-
PDF
- Split View
-
Views
-
Cite
Cite
Luigi Di Biase, Angelo Auricchio, Prasant Mohanty, Rong Bai, Josef Kautzner, Paolo Pieragnoli, Francois Regoli, Antonio Sorgente, Giulio Spinucci, Giuseppe Ricciardi, Antonio Michelucci, Laura Perrotta, Francesco Faletra, Hancha Mlcochová, Kamil Sedlacek, Robert Canby, Javier E. Sanchez, Rodney Horton, J. David Burkhardt, Tiziano Moccetti, Luigi Padeletti, Andrea Natale, Impact of cardiac resynchronization therapy on the severity of mitral regurgitation, EP Europace, Volume 13, Issue 6, June 2011, Pages 829–838, https://doi.org/10.1093/europace/eur047
Close - Share Icon Share
Abstract
Functional mitral regurgitation (MR) could be managed by both cardiac resynchronization therapy (CRT) and mitral-valve surgery. Clinical decision making regarding the appropriateness of mitral-valve surgery vs. CRT is a challenging task. This study assessed the prevalence and prognosis of various degrees of functional MR in CRT candidates. Additionally, we sought to identify functional MR patients who either can be adequately managed by CRT only or will need surgery.
Cardiac resynchronization therapy recipients (n= 794) were followed-up for 26 ± 18 months. Mitral regurgitation severity was quantified on scale 0–4. Cardiac resynchronization therapy responders were identified based on improvement in the New York Heart Association class and left-ventricular ejection fraction. Severity of MR and LV reverse remodelling were assessed at 3 and 12 months. Predictors of long-term MR change and CRT response were explored with multivariable models. Mitral regurgitation was present in 86%, with 35% prevalence of advanced MR (grade 3–4). Improvement of MR ≥1° after 12 months occurred in 46% of patients. It was relatively more frequent in patients with advanced MR at baseline (63%, P< 0.01). Baseline MR severity and change in MR at 3-month follow-up predicted response to CRT. Patients with ≥1° MR improvement at 12 months had more reverse remodelling compared with those with no change or worsening of MR.
Mitral regurgitation improvement at 3 months predicts CRT response and MR improvement at 12-month follow-up. This finding could have implications for subsequent MR surgical therapies.
Introduction
Functional mitral regurgitation (MR) is a common finding in patients with heart failure (HF).1,2 It results from an imbalance between closing and tethering forces that ensure valve competency. Functional MR may be a consequence of systolic dysfunction, changed geometry, and size of the left ventricle or it may occur in the presence of dyssynchrony.3 Vice versa, MR per se might be responsible for the HF progression. Moreover, it is known that moderate-to-severe MR can aggravate symptoms and it is a marker of adverse outcome.1,2,4,5
Cardiac resynchronization therapy (CRT), on the contrary, has the potential to reverse the vicious cycle resulting in MR worsening. Cardiac resynchronization therapy leads to reverse remodelling and reduces morbidity and mortality, in addition to improvement of symptoms and exercise capacity.6–8 Moreover, CRT-induced resynchronization is able to improve MR due to an increased closing force and restoration of a more coordinated activation of the components of the mitral-valve apparatus.7,9,10
Smaller studies described the mechanism by which CRT improves MR.9–11 However, there are still rather limited data on the prevalence of functional MR of various degrees in CRT candidates, frequency of CRT-induced MR reduction in the long term, and its relationship to LV reverse remodelling and mortality. This study aimed at identifying patients with functional MR who can be adequately managed with CRT before any surgical procedure is planned. 12–14
Methods
Study population
The study population included consecutive patients who received biventricular pacemaker/defibrillator in the four participating institutions (Texas Cardiac Arrhythmia Institute at St Davids Medical Center, Austin, TX, USA; Institute for Clinical and Experimental Medicine, Prague, CZ; Cardiocentro Ticino Lugano, Switzerland; and University of Florence, Florence, Italy). All patients had evidence of HF for at least 6 months before CRT implantation, they remained symptomatic in functional New York Heart Association (NYHA) class III–IV despite optimized medical therapy, had left-ventricular systolic dysfunction [left-ventricular ejection fraction (LVEF) <35%], dilatation (left-ventricular end-diastolic diameter (LVEDD) >50 mm), and presented with intraventricular conduction delay with a QRS duration ≥120 ms. Patients were on stable medical therapy consisting of beta-blockers in 85%, angiotensin-converting enzyme inhibitors/AT blockers in 79%, diuretics in 91%, and digoxin in 44% that remained stable also during the follow-up.
Patients with a significant structural disease of the mitral valve, moderate–severe annular calcifications, rheumatic valve disease, mitral prolapse, or flail leaflet/chordae were excluded from the analysis. In addition, patients with a history of valve repair before CRT implantation, cases with missing data from the long-term follow-up, and patients with insufficient echocardiographic evaluation were excluded.
Echocardiography and data collected
Standard 2D and Doppler echocardiography was performed in partial left decubitus. A variable frequency phased-array transducer was used. Echocardiographic study included standard measurements of LVEDD and left-ventricular end-systolic diameters (LVESD) in the parasternal long-axis view, LVEF was quantified using modified biplane Simpson's rule in two- and four-chamber apical views. Mitral regurgitation was graded as follows: no or trace MR was classified as grade 0, grade 1 was used for mild, grade 2 for moderate, grade 3 for moderate–severe, and grade 4 for severe MR. Routine assessment of MR incorporated semiquantitative methods (colour flow mapping of the regurgitant jet in two orthogonal views with the Nyquist limit set at 50–60 cm/s, with pulmonary venous flow pulsed wave doppler integration) and quantitative methods (quantitative Doppler and proximal isovelocity surface area or width of the vena contracta). Area of the left atrium was further assessed in the apical four-chamber view [left atrium (LA) area]. Right-ventricular (RV) dimensions were assessed by observing RV diameter in the long-axis parasternal view and the ratio of RV and left-ventricular area in the apical four-chamber view. Right-ventricular dilatation was qualified as being present or absent based on a diastolic ventricular ratio cut-off value ≥1/<1. Right-ventricular dysfunction was evaluated using both visual assessment of endo-myocardial thickening of the RV free wall, tricuspid annular plane systolic excursion (TAPSE), and/or pulsed tissue Doppler. Patients with TAPSE 20 mm or longer were considered as not having RV dysfunction, between 20 and 15 mm as mild (Grade 1), <15–10 mm moderate (Grade 2), and those with <10 mm as severe (Grade 3) RV dysfunction.
