure 1 shows the 12-lead electrocardiogram (ECG) and ICD rhythm strip of a 16-year-old boy with congenital LQTS who underwent CA at our centre for recurrent VF episodes triggered by a unifocal PVC, despite being on maximally tolerated doses of a β-blocker. Electrophysiological study and CA of the triggering PVCs, involving activation and pace maps, demonstrated an unusual site of origin, at the left ventricular outflow tract region just below the aortic valve area (Figure 2). Following successful CA of the triggering PVCs, the patient made a good recovery and has had no further episodes of VF or electrical storm during an 18-month follow-up period. Catheter ablation therefore may have a role to play in rare cases of congenital LQTS where the triggering PVCs responsible for the VF episodes can be mapped and targeted during ablation. Experience, however, is limited and only a few cases have so far been reported.
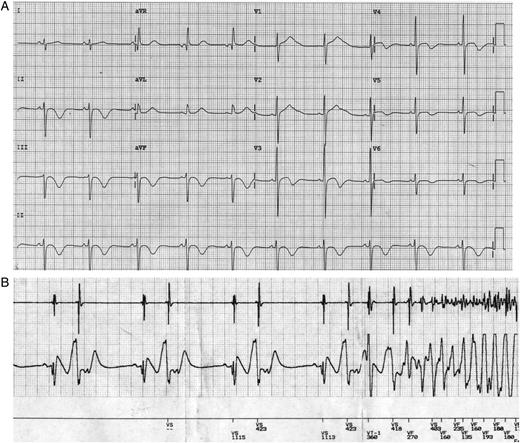
Twelve-lead electrocardiograph of a patient with long QT syndrome (A) and cardioverter defibrillator rhythm strip showing a run of ventricular bigeminy triggering ventricular fibrillation (B). The multiple ventricular fibrillation episodes required cardioverter defibrillator shocks to restore sinus rhythm, despite use of a regular β-blocker. Corrected QT interval on this electrocardiograph measured 580 ms.
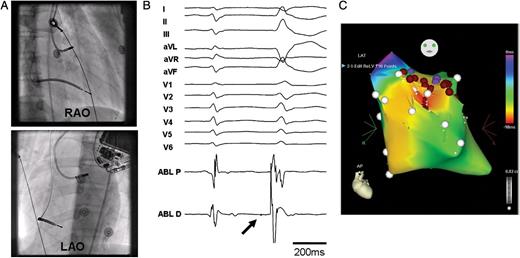
Catheter ablation of the PVCs triggering recurrent VF episodes in a patient with long QT syndrome (same patient as in Figure 1). (A) Fluoroscopic images (right and left anterior oblique views) of the ablation catheter at the site of origin of the premature ventricular complexes. (B) Twelve-lead surface electrocardiograph and ablation signal at earliest site (30 ms ahead of PVC onset). Note inferior axis of PVC and small Purkinje potential (PP- thick arrow) visible just before premature ventricular complex onset. (C) CARTO map of left ventricle (anterior view) showing multiple ablation points (brown circles) below the aortic valve area at the earliest site of triggering premature ventricular complexes. Coronary angiography (not shown) was performed to confirm that the ablation site was at a safe distance away from the epicardial coronary arteries. RAO, right anterior oblique view; LAO, left anterior oblique view.
Brugada syndrome
Brugada syndrome may present with recurrent VF initiated by PVCs that most frequently arise from the RVOT region. A number of investigators have reported isolated cases or small series on successful ablation of PVCs at RVOT sites in patients with this condition.44,46,47 The largest prospective study of VF ablation in Brugada syndrome patients was recently reported by Nademanee et al.36 in nine patients (all male; median age 38 years) with recurrent VF that required multiple ICD shocks. The investigators performed endocardial and epicardial electroanatomic mapping of the right and left ventricle in conjunction with CT image integration found that all subjects exhibited abnormal low-voltage areas with prolonged duration and fractionated late potentials around the anterior aspect of the RVOT epicardium. Catheter ablation targeted at the abnormal arrhythmogenic substrate in this location successfully abolished further ventricular tachycardia (VT)/VF episodes in all but one patient during a follow-up period of 20 ± 6 months. Interestingly, CA also resulted in normalization of the Brugada ECG pattern (all had type 1 pattern pre-ablation). This study provides important evidence that the increasingly recognized subtle structural abnormalities observed in the RVOT region of patients with Brugada syndrome48 may be a potential target to treat recurrent VF in this condition and opens up the possibility of ‘substrate modification’ to treat recurrent VF, even if pathological PVCs are not present at the time of the EP study.