The following variables were collected at baseline, 3 months, 12 months of post-CRT and at the last available follow-up: NYHA class, medication, LVEDD, LVESD, LVEF, severity of MR, left-atrial size, RV dilatation and dysfunction, hospitalizations for HF, mortality and history of atrial fibrillation, and QRS duration.
Responders to CRT were defined by an improvement of NYHA ≥1 class and/or absolute increase of LVEF ≥5% points.15,16 Patients who died before 12th month of follow-up from cardiovascular reason were considered as non-responders.
The study had Institutional Review Board approval.
Statistical analysis
Continuous data were expressed as mean ± standard deviation. Comparison of continuous data for two groups was performed using the Student's t-test for paired or unpaired data sets, when appropriate. Non-normally distributed continuous data were compared using the Wilcoxon (for paired data) and Mann–Whitney non-parametric test. Three groups were compared by analysis of variance (ANOVA) or Kruskal–Wallis ANOVA for skewed data. For comparison of discrete data between groups, Pearson's χ2 test was used. For paired data, Mc Nemar's test was applied. Correlation between parameters was evaluated using Spearman's correlation coefficients. Multivariate regression was performed to identify predictors of response to CRT and MR improvement/worsening. Potential confounders were entered into the model based on known or expected clinical relevance, regardless of their statistical significance. Variables included in the model were: age, aetiology of cardiomyopathy, NYHA class, QRS duration before CRT, left-atrial size, LV diameters, LVEF, and change in MR at 3 months. The Kaplan–Meier method was used to estimate survival curves and log-rank test was applied to test the equivalence of survival curves. All tests were two sided and P value <0.05 was considered significant, and performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Characteristic of the study population, distribution of mitral regurgitation
Out of the 1103 consecutive patients from the four CRT registries, a total of 794 patients fulfilled inclusion criteria into this study. Patients were followed for a mean of 26 ± 18 months (median 25 months). Distribution of patients between the institutions and characteristics and outcome of patients in each centre are summarized in Table 1. The statistically significant differences found in the patients’ characteristics between centres were mostly attributed to the cohort size. The only clinically important difference was that patients from Centres A and B tended to have more dilated ventricles and there were higher proportion of patients with no/trace MR in Centre D than in the other three Centres.
| Baseline characteristics (n= 794) . | Centre A (n= 284) . | Centre B (n= 176) . | Centre C (n= 127) . | Centre D (n= 207) . | P value . |
|---|---|---|---|---|---|
| Male | 242 (85%) | 137 (78%) | 68 (54%) | 147 (71%) | <0.001 |
| Age (year) | 64 ± 10 | 70 ± 9 | 70 ± 10 | 64 ± 11 | <0.001 |
| Prevalence of ischaemic cardiomyopathy | 156 (55%) | 104 (59%) | 61 (48%) | 113 (55%) | 0.301 |
| History of revascularization in ischaemic patients | 43 (15%) | 32 (18%) | 51 (40%) | 33 (16%) | <0.001 |
| Severity of MR at baseline | |||||
| No MR | 29 (10%) | 17 (10%) | 17 (13%) | 47 (23%) | <0.001 |
| Mild–moderate MR | 103 (36%) | 129 (73%) | 73 (58%) | 104 (50%) | <0.001 |
| Advanced MR | 152 (54%) | 30 (17%) | 37 (29%) | 56 (27%) | <0.001 |
| NYHA class | 3.01 ± .52 | 3.09 ± 0.49 | 2.87 ± 0.50 | 2.97 ± 0.47 | 0.002 |
| QRS duration (ms) | 178 ± 28 | 142 ± 31 | 154 ± 30 | 166 ± 32 | <0.001 |
| LVEDD (mm/m) | 74 ± 9 | 68 ± 9 | 66 ± 9 | 65 ± 11 | <0.002 |
| LVESD (mm/m) | 65 ± 7 | 57 ± 9 | 56 ± 9 | 55 ± 13 | 0.133 |
| LVEF (%) | 22 ± 4 | 27 ± 6 | 28 ± 8 | 22 ± 9 | <0.001 |
| Prevalence of RV dilatation | 68 (24%) | 124 (71%) | 11 (9%) | 29 (14%) | <0.001 |
| Follow-up | |||||
| Change in MR | |||||
| MR improvement ≥1° | 69 (24%) | 50 (28%) | 30 (24%) | 73 (35%) | <0.001 |
| No change in MR | 59 (21%) | 76 (43%) | 30 (24%) | 67 (32%) | <0.001 |
| MR worsening ≥1° | 28 (10%) | 17 (10%) | 4 (3%) | 43 (21%) | <0.001 |
| MR from FU not available | 128 (45%) | 33 (19%) | 63 (50%) | 24 (12%) | <0.001 |
| NYHA | 2.42 ± 0.6 | 2.39 ± 0.81 | 2.53 ± 0.60 | 2.25 ± 0.63 | 0.008 |
| LVEDD (mm/m) | 72 ± 10 | 65 ± 9 | 64 ± 11 | 63 ± 12 | <0.001 |
| LVESD (mm/m) | 63 ± 12 | 53 ± 11 | 52 ± 13 | 51 ± 14 | 0.212 |
| LVEF (%) | 25 ± 8 | 33 ± 11 | 39 ± 13 | 26 ± 12 | <0.001 |
| Baseline characteristics (n= 794) . | Centre A (n= 284) . | Centre B (n= 176) . | Centre C (n= 127) . | Centre D (n= 207) . | P value . |
|---|---|---|---|---|---|
| Male | 242 (85%) | 137 (78%) | 68 (54%) | 147 (71%) | <0.001 |
| Age (year) | 64 ± 10 | 70 ± 9 | 70 ± 10 | 64 ± 11 | <0.001 |
| Prevalence of ischaemic cardiomyopathy | 156 (55%) | 104 (59%) | 61 (48%) | 113 (55%) | 0.301 |
| History of revascularization in ischaemic patients | 43 (15%) | 32 (18%) | 51 (40%) | 33 (16%) | <0.001 |
| Severity of MR at baseline | |||||
| No MR | 29 (10%) | 17 (10%) | 17 (13%) | 47 (23%) | <0.001 |
| Mild–moderate MR | 103 (36%) | 129 (73%) | 73 (58%) | 104 (50%) | <0.001 |
| Advanced MR | 152 (54%) | 30 (17%) | 37 (29%) | 56 (27%) | <0.001 |
| NYHA class | 3.01 ± .52 | 3.09 ± 0.49 | 2.87 ± 0.50 | 2.97 ± 0.47 | 0.002 |
| QRS duration (ms) | 178 ± 28 | 142 ± 31 | 154 ± 30 | 166 ± 32 | <0.001 |
| LVEDD (mm/m) | 74 ± 9 | 68 ± 9 | 66 ± 9 | 65 ± 11 | <0.002 |
| LVESD (mm/m) | 65 ± 7 | 57 ± 9 | 56 ± 9 | 55 ± 13 | 0.133 |
| LVEF (%) | 22 ± 4 | 27 ± 6 | 28 ± 8 | 22 ± 9 | <0.001 |
| Prevalence of RV dilatation | 68 (24%) | 124 (71%) | 11 (9%) | 29 (14%) | <0.001 |
| Follow-up | |||||
| Change in MR | |||||
| MR improvement ≥1° | 69 (24%) | 50 (28%) | 30 (24%) | 73 (35%) | <0.001 |
| No change in MR | 59 (21%) | 76 (43%) | 30 (24%) | 67 (32%) | <0.001 |
| MR worsening ≥1° | 28 (10%) | 17 (10%) | 4 (3%) | 43 (21%) | <0.001 |
| MR from FU not available | 128 (45%) | 33 (19%) | 63 (50%) | 24 (12%) | <0.001 |
| NYHA | 2.42 ± 0.6 | 2.39 ± 0.81 | 2.53 ± 0.60 | 2.25 ± 0.63 | 0.008 |
| LVEDD (mm/m) | 72 ± 10 | 65 ± 9 | 64 ± 11 | 63 ± 12 | <0.001 |
| LVESD (mm/m) | 63 ± 12 | 53 ± 11 | 52 ± 13 | 51 ± 14 | 0.212 |
| LVEF (%) | 25 ± 8 | 33 ± 11 | 39 ± 13 | 26 ± 12 | <0.001 |
MR, mitral regurgitation; NYHA, New York Heart Association; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; RV, right ventricular; FU, follow-up.