Ventricular fibrillation ablation in structural heart disease
In patients with structural heart disease, the usual presentation of electrical storm which necessitates CA is VT which degenerates into VF.49,50 CA of electrical storm in patients with structural heart disease of various aetiologies (e.g. coronary artery disease, idiopathic dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy) had been reported to be effective in acutely suppressing electrical storm.49,50
Ischaemic heart disease
Ventricular fibrillation can potentially occur at an early stage soon after an AMI or at a later stage post-myocardial infarction after scar formation. During an AMI, ischaemia of the His–Purkinje system may increase susceptibility to VF. In the majority of cases, frequent episodes of VF can be controlled with medication including β-blockers and/or amiodarone, and adequate coronary revascularization. Ablation is rarely required for cases that are refractory to medical therapy or successful revascularization. Bansch et al.51 reported their experience of CA in four patients with incessant VF and VT triggered by monomorphic PVCs after an AMI which could not be controlled despite successful reperfusion, intravenous amiodarone, and use of β-blockers. They demonstrated that CA of the triggering PVCs successfully controlled the ventricular arrhythmic storms, with none of the patients experiencing further VF after a follow-up period of between 5 and 33 months. The authors added that, although successful, CA is rarely required in patients following AMI, and in their experience, was only required in their four reported patients out of a total of 2340 post-AMI patients (i.e. 0.17% of cases). Enjoji et al.52 similarly reported their experience of four patients with acute coronary syndrome and low ejection fraction who suffered from multiple VF/VT episodes triggered by unifocal PVCs, despite successful revascularization. The PVCs were located in the left ventricular posteroinferior region and all related to the Purkinje fibre network. No further episodes of VT/VF were noted post-CA.
In patients with previous myocardial infarction with scar formation, VF occurrence is often due to presence of re-entrant circuits surrounding the scar. Ablation targets include the initiating PVCs that arise from the His–Purkinje system or the border of the scar. Marrouche et al.53 investigated the mode of initiation of VF storm in patients with ischaemic cardiomyopathy who had suffered their myocardial infarction more than 6 months prior to the EP study. Using a three-dimensional (3D) mapping system to perform activation maps of the PVCs or pace-mapping (in cases where the PVC frequency was too low to allow for activation mapping), they demonstrated that in most cases (five of eight who underwent CA), the culprit PVCs originated from the scar border zone and were often preceded by Purkinje potentials (PPs). Their ablation strategy included additional lesions along the length of the border zone in order to eliminate all detected potentials. This appeared to be successful and over a 10 ± 6-month follow-up period, VF only recurred in one patient.
Non-ischaemic dilated cardiomyopathy
Kirubakaran et al.54 reported a case of successful CA of focal VF in a patient with non-ischemic dilated cardiomyopathy. Ventricular fibrillation ablation was also described in another series of patients with dilated cardiomyopathy who experienced recurrent VF despite optimal heart failure medication and the use of anti-arrhythmic medication.55 The investigators performed EP study in five patients using 3D electroanatomical mapping and recorded PPs in sinus rhythm around the scar border (left posterior wall near the mitral annulus) in four patients. These four patients underwent successful CA targeted at the PPs and none had any recurrence of VF during a 12 ± 5-month follow-up period.
Other conditions associated with ventricular fibrillation storm requiring CA
Cardiac amyloidosis may be an under-recognized cause of VF due to it being a relatively rare diagnosis. To date, there has only been one report of two patients with cardiac amyloidosis and VF storm who were successfully treated with CA targeting the initiating monomorphic PVCs.56 Ventricular fibrillation storm can also rarely occur after cardiac surgery, most commonly coronary artery bypass grafting, and may necessitate CA for full control. In general, the most common location of the initiating PVC in the post-surgical patient is at His–Purkinje fibres.57,58
Technique of catheter ablation
From the preceding sections, it can be appreciated that the main clinical use of CA at present in the treatment of VF is in the targeting and ablation of the critically timed PVCs that are responsible for triggering the VF episodes in patients with both structural heart disease (e.g. ischaemic heart disease) and structurally normal hearts (inherited arrhythmogenic diseases, idiopathic VF). Most cases of these triggering PVCs appear to originate from the His–Purkinje system. Electrophysiological mapping and CA of these culprit PVCs can be very effective at reducing the VF episodes and may be life-saving in cases of electrical storm. Mapping can be performed using conventional EP techniques involving the use of between two and four intracardiac catheters to identify the earliest site of activation of the culprit PVCs and/or small, sharp PPs which may precede PVC onset by tens of milliseconds.39 In addition, use of a 3D electroanatomical mapping system may be helpful in providing information on low-voltage areas and scarred regions, especially in patients with structural heart disease, which may be the sites of the triggering PVCs. Determining the earliest potential is the key to successful ablation. Activation mapping can be performed if there are multiple clinical PVCs present at the time of the EP study, although pace-mapping may be required if the PVCs are either not present at the time of the EP study or of insufficient frequency to allow accurate mapping. In either case, it is essential that the electrophysiologist has a clear 12-lead ECG of the triggering PVC in order to maximize chances of success.
Owing to the unpredictable nature of PVCs, the optimal time for ablation is often at the time of an electrical storm when the PVCs tend to be most frequent and easily captured during the EP study. Difficulties with conventional mapping and ablation may arise in cases of polymorphic PVCs or recurrent episodes of VF during the procedure, requiring frequent external or ICD shocks. In these situations, identification of the substrate during sinus rhythm may be a more important strategy. This may include identification and ablation of PPs that precede the culprit PVCs responsible for triggering the VF episodes in cases of idiopathic VF41 or mapping out and ablation of scar border zones in patients with structural heart disease.59 As the His–Purkinje system is located within the endocardium, the fibres tend to be relatively responsive to radiofrequency energy without the need for prolonged ablation times or high powers.
Limitations of catheter ablation and future directions
CA of triggering PVCs remains a challenging task for eletrophysiologists due to the difficulties of mapping infrequent triggers in the setting of an electrically unstable myocardium. The technique as a treatment for recurrent VF and electrical storm is still at an early stage and methods and strategies are likely to evolve as our understanding of the mechanisms and triggers for VF improve. To date, there have been only 13 reports involving CA of VF in series consisting of four or more patients—these are summarized in Table 1. It can be seen that most of the ablation strategies involve targeting culprit PVCs, which may or may not be related to the Purkinje system or preceded by a PP. Exceptions to the strategy of targeting triggering PVCs include cases of Brugada syndrome (in which the occurrence of PVCs may be infrequent) and VF in structural heart disease (ischaemic and non-ischaemic cardiomyopathy and in the immediate post-infarct period). In these cases, the ablation strategy may involve targeting abnormal areas of delayed depolarization in the RVOT region (in cases of Brugada syndrome) or scar borderzone areas. It should be noted that although CA of VF appears to have good initial success rates in these published series, some patients nevertheless experience VF recurrence during follow-up. Catheter ablation for VF therefore cannot at present be used as an alternative to ICD insertion. In addition, the reports summarized in Table 1 are all from highly specialized centres with considerable expertise in invasive cardiac EP and in particular ventricular arrhythmia ablation.
Summary of case series to date reporting on catheter ablation of ventricular fibrillation and outcomesa
| Authors . | Year of publication . | Number of patients . | Aetiologies of VF . | Ablation site . | Follow-up duration . | Outcome . |
|---|---|---|---|---|---|---|
| Non-structural heart disease | ||||||
| Nademanee et al.36 | 2011 | 9 | Brugada syndrome | Areas of delayed depolarization over RVOT (anterior aspect) | 20 ± 6 months | No recurrent VF/VT in all patients off medication (except for one patient on amiodarone) |
| Knecht et al.41 | 2009 | 38 | Idiopathic VF | Targeted PVCs originating from Purkinje system | 63 months (median) | Seven patients (18%) experienced VF recurrence at median of 4 months. Five of these seven patients underwent repeat ablation without VF recurrence |
| Haissaguerre et al.40 | 2008 | 8 | Idiopathic VF (with early repolarization) | Purkinje tissue, ventricular myocardium or multiple sites | Specific follow-up of these patients not given | All PVCs eliminated in five patients; unsuccessful in three patients |
| Haissaguerre et al.44 | 2003 | 7 (three with Brugada syndrome; four with LQTS) | Brugada syndrome and long QT syndrome | Targeted PVCs originating from Purkinje system (1 Brugada syndrome and three LQTS) or RVOT | 17 ± 17 months | No patient had recurrence of symptomatic ventricular arrhythmia but one had persistent PVC |
| Haissaguerre et al.39 | 2002 | 27 | Idiopathic VF | Targeted Purkinje-like potentials originating from distal Purkinje system | 24 ± 28 months | Twenty-four patients (89%) had no recurrence of VF off medication |
| Haissaguerre et al.38 | 2002 | 16 | Idiopathic VF | Earliest site of PVC activation at Purkinje system | 32 months | Successful in 13 patients—no VF recurrence or syncope |