| Baseline characteristics (n= 794) . | Centre A (n= 284) . | Centre B (n= 176) . | Centre C (n= 127) . | Centre D (n= 207) . | P value . |
|---|---|---|---|---|---|
| Male | 242 (85%) | 137 (78%) | 68 (54%) | 147 (71%) | <0.001 |
| Age (year) | 64 ± 10 | 70 ± 9 | 70 ± 10 | 64 ± 11 | <0.001 |
| Prevalence of ischaemic cardiomyopathy | 156 (55%) | 104 (59%) | 61 (48%) | 113 (55%) | 0.301 |
| History of revascularization in ischaemic patients | 43 (15%) | 32 (18%) | 51 (40%) | 33 (16%) | <0.001 |
| Severity of MR at baseline | |||||
| No MR | 29 (10%) | 17 (10%) | 17 (13%) | 47 (23%) | <0.001 |
| Mild–moderate MR | 103 (36%) | 129 (73%) | 73 (58%) | 104 (50%) | <0.001 |
| Advanced MR | 152 (54%) | 30 (17%) | 37 (29%) | 56 (27%) | <0.001 |
| NYHA class | 3.01 ± .52 | 3.09 ± 0.49 | 2.87 ± 0.50 | 2.97 ± 0.47 | 0.002 |
| QRS duration (ms) | 178 ± 28 | 142 ± 31 | 154 ± 30 | 166 ± 32 | <0.001 |
| LVEDD (mm/m) | 74 ± 9 | 68 ± 9 | 66 ± 9 | 65 ± 11 | <0.002 |
| LVESD (mm/m) | 65 ± 7 | 57 ± 9 | 56 ± 9 | 55 ± 13 | 0.133 |
| LVEF (%) | 22 ± 4 | 27 ± 6 | 28 ± 8 | 22 ± 9 | <0.001 |
| Prevalence of RV dilatation | 68 (24%) | 124 (71%) | 11 (9%) | 29 (14%) | <0.001 |
| Follow-up | |||||
| Change in MR | |||||
| MR improvement ≥1° | 69 (24%) | 50 (28%) | 30 (24%) | 73 (35%) | <0.001 |
| No change in MR | 59 (21%) | 76 (43%) | 30 (24%) | 67 (32%) | <0.001 |
| MR worsening ≥1° | 28 (10%) | 17 (10%) | 4 (3%) | 43 (21%) | <0.001 |
| MR from FU not available | 128 (45%) | 33 (19%) | 63 (50%) | 24 (12%) | <0.001 |
| NYHA | 2.42 ± 0.6 | 2.39 ± 0.81 | 2.53 ± 0.60 | 2.25 ± 0.63 | 0.008 |
| LVEDD (mm/m) | 72 ± 10 | 65 ± 9 | 64 ± 11 | 63 ± 12 | <0.001 |
| LVESD (mm/m) | 63 ± 12 | 53 ± 11 | 52 ± 13 | 51 ± 14 | 0.212 |
| LVEF (%) | 25 ± 8 | 33 ± 11 | 39 ± 13 | 26 ± 12 | <0.001 |
| Baseline characteristics (n= 794) . | Centre A (n= 284) . | Centre B (n= 176) . | Centre C (n= 127) . | Centre D (n= 207) . | P value . |
|---|---|---|---|---|---|
| Male | 242 (85%) | 137 (78%) | 68 (54%) | 147 (71%) | <0.001 |
| Age (year) | 64 ± 10 | 70 ± 9 | 70 ± 10 | 64 ± 11 | <0.001 |
| Prevalence of ischaemic cardiomyopathy | 156 (55%) | 104 (59%) | 61 (48%) | 113 (55%) | 0.301 |
| History of revascularization in ischaemic patients | 43 (15%) | 32 (18%) | 51 (40%) | 33 (16%) | <0.001 |
| Severity of MR at baseline | |||||
| No MR | 29 (10%) | 17 (10%) | 17 (13%) | 47 (23%) | <0.001 |
| Mild–moderate MR | 103 (36%) | 129 (73%) | 73 (58%) | 104 (50%) | <0.001 |
| Advanced MR | 152 (54%) | 30 (17%) | 37 (29%) | 56 (27%) | <0.001 |
| NYHA class | 3.01 ± .52 | 3.09 ± 0.49 | 2.87 ± 0.50 | 2.97 ± 0.47 | 0.002 |
| QRS duration (ms) | 178 ± 28 | 142 ± 31 | 154 ± 30 | 166 ± 32 | <0.001 |
| LVEDD (mm/m) | 74 ± 9 | 68 ± 9 | 66 ± 9 | 65 ± 11 | <0.002 |
| LVESD (mm/m) | 65 ± 7 | 57 ± 9 | 56 ± 9 | 55 ± 13 | 0.133 |
| LVEF (%) | 22 ± 4 | 27 ± 6 | 28 ± 8 | 22 ± 9 | <0.001 |
| Prevalence of RV dilatation | 68 (24%) | 124 (71%) | 11 (9%) | 29 (14%) | <0.001 |
| Follow-up | |||||
| Change in MR | |||||
| MR improvement ≥1° | 69 (24%) | 50 (28%) | 30 (24%) | 73 (35%) | <0.001 |
| No change in MR | 59 (21%) | 76 (43%) | 30 (24%) | 67 (32%) | <0.001 |
| MR worsening ≥1° | 28 (10%) | 17 (10%) | 4 (3%) | 43 (21%) | <0.001 |
| MR from FU not available | 128 (45%) | 33 (19%) | 63 (50%) | 24 (12%) | <0.001 |
| NYHA | 2.42 ± 0.6 | 2.39 ± 0.81 | 2.53 ± 0.60 | 2.25 ± 0.63 | 0.008 |
| LVEDD (mm/m) | 72 ± 10 | 65 ± 9 | 64 ± 11 | 63 ± 12 | <0.001 |
| LVESD (mm/m) | 63 ± 12 | 53 ± 11 | 52 ± 13 | 51 ± 14 | 0.212 |
| LVEF (%) | 25 ± 8 | 33 ± 11 | 39 ± 13 | 26 ± 12 | <0.001 |
MR, mitral regurgitation; NYHA, New York Heart Association; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; RV, right ventricular; FU, follow-up.
Patients were divided into three groups based on the degree of their MR before CRT implantation: group 1 included patients with no/trace MR (n= 110), group 2 consisted of patients with mild or moderate MR (MR of first and second degree, n= 409), and group 3 comprised those with moderate-to-severe or severe MR (MR of third and fourth degree, n= 275). The distribution of MR in CRT population is depicted in Figure 1.
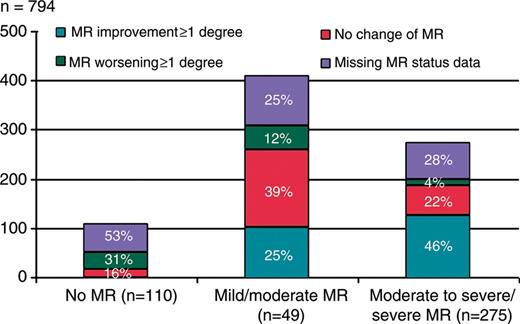
The distribution of mitral regurgitation in the cardiac resynchronization therapy population. The rate of certain degree of mitral regurgitation is expressed as both absolute value and as a percentage of the study population under each bar. Percentage of patients whose mitral regurgitation improved, not changed or worsened, as well as percentage of cases with missing data about the mitral regurgitation severity after 12 months of cardiac resynchronization therapy are marked in each bar.
Clinical characteristic and outcome of patients with advanced mitral regurgitation as compared with patients with milder mitral regurgitation at baseline
The demographic data were comparable between groups. Patients with advanced MR tended to have more dilated ventricles, more dilated left atrium, and lower LVEF, as compared with the other two groups (Table 2).
| . | All patients (n= 794) . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate-severe/severe MR (n= 275) . | P value . |
|---|---|---|---|---|---|
| Age | 66 ± 11 | 65 ± 11 | 66 ± 10 | 66 ± 11 | 0.506 |
| Men (%) | 594 (75%) | 84 (76%) | 309 (76%) | 201 (73%) | 0.875 |
| Follow-up duration (months) | 26 ± 18 | 25 ± 18 | 26 ± 17 | 26 ± 20 | 0.814 |
| Aetiology of HF | |||||
| CAD | 397 (50%) | 57 (52%) | 205 (50%) | 135 (49%) | 0.971 |
| DCM | 341 (43%) | 39 (35%) | 179 (44%) | 123 (45%) | 0.394 |
| Revascularization in CAD (CABG and/or PCI) | 162 (20%) | 17 (15%) | 82 (20%) | 63 (23%) | 0.431 |
| History of atrial fibrillation | 349 (44%) | 53 (48%) | 169 (41%) | 126 (46%) | 0.506 |
| Paroxysmal | 203 (26%) | 32 | 98 | 73 | |
| Persistent/LS persistent | 146 (18%) | 21 | 71 | 54 | |
| NYHA class | 2.99 ± 0.50 | 3.00 ± 0.50 | 2.94 ± 0.48 | 3.00 ± 0.51 | 0.003 |
| QRS duration (ms) | 162 ± 33 | 164 ± 33 | 158 ± 34 | 168 ± 31 | <0.001 |
| LVEDD (mm/m) | 69 ± 10 | 67 ± 11 | 66 ± 10 | 73 ± 10 | <0.001 |
| LVESD (mm/m) | 56 ± 11 | 54 ± 11 | 55 ± 11 | 58 ± 10 | 0.006 |
| LVEF (%) | 24 ± 7 | 24 ± 9 | 25 ± 7 | 23 ± 7 | 0.014 |
| LA area (mm2) | 48 ± 8 | 49 ± 10 | 46 ± 8 | 50 ± 8 | <0.001 |
| RV dilatation | 231 (29%) | 20 (18%) | 127 (31%) | 84 (31%) | 0.061 |
| RV dysfunction | |||||
| No | 425 (53%) | 74 (67%) | 234 (57%) | 117 (43%) | <0.001 |
| Mild | 176 (22%) | 15 (14%) | 92 (22%) | 69 (25%) | |
| Moderate | 141 (18%) | 13 (11%) | 66 (16%) | 63 (23%) | |
| Severe | 53 (7%) | 08 (8%) | 18 (4%) | 26 (10%) | |
| . | All patients (n= 794) . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate-severe/severe MR (n= 275) . | P value . |
|---|---|---|---|---|---|
| Age | 66 ± 11 | 65 ± 11 | 66 ± 10 | 66 ± 11 | 0.506 |
| Men (%) | 594 (75%) | 84 (76%) | 309 (76%) | 201 (73%) | 0.875 |
| Follow-up duration (months) | 26 ± 18 | 25 ± 18 | 26 ± 17 | 26 ± 20 | 0.814 |
| Aetiology of HF | |||||
| CAD | 397 (50%) | 57 (52%) | 205 (50%) | 135 (49%) | 0.971 |
| DCM | 341 (43%) | 39 (35%) | 179 (44%) | 123 (45%) | 0.394 |
| Revascularization in CAD (CABG and/or PCI) | 162 (20%) | 17 (15%) | 82 (20%) | 63 (23%) | 0.431 |
| History of atrial fibrillation | 349 (44%) | 53 (48%) | 169 (41%) | 126 (46%) | 0.506 |
| Paroxysmal | 203 (26%) | 32 | 98 | 73 | |
| Persistent/LS persistent | 146 (18%) | 21 | 71 | 54 | |
| NYHA class | 2.99 ± 0.50 | 3.00 ± 0.50 | 2.94 ± 0.48 | 3.00 ± 0.51 | 0.003 |
| QRS duration (ms) | 162 ± 33 | 164 ± 33 | 158 ± 34 | 168 ± 31 | <0.001 |
| LVEDD (mm/m) | 69 ± 10 | 67 ± 11 | 66 ± 10 | 73 ± 10 | <0.001 |
| LVESD (mm/m) | 56 ± 11 | 54 ± 11 | 55 ± 11 | 58 ± 10 | 0.006 |