| Structural heart disease | ||||||
| Kozeluhova et al.50 | 2011 | 50 | Coronary artery disease,38 idiopathic dilated cardiomyopathy,5 arrhythmogenic right ventricular cardiomyopathy,6 and/or with combined aetiology1 | Predominantly His–Purkinje network | 18 ± 16 months | Twenty-four patients(48%) had no recurrences |
| Peichl et al.59 | 2010 | 9 | Previous AMI with depressed LVEF | Scar border zone of interventricular septum, lateral wall or inferior wall. Linear lesions created within infarct border zone | 13 ± 7 months | Two patients died due to progressive heart failure; one patient had recurrence of VT/VF storm from a different site |
| Sinha et al.55 | 2009 | 4 | Nonischemic cardiomyopathy | Purkinje-like potentials around left ventricular posterior wall scar near the mitral annulus | 12 ± 5 months | No VF recurrence in the 4 patients that underwent ablation |
| Enjoji et al.52 | 2009 | 4 | Post-AMI | Left ventricular posteroinferior region | 1–4 years | No further ventricular arrhythmias (ICD inserted in two patients) |
| Bode et al.49 | 2008 | 7 | Structural heart disease (four IHD, two chronic myocarditis, one post-AVR) | Triggering PVCs from Purkinje system at midseptal, mid-inferoseptal and RV-free wall sites | 1–27 months (median, 10 months) | No recurrence of polymorphic VT/VF in five patients; two patients with IHD died due to refractory heart failure |
| Marrouche et al.53 | 2004 | 5 | Ischaemic cardiomyopathy | Targeted monomorphic PVCs originating from the scar border zone with or without preceding Purkinje-like potentials | 10 ± 6 months | One patient had a single VF episode and another developed sustained monomorphic VT |
| Bansch et al.51 | 2003 | 4 | Post-AMI | Border of infarct site in left ventricle | 5, 6, 14, and 33 months for each patient | No further ventricular arrhythmias |
| Authors . | Year of publication . | Number of patients . | Aetiologies of VF . | Ablation site . | Follow-up duration . | Outcome . |
|---|---|---|---|---|---|---|
| Non-structural heart disease | ||||||
| Nademanee et al.36 | 2011 | 9 | Brugada syndrome | Areas of delayed depolarization over RVOT (anterior aspect) | 20 ± 6 months | No recurrent VF/VT in all patients off medication (except for one patient on amiodarone) |
| Knecht et al.41 | 2009 | 38 | Idiopathic VF | Targeted PVCs originating from Purkinje system | 63 months (median) | Seven patients (18%) experienced VF recurrence at median of 4 months. Five of these seven patients underwent repeat ablation without VF recurrence |
| Haissaguerre et al.40 | 2008 | 8 | Idiopathic VF (with early repolarization) | Purkinje tissue, ventricular myocardium or multiple sites | Specific follow-up of these patients not given | All PVCs eliminated in five patients; unsuccessful in three patients |
| Haissaguerre et al.44 | 2003 | 7 (three with Brugada syndrome; four with LQTS) | Brugada syndrome and long QT syndrome | Targeted PVCs originating from Purkinje system (1 Brugada syndrome and three LQTS) or RVOT | 17 ± 17 months | No patient had recurrence of symptomatic ventricular arrhythmia but one had persistent PVC |
| Haissaguerre et al.39 | 2002 | 27 | Idiopathic VF | Targeted Purkinje-like potentials originating from distal Purkinje system | 24 ± 28 months | Twenty-four patients (89%) had no recurrence of VF off medication |
| Haissaguerre et al.38 | 2002 | 16 | Idiopathic VF | Earliest site of PVC activation at Purkinje system | 32 months | Successful in 13 patients—no VF recurrence or syncope |
| Structural heart disease | ||||||
| Kozeluhova et al.50 | 2011 | 50 | Coronary artery disease,38 idiopathic dilated cardiomyopathy,5 arrhythmogenic right ventricular cardiomyopathy,6 and/or with combined aetiology1 | Predominantly His–Purkinje network | 18 ± 16 months | Twenty-four patients(48%) had no recurrences |
| Peichl et al.59 | 2010 | 9 | Previous AMI with depressed LVEF | Scar border zone of interventricular septum, lateral wall or inferior wall. Linear lesions created within infarct border zone | 13 ± 7 months | Two patients died due to progressive heart failure; one patient had recurrence of VT/VF storm from a different site |
| Sinha et al.55 | 2009 | 4 | Nonischemic cardiomyopathy | Purkinje-like potentials around left ventricular posterior wall scar near the mitral annulus | 12 ± 5 months | No VF recurrence in the 4 patients that underwent ablation |