| LVEF (%) | 24 ± 7 | 24 ± 9 | 25 ± 7 | 23 ± 7 | 0.014 |
| LA area (mm2) | 48 ± 8 | 49 ± 10 | 46 ± 8 | 50 ± 8 | <0.001 |
| RV dilatation | 231 (29%) | 20 (18%) | 127 (31%) | 84 (31%) | 0.061 |
| RV dysfunction | |||||
| No | 425 (53%) | 74 (67%) | 234 (57%) | 117 (43%) | <0.001 |
| Mild | 176 (22%) | 15 (14%) | 92 (22%) | 69 (25%) | |
| Moderate | 141 (18%) | 13 (11%) | 66 (16%) | 63 (23%) | |
| Severe | 53 (7%) | 08 (8%) | 18 (4%) | 26 (10%) | |
HF, heart failure; CAD, coronary artery disease; DCM, dilated cardiomyopathy; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; LS Persistent, long-standing persistent; LA, left atrium; other abbreviations as in Table 1.
| . | All patients (n= 794) . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate-severe/severe MR (n= 275) . | P value . |
|---|---|---|---|---|---|
| Age | 66 ± 11 | 65 ± 11 | 66 ± 10 | 66 ± 11 | 0.506 |
| Men (%) | 594 (75%) | 84 (76%) | 309 (76%) | 201 (73%) | 0.875 |
| Follow-up duration (months) | 26 ± 18 | 25 ± 18 | 26 ± 17 | 26 ± 20 | 0.814 |
| Aetiology of HF | |||||
| CAD | 397 (50%) | 57 (52%) | 205 (50%) | 135 (49%) | 0.971 |
| DCM | 341 (43%) | 39 (35%) | 179 (44%) | 123 (45%) | 0.394 |
| Revascularization in CAD (CABG and/or PCI) | 162 (20%) | 17 (15%) | 82 (20%) | 63 (23%) | 0.431 |
| History of atrial fibrillation | 349 (44%) | 53 (48%) | 169 (41%) | 126 (46%) | 0.506 |
| Paroxysmal | 203 (26%) | 32 | 98 | 73 | |
| Persistent/LS persistent | 146 (18%) | 21 | 71 | 54 | |
| NYHA class | 2.99 ± 0.50 | 3.00 ± 0.50 | 2.94 ± 0.48 | 3.00 ± 0.51 | 0.003 |
| QRS duration (ms) | 162 ± 33 | 164 ± 33 | 158 ± 34 | 168 ± 31 | <0.001 |
| LVEDD (mm/m) | 69 ± 10 | 67 ± 11 | 66 ± 10 | 73 ± 10 | <0.001 |
| LVESD (mm/m) | 56 ± 11 | 54 ± 11 | 55 ± 11 | 58 ± 10 | 0.006 |
| LVEF (%) | 24 ± 7 | 24 ± 9 | 25 ± 7 | 23 ± 7 | 0.014 |
| LA area (mm2) | 48 ± 8 | 49 ± 10 | 46 ± 8 | 50 ± 8 | <0.001 |
| RV dilatation | 231 (29%) | 20 (18%) | 127 (31%) | 84 (31%) | 0.061 |
| RV dysfunction | |||||
| No | 425 (53%) | 74 (67%) | 234 (57%) | 117 (43%) | <0.001 |
| Mild | 176 (22%) | 15 (14%) | 92 (22%) | 69 (25%) | |
| Moderate | 141 (18%) | 13 (11%) | 66 (16%) | 63 (23%) | |
| Severe | 53 (7%) | 08 (8%) | 18 (4%) | 26 (10%) | |
| . | All patients (n= 794) . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate-severe/severe MR (n= 275) . | P value . |
|---|---|---|---|---|---|
| Age | 66 ± 11 | 65 ± 11 | 66 ± 10 | 66 ± 11 | 0.506 |
| Men (%) | 594 (75%) | 84 (76%) | 309 (76%) | 201 (73%) | 0.875 |
| Follow-up duration (months) | 26 ± 18 | 25 ± 18 | 26 ± 17 | 26 ± 20 | 0.814 |
| Aetiology of HF | |||||
| CAD | 397 (50%) | 57 (52%) | 205 (50%) | 135 (49%) | 0.971 |
| DCM | 341 (43%) | 39 (35%) | 179 (44%) | 123 (45%) | 0.394 |
| Revascularization in CAD (CABG and/or PCI) | 162 (20%) | 17 (15%) | 82 (20%) | 63 (23%) | 0.431 |
| History of atrial fibrillation | 349 (44%) | 53 (48%) | 169 (41%) | 126 (46%) | 0.506 |
| Paroxysmal | 203 (26%) | 32 | 98 | 73 | |
| Persistent/LS persistent | 146 (18%) | 21 | 71 | 54 | |
| NYHA class | 2.99 ± 0.50 | 3.00 ± 0.50 | 2.94 ± 0.48 | 3.00 ± 0.51 | 0.003 |
| QRS duration (ms) | 162 ± 33 | 164 ± 33 | 158 ± 34 | 168 ± 31 | <0.001 |
| LVEDD (mm/m) | 69 ± 10 | 67 ± 11 | 66 ± 10 | 73 ± 10 | <0.001 |
| LVESD (mm/m) | 56 ± 11 | 54 ± 11 | 55 ± 11 | 58 ± 10 | 0.006 |
| LVEF (%) | 24 ± 7 | 24 ± 9 | 25 ± 7 | 23 ± 7 | 0.014 |
| LA area (mm2) | 48 ± 8 | 49 ± 10 | 46 ± 8 | 50 ± 8 | <0.001 |
| RV dilatation | 231 (29%) | 20 (18%) | 127 (31%) | 84 (31%) | 0.061 |
| RV dysfunction | |||||
| No | 425 (53%) | 74 (67%) | 234 (57%) | 117 (43%) | <0.001 |
| Mild | 176 (22%) | 15 (14%) | 92 (22%) | 69 (25%) | |
| Moderate | 141 (18%) | 13 (11%) | 66 (16%) | 63 (23%) | |
| Severe | 53 (7%) | 08 (8%) | 18 (4%) | 26 (10%) | |
HF, heart failure; CAD, coronary artery disease; DCM, dilated cardiomyopathy; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; LS Persistent, long-standing persistent; LA, left atrium; other abbreviations as in Table 1.