| Enjoji et al.52 | 2009 | 4 | Post-AMI | Left ventricular posteroinferior region | 1–4 years | No further ventricular arrhythmias (ICD inserted in two patients) |
| Bode et al.49 | 2008 | 7 | Structural heart disease (four IHD, two chronic myocarditis, one post-AVR) | Triggering PVCs from Purkinje system at midseptal, mid-inferoseptal and RV-free wall sites | 1–27 months (median, 10 months) | No recurrence of polymorphic VT/VF in five patients; two patients with IHD died due to refractory heart failure |
| Marrouche et al.53 | 2004 | 5 | Ischaemic cardiomyopathy | Targeted monomorphic PVCs originating from the scar border zone with or without preceding Purkinje-like potentials | 10 ± 6 months | One patient had a single VF episode and another developed sustained monomorphic VT |
| Bansch et al.51 | 2003 | 4 | Post-AMI | Border of infarct site in left ventricle | 5, 6, 14, and 33 months for each patient | No further ventricular arrhythmias |
aOnly reports with four or more than four patients are included.
AVR, aortic valve replacement; PVC, premature ventricular complex; RVOT, right ventricular outflow tract; AMI, acute myocardial infarction; LQTS, long QT syndrome; IHD, ischaemic heart disease; VF, ventricular fibrillation; VT, ventricular tachycardia; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter defibrillator.
Summary of case series to date reporting on catheter ablation of ventricular fibrillation and outcomesa
| Authors . | Year of publication . | Number of patients . | Aetiologies of VF . | Ablation site . | Follow-up duration . | Outcome . |
|---|---|---|---|---|---|---|
| Non-structural heart disease | ||||||
| Nademanee et al.36 | 2011 | 9 | Brugada syndrome | Areas of delayed depolarization over RVOT (anterior aspect) | 20 ± 6 months | No recurrent VF/VT in all patients off medication (except for one patient on amiodarone) |
| Knecht et al.41 | 2009 | 38 | Idiopathic VF | Targeted PVCs originating from Purkinje system | 63 months (median) | Seven patients (18%) experienced VF recurrence at median of 4 months. Five of these seven patients underwent repeat ablation without VF recurrence |
| Haissaguerre et al.40 | 2008 | 8 | Idiopathic VF (with early repolarization) | Purkinje tissue, ventricular myocardium or multiple sites | Specific follow-up of these patients not given | All PVCs eliminated in five patients; unsuccessful in three patients |
| Haissaguerre et al.44 | 2003 | 7 (three with Brugada syndrome; four with LQTS) | Brugada syndrome and long QT syndrome | Targeted PVCs originating from Purkinje system (1 Brugada syndrome and three LQTS) or RVOT | 17 ± 17 months | No patient had recurrence of symptomatic ventricular arrhythmia but one had persistent PVC |
| Haissaguerre et al.39 | 2002 | 27 | Idiopathic VF | Targeted Purkinje-like potentials originating from distal Purkinje system | 24 ± 28 months | Twenty-four patients (89%) had no recurrence of VF off medication |
| Haissaguerre et al.38 | 2002 | 16 | Idiopathic VF | Earliest site of PVC activation at Purkinje system | 32 months | Successful in 13 patients—no VF recurrence or syncope |
| Structural heart disease | ||||||
| Kozeluhova et al.50 | 2011 | 50 | Coronary artery disease,38 idiopathic dilated cardiomyopathy,5 arrhythmogenic right ventricular cardiomyopathy,6 and/or with combined aetiology1 | Predominantly His–Purkinje network | 18 ± 16 months | Twenty-four patients(48%) had no recurrences |
| Peichl et al.59 | 2010 | 9 | Previous AMI with depressed LVEF | Scar border zone of interventricular septum, lateral wall or inferior wall. Linear lesions created within infarct border zone | 13 ± 7 months | Two patients died due to progressive heart failure; one patient had recurrence of VT/VF storm from a different site |
| Sinha et al.55 | 2009 | 4 | Nonischemic cardiomyopathy | Purkinje-like potentials around left ventricular posterior wall scar near the mitral annulus | 12 ± 5 months | No VF recurrence in the 4 patients that underwent ablation |
| Enjoji et al.52 | 2009 | 4 | Post-AMI | Left ventricular posteroinferior region | 1–4 years | No further ventricular arrhythmias (ICD inserted in two patients) |