The outcome of the three groups is summarized in Table 3. Improvement in NYHA class, LVEDD, LA size, and LVEF were present in all three groups. The extent of reduction of LVESD tended to be comparable in three groups. At 12-month follow-up, the RV dilatation disappeared in 15% (P= 0.009) of patients with advanced MR (group 3), whereas its improvement was relatively small in patients with no (group 1) or mild/moderate MR (group 2) [2.9% (P= 0.003) and 7.5% (P= 0.144), respectively]. Right-ventricular dysfunction improved in 32% (P= 0.010) of patients in group 3 and 25% (0.002) in group 2, whereas no significant improvement was recorded in group 1. When all the three groups were compared, no statistically significant trend in RV dilation or dysfunction was observed across the groups (P = 0.157 and P= 0.175, respectively).
The outcome of patients with advanced mitral regurgitation as compared with patients presenting with no or mild/moderate mitral regurgitation at baseline; prevalence of mitral regurgitation improvement and worsening in the groups
| . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate to severe/severe MR (n= 275) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | P value (between groups) . |
| NYHA class | 2.4 ± 0.7 | ( − 19.0)± 21% | <0.001 | 2.3 ± 0.6 | ( − 19.4) ± 22% | <0.001 | 2.4 ± 0.6 | ( − 18.9) ± 22% | <0.001 | 0.003 |
| QRS duration (ms) | 147 ± 27 | ( − 10) ± 45% | <0.001 | 142 ± 27 | ( − 11) ± 49% | <0.001 | 144 ± 26 | ( − 14) ± 46% | <0.001 | 0.306 |
| LVEDD (mm/m) | 57 ± 23 | ( − 6.2) ± 25.2% | 0.025 | 57 ± 22 | ( − 13.5) ± 30.4% | <0.001 | 65 ± 21 | (−9.6) ± 26.8% | <0.001 | <0.001 |
| LVESD (mm/m) | 51±10 | ( − 11.9) ± 32.7% | 0.004 | 44 ± 21 | ( − 18.6) ± 34.4% | <0.001 | 45 ± 23 | (− 21.2) ± 38.5% | <0.001 | 0.819 |
| LVEF (%) | 28 ± 11 | (20) ± 44% | <0.001 | 30 ± 12 | (27) ± 49% | <0.001 | 26 ± 9 | (17) ± 46% | <0.001 | <0.001 |
| LA area (mm2) | 49 ± 9 | 2.7 ± 17% | 0.730 | 46 ± 7 | 9 ± 90% | 0.816 | 48 ± 8 | (− 2) ± 15% | 0.005 | 0.001 |
| Absolute value at 12 months | Improvement/ worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | P value (between groups) | |
| RV dilatation | 33 (30%) | 2.9%/14.5% | 0.003 | 136 (33%) | 7.6%/10.5% | 0.144 | 69 (25%) | 14.6%/7.1% | 0.009 | 0.157 |
| RV dysfunction | 44 (40%) | 18.0%/19.7% | 0.732 | 159 (39%) | 24.9%/16.2% | 0.002 | 130 (47%) | 32.3%/22.6% | 0.010 | 0.175 |
| Mild/moderate | 31 | 142 | 100 | |||||||
| Severe | 13 | 16 | 31 | |||||||
| Atrial fibrillation: | 58 (53%) | 28%/17% | 0.054 | 162 (40%) | 14.2%/8.9% | 0.016 | 103 (37%) | 18.9%/10.7% | 0.006 | 0.046 |
| Paroxysmal | 23 | 41 | 30 | |||||||
| LSpersistent | 35 | 122 | 73 | |||||||
| Change in MR after 12 months | ||||||||||
| MR improvement ≥1° | – | 101 (25%) | 126 (46%) | <0.001 | ||||||
| No change in MR | 18 (16%) | 159 (39%) | 61 (22%) | <0.001 | ||||||
| MR worsening ≥1° | 34 (31%) | 48 (12%) | 12 (4%) | <0.001 | ||||||
| Missing MR status data | 58 (53%) | 102 (25%) | 76 (28%) | |||||||
| . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate to severe/severe MR (n= 275) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | P value (between groups) . |
| NYHA class | 2.4 ± 0.7 | ( − 19.0)± 21% | <0.001 | 2.3 ± 0.6 | ( − 19.4) ± 22% | <0.001 | 2.4 ± 0.6 | ( − 18.9) ± 22% | <0.001 | 0.003 |
| QRS duration (ms) | 147 ± 27 | ( − 10) ± 45% | <0.001 | 142 ± 27 | ( − 11) ± 49% | <0.001 | 144 ± 26 | ( − 14) ± 46% | <0.001 | 0.306 |
| LVEDD (mm/m) | 57 ± 23 | ( − 6.2) ± 25.2% | 0.025 | 57 ± 22 | ( − 13.5) ± 30.4% | <0.001 | 65 ± 21 | (−9.6) ± 26.8% | <0.001 | <0.001 |
| LVESD (mm/m) | 51±10 | ( − 11.9) ± 32.7% | 0.004 | 44 ± 21 | ( − 18.6) ± 34.4% | <0.001 | 45 ± 23 | (− 21.2) ± 38.5% | <0.001 | 0.819 |
| LVEF (%) | 28 ± 11 | (20) ± 44% | <0.001 | 30 ± 12 | (27) ± 49% | <0.001 | 26 ± 9 | (17) ± 46% | <0.001 | <0.001 |
| LA area (mm2) | 49 ± 9 | 2.7 ± 17% | 0.730 | 46 ± 7 | 9 ± 90% | 0.816 | 48 ± 8 | (− 2) ± 15% | 0.005 | 0.001 |
| Absolute value at 12 months | Improvement/ worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | P value (between groups) | |
| RV dilatation | 33 (30%) | 2.9%/14.5% | 0.003 | 136 (33%) | 7.6%/10.5% | 0.144 | 69 (25%) | 14.6%/7.1% | 0.009 | 0.157 |
| RV dysfunction | 44 (40%) | 18.0%/19.7% | 0.732 | 159 (39%) | 24.9%/16.2% | 0.002 | 130 (47%) | 32.3%/22.6% | 0.010 | 0.175 |
| Mild/moderate | 31 | 142 | 100 | |||||||
| Severe | 13 | 16 | 31 | |||||||
| Atrial fibrillation: | 58 (53%) | 28%/17% | 0.054 | 162 (40%) | 14.2%/8.9% | 0.016 | 103 (37%) | 18.9%/10.7% | 0.006 | 0.046 |
| Paroxysmal | 23 | 41 | 30 | |||||||
| LSpersistent | 35 | 122 | 73 | |||||||
| Change in MR after 12 months | ||||||||||
| MR improvement ≥1° | – | 101 (25%) | 126 (46%) | <0.001 | ||||||
| No change in MR | 18 (16%) | 159 (39%) | 61 (22%) | <0.001 | ||||||
| MR worsening ≥1° | 34 (31%) | 48 (12%) | 12 (4%) | <0.001 | ||||||
| Missing MR status data | 58 (53%) | 102 (25%) | 76 (28%) | |||||||
Abbreviations as in Table 1.