| Bode et al.49 | 2008 | 7 | Structural heart disease (four IHD, two chronic myocarditis, one post-AVR) | Triggering PVCs from Purkinje system at midseptal, mid-inferoseptal and RV-free wall sites | 1–27 months (median, 10 months) | No recurrence of polymorphic VT/VF in five patients; two patients with IHD died due to refractory heart failure |
| Marrouche et al.53 | 2004 | 5 | Ischaemic cardiomyopathy | Targeted monomorphic PVCs originating from the scar border zone with or without preceding Purkinje-like potentials | 10 ± 6 months | One patient had a single VF episode and another developed sustained monomorphic VT |
| Bansch et al.51 | 2003 | 4 | Post-AMI | Border of infarct site in left ventricle | 5, 6, 14, and 33 months for each patient | No further ventricular arrhythmias |
| Authors . | Year of publication . | Number of patients . | Aetiologies of VF . | Ablation site . | Follow-up duration . | Outcome . |
|---|---|---|---|---|---|---|
| Non-structural heart disease | ||||||
| Nademanee et al.36 | 2011 | 9 | Brugada syndrome | Areas of delayed depolarization over RVOT (anterior aspect) | 20 ± 6 months | No recurrent VF/VT in all patients off medication (except for one patient on amiodarone) |
| Knecht et al.41 | 2009 | 38 | Idiopathic VF | Targeted PVCs originating from Purkinje system | 63 months (median) | Seven patients (18%) experienced VF recurrence at median of 4 months. Five of these seven patients underwent repeat ablation without VF recurrence |
| Haissaguerre et al.40 | 2008 | 8 | Idiopathic VF (with early repolarization) | Purkinje tissue, ventricular myocardium or multiple sites | Specific follow-up of these patients not given | All PVCs eliminated in five patients; unsuccessful in three patients |
| Haissaguerre et al.44 | 2003 | 7 (three with Brugada syndrome; four with LQTS) | Brugada syndrome and long QT syndrome | Targeted PVCs originating from Purkinje system (1 Brugada syndrome and three LQTS) or RVOT | 17 ± 17 months | No patient had recurrence of symptomatic ventricular arrhythmia but one had persistent PVC |
| Haissaguerre et al.39 | 2002 | 27 | Idiopathic VF | Targeted Purkinje-like potentials originating from distal Purkinje system | 24 ± 28 months | Twenty-four patients (89%) had no recurrence of VF off medication |
| Haissaguerre et al.38 | 2002 | 16 | Idiopathic VF | Earliest site of PVC activation at Purkinje system | 32 months | Successful in 13 patients—no VF recurrence or syncope |
| Structural heart disease | ||||||
| Kozeluhova et al.50 | 2011 | 50 | Coronary artery disease,38 idiopathic dilated cardiomyopathy,5 arrhythmogenic right ventricular cardiomyopathy,6 and/or with combined aetiology1 | Predominantly His–Purkinje network | 18 ± 16 months | Twenty-four patients(48%) had no recurrences |
| Peichl et al.59 | 2010 | 9 | Previous AMI with depressed LVEF | Scar border zone of interventricular septum, lateral wall or inferior wall. Linear lesions created within infarct border zone | 13 ± 7 months | Two patients died due to progressive heart failure; one patient had recurrence of VT/VF storm from a different site |
| Sinha et al.55 | 2009 | 4 | Nonischemic cardiomyopathy | Purkinje-like potentials around left ventricular posterior wall scar near the mitral annulus | 12 ± 5 months | No VF recurrence in the 4 patients that underwent ablation |
| Enjoji et al.52 | 2009 | 4 | Post-AMI | Left ventricular posteroinferior region | 1–4 years | No further ventricular arrhythmias (ICD inserted in two patients) |
| Bode et al.49 | 2008 | 7 | Structural heart disease (four IHD, two chronic myocarditis, one post-AVR) | Triggering PVCs from Purkinje system at midseptal, mid-inferoseptal and RV-free wall sites | 1–27 months (median, 10 months) | No recurrence of polymorphic VT/VF in five patients; two patients with IHD died due to refractory heart failure |
| Marrouche et al.53 | 2004 | 5 | Ischaemic cardiomyopathy | Targeted monomorphic PVCs originating from the scar border zone with or without preceding Purkinje-like potentials | 10 ± 6 months | One patient had a single VF episode and another developed sustained monomorphic VT |
| Bansch et al.51 | 2003 | 4 | Post-AMI | Border of infarct site in left ventricle | 5, 6, 14, and 33 months for each patient | No further ventricular arrhythmias |
aOnly reports with four or more than four patients are included.
AVR, aortic valve replacement; PVC, premature ventricular complex; RVOT, right ventricular outflow tract; AMI, acute myocardial infarction; LQTS, long QT syndrome; IHD, ischaemic heart disease; VF, ventricular fibrillation; VT, ventricular tachycardia; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter defibrillator.
Despite improvements in our understanding of the mechanisms responsible for VF and major advancements in the technology used for CA, several limitations remain. These are partly related to gaps in our current knowledge of the underlying basic pathophysiology of VF. As previously discussed, a number of theories still exist regarding the exact mechanism(s) responsible for initiation and maintenance of VF with none proven conclusively to be the most important or predominant. In our view, VF is probably the ‘final common pathway’ of a number of distinct cardiological and metabolic disease processes as reflected by the wide variety of conditions that predispose to VF. It is therefore probably too simplistic to expect a single, relatively straightforward technique, such as CA, to be the sole answer in the management of this complex arrhythmia. A number of key questions remain unanswered. Should all patients with symptomatic PVCs or PVCs that trigger VF episodes or non-sustained VT be subjected to radiofrequency ablation? Which patients with PVCs are at greatest risk of VF episodes or VF recurrence and what is the best and most complete ablation strategy? Not all patients with asymptomatic PVCs are prone to develop VF, possibly due to the absence of the electrical substrate responsible for initiating and maintaining VF.17–22 In contrast, a small proportion of patients (<20%) with VF of various aetiologies may remain stable for months or even years following CA of triggering PVCs and then suddenly experience recurrent VF episodes.39,41,53,59 These observations suggest that modulation of the site of initiation of PVCs and surrounding tissue may not be sufficient to avoid VF recurrence. Future directions to improve our understanding of the basic mechanisms underlying VF and thereby allow for better and more effective therapies include using computer-simulation models of arrhythmias60 and in vitro studies of isolated ventricular myocytes,61 ventricular myocyte monolayers62 and whole hearts.63 Another interesting angle is to assess the combination of novel drugs for ventricular arrhythmias, such as the late sodium channel blocker ranolazine,64 with CA strategies, such as modulation of the ventricular substrate, on suppressing or reducing the incidence of VF. Ideally such trials should initially be targeted towards a subset of patients with recurrent VF (e.g. those with or without structural heart disease) and involve a standardized CA protocol, endpoint, and follow-up.
Conclusions
Catheter ablation has been used in a limited number of centres worldwide to target clearly identifiable EP triggers of VF and electrical storm, predominantly in the form of unifocal PVCs, with relatively good short-term success rates. Unlike cases of structural heart disease in which there is a clear substrate for PVCs and ventricular arrhythmias, the role of CA in patients with idiopathic VF or cardiac ion channelopathies, in which the underlying abnormality is likely to be a genetic condition predisposing to widespread electrical instability, remains to be defined. Nonetheless, some case series and reports lend support for a role of CA in these conditions in highly selected cases in which triggering PVC can be identified. In view of the invasive nature of CA for VF, potential complications and expertise required, patients presenting with VF storm should still be managed along conventional lines in the first instance. These measures, which include anti-arrhythmic medication, over-drive ventricular pacing and sympathetic blockade, may be effective in the majority of cases. However, as highlighted in this review, there remains an important and potentially life-saving role for CA in patients with recurrent VF and electrical storm, many of whom have tried and failed conventional treatments.
Conflict of interest: none declared.