The outcome of patients with advanced mitral regurgitation as compared with patients presenting with no or mild/moderate mitral regurgitation at baseline; prevalence of mitral regurgitation improvement and worsening in the groups
| . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate to severe/severe MR (n= 275) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | P value (between groups) . |
| NYHA class | 2.4 ± 0.7 | ( − 19.0)± 21% | <0.001 | 2.3 ± 0.6 | ( − 19.4) ± 22% | <0.001 | 2.4 ± 0.6 | ( − 18.9) ± 22% | <0.001 | 0.003 |
| QRS duration (ms) | 147 ± 27 | ( − 10) ± 45% | <0.001 | 142 ± 27 | ( − 11) ± 49% | <0.001 | 144 ± 26 | ( − 14) ± 46% | <0.001 | 0.306 |
| LVEDD (mm/m) | 57 ± 23 | ( − 6.2) ± 25.2% | 0.025 | 57 ± 22 | ( − 13.5) ± 30.4% | <0.001 | 65 ± 21 | (−9.6) ± 26.8% | <0.001 | <0.001 |
| LVESD (mm/m) | 51±10 | ( − 11.9) ± 32.7% | 0.004 | 44 ± 21 | ( − 18.6) ± 34.4% | <0.001 | 45 ± 23 | (− 21.2) ± 38.5% | <0.001 | 0.819 |
| LVEF (%) | 28 ± 11 | (20) ± 44% | <0.001 | 30 ± 12 | (27) ± 49% | <0.001 | 26 ± 9 | (17) ± 46% | <0.001 | <0.001 |
| LA area (mm2) | 49 ± 9 | 2.7 ± 17% | 0.730 | 46 ± 7 | 9 ± 90% | 0.816 | 48 ± 8 | (− 2) ± 15% | 0.005 | 0.001 |
| Absolute value at 12 months | Improvement/ worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | P value (between groups) | |
| RV dilatation | 33 (30%) | 2.9%/14.5% | 0.003 | 136 (33%) | 7.6%/10.5% | 0.144 | 69 (25%) | 14.6%/7.1% | 0.009 | 0.157 |
| RV dysfunction | 44 (40%) | 18.0%/19.7% | 0.732 | 159 (39%) | 24.9%/16.2% | 0.002 | 130 (47%) | 32.3%/22.6% | 0.010 | 0.175 |
| Mild/moderate | 31 | 142 | 100 | |||||||
| Severe | 13 | 16 | 31 | |||||||
| Atrial fibrillation: | 58 (53%) | 28%/17% | 0.054 | 162 (40%) | 14.2%/8.9% | 0.016 | 103 (37%) | 18.9%/10.7% | 0.006 | 0.046 |
| Paroxysmal | 23 | 41 | 30 | |||||||
| LSpersistent | 35 | 122 | 73 | |||||||
| Change in MR after 12 months | ||||||||||
| MR improvement ≥1° | – | 101 (25%) | 126 (46%) | <0.001 | ||||||
| No change in MR | 18 (16%) | 159 (39%) | 61 (22%) | <0.001 | ||||||
| MR worsening ≥1° | 34 (31%) | 48 (12%) | 12 (4%) | <0.001 | ||||||
| Missing MR status data | 58 (53%) | 102 (25%) | 76 (28%) | |||||||
| . | No MR (n= 110) . | Mild/moderate MR (n= 409) . | Moderate to severe/severe MR (n= 275) . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | Absolute value at 12 months . | Change from baseline in % . | P value . | P value (between groups) . |
| NYHA class | 2.4 ± 0.7 | ( − 19.0)± 21% | <0.001 | 2.3 ± 0.6 | ( − 19.4) ± 22% | <0.001 | 2.4 ± 0.6 | ( − 18.9) ± 22% | <0.001 | 0.003 |
| QRS duration (ms) | 147 ± 27 | ( − 10) ± 45% | <0.001 | 142 ± 27 | ( − 11) ± 49% | <0.001 | 144 ± 26 | ( − 14) ± 46% | <0.001 | 0.306 |
| LVEDD (mm/m) | 57 ± 23 | ( − 6.2) ± 25.2% | 0.025 | 57 ± 22 | ( − 13.5) ± 30.4% | <0.001 | 65 ± 21 | (−9.6) ± 26.8% | <0.001 | <0.001 |
| LVESD (mm/m) | 51±10 | ( − 11.9) ± 32.7% | 0.004 | 44 ± 21 | ( − 18.6) ± 34.4% | <0.001 | 45 ± 23 | (− 21.2) ± 38.5% | <0.001 | 0.819 |
| LVEF (%) | 28 ± 11 | (20) ± 44% | <0.001 | 30 ± 12 | (27) ± 49% | <0.001 | 26 ± 9 | (17) ± 46% | <0.001 | <0.001 |
| LA area (mm2) | 49 ± 9 | 2.7 ± 17% | 0.730 | 46 ± 7 | 9 ± 90% | 0.816 | 48 ± 8 | (− 2) ± 15% | 0.005 | 0.001 |
| Absolute value at 12 months | Improvement/ worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | Absolute value at 12 months | Improvement/worsening after 12 months in % | P value | P value (between groups) | |
| RV dilatation | 33 (30%) | 2.9%/14.5% | 0.003 | 136 (33%) | 7.6%/10.5% | 0.144 | 69 (25%) | 14.6%/7.1% | 0.009 | 0.157 |
| RV dysfunction | 44 (40%) | 18.0%/19.7% | 0.732 | 159 (39%) | 24.9%/16.2% | 0.002 | 130 (47%) | 32.3%/22.6% | 0.010 | 0.175 |
| Mild/moderate | 31 | 142 | 100 | |||||||
| Severe | 13 | 16 | 31 | |||||||
| Atrial fibrillation: | 58 (53%) | 28%/17% | 0.054 | 162 (40%) | 14.2%/8.9% | 0.016 | 103 (37%) | 18.9%/10.7% | 0.006 | 0.046 |
| Paroxysmal | 23 | 41 | 30 | |||||||
| LSpersistent | 35 | 122 | 73 | |||||||
| Change in MR after 12 months | ||||||||||
| MR improvement ≥1° | – | 101 (25%) | 126 (46%) | <0.001 | ||||||
| No change in MR | 18 (16%) | 159 (39%) | 61 (22%) | <0.001 | ||||||
| MR worsening ≥1° | 34 (31%) | 48 (12%) | 12 (4%) | <0.001 | ||||||
| Missing MR status data | 58 (53%) | 102 (25%) | 76 (28%) | |||||||
Abbreviations as in Table 1.
A total of 508 (64%) patients responded to CRT. Out of 397 patients with ischaemic heart disease (HD), 218 (55%) were CRT responders and 179 (45%) were non-responders.
Similarly, among the 397 patients without ischaemic HD-290 (73%) patients were responders and 107 (27%) were non-responders. Compared with the ischaemic group, non-ischaemic population had a significantly higher rate of CRT responders (P> 0.001). Multivariable regression analysis was performed to identify predictors of response to CRT. After adjusting for clinical characteristics (described in statistical analysis section), baseline QRS, LVEDD, MR at baseline, and change in MR at 3-month follow-up were strongly associated with CRT response. However, although QRS and LVEDD demonstrated statistically significant association with CRT response, their impact was extremely small (odds ratio 1.03 and 0.95, respectively). Significant predictors from multivariable regression analysis are presented in Table 4.
| Effect . | Odds ratio . | 95% Confidence limits . | P value . | |
|---|---|---|---|---|
| . | . | Lower CL . | Upper CL . | . |
| Baseline QRS | 1.03 | 1.01 | 1.06 | 0.043 |
| Pre-CRT LVEDD | 0.95 | 0.93 | 0.97 | <0.0001 |
| Baseline MR | 0.82 | 0.72 | 0.94 | 0.004 |
| MR change at 3 months follow-up | 1.27 | 1.04 | 1.55 | 0.019 |
| Effect . | Odds ratio . | 95% Confidence limits . | P value . | |
|---|---|---|---|---|
| . | . | Lower CL . | Upper CL . | . |
| Baseline QRS | 1.03 | 1.01 | 1.06 | 0.043 |
| Pre-CRT LVEDD | 0.95 | 0.93 | 0.97 | <0.0001 |
| Baseline MR | 0.82 | 0.72 | 0.94 | 0.004 |
| MR change at 3 months follow-up | 1.27 | 1.04 | 1.55 | 0.019 |
CRT, cardiac resynchronization therapy; CL, confidence limit; other abbreviations as in Table 1.
| Effect . | Odds ratio . | 95% Confidence limits . | P value . | |
|---|---|---|---|---|
| . | . | Lower CL . | Upper CL . | . |
| Baseline QRS | 1.03 | 1.01 | 1.06 | 0.043 |
| Pre-CRT LVEDD | 0.95 | 0.93 | 0.97 | <0.0001 |
| Baseline MR | 0.82 | 0.72 | 0.94 | 0.004 |
| MR change at 3 months follow-up | 1.27 | 1.04 | 1.55 | 0.019 |
| Effect . | Odds ratio . | 95% Confidence limits . | P value . | |
|---|---|---|---|---|
| . | . | Lower CL . | Upper CL . | . |
| Baseline QRS | 1.03 | 1.01 | 1.06 | 0.043 |
| Pre-CRT LVEDD | 0.95 | 0.93 | 0.97 | <0.0001 |
| Baseline MR | 0.82 | 0.72 | 0.94 | 0.004 |
| MR change at 3 months follow-up | 1.27 | 1.04 | 1.55 | 0.019 |
CRT, cardiac resynchronization therapy; CL, confidence limit; other abbreviations as in Table 1.
Frequency of mitral regurgitation improvement during cardiac resynchronization therapy, features of patients with mitral regurgitation improvement, predictors of mitral regurgitation improvement
Severity of MR after 3 and 12 months of CRT was available in 558 patients (Table 5). Based on MR status at follow-up, these 558 patients were stratified into three categories: MR worsening ≥1°, no MR change, and MR improving ≥1°. Patients with no/trace MR at baseline were excluded from this subanalysis leaving a population of 506 patients.
The baseline characteristic and outcome of patients with mitral regurgitation improvement as compared with those with not changed or worsened mitral regurgitation after 12 months of cardiac resynchronization therapy
| . | MR improvement ≥1° at 12 months (n= 227) . | P value (within group) . | No change in MR at 12 months (n= 219) . | P value (within group) . | MR worsening ≥1° at 12 months (n= 60) . | P value (within group) . | P value (between groups) . |
|---|---|---|---|---|---|---|---|
| Prevalence of CAD | 36% | 36% | 42% | 0.581 | |||
| MR at baseline | |||||||
| Mild/moderate | 101/308 (33%) | 159/308 (52%) | 48/308 (16%) | <0.0001 | |||
| Advanced (MR 3–4) | 126/199 (63%) | 61/199 (31%) | 12/199 (6%) | <0.001 | |||
| NYHA class | |||||||
| Baseline | 3.04 ± 0.49 | <0.001 | 2.96 ± 0.52 | <0.001 | 2.96 ± 0.49 | <0.001 | 0.348 |
| 12 month | 2.29 ± 0.64 | 2.35 ± 0.65 | 2.54 ± 0.63 | 0.054 | |||
| QRS (ms) | |||||||
| Baseline | 161 ± 35 | <0.001 | 162 ± 32 | <0.0001 | 162 ± 37 | 0.012 | 0.986 |
| 12 month | 141 ± 23 | 144 ± 21 | 147 ± 40 | 0.283 | |||
| LVEDD (mm/m) | |||||||
| Baseline | 69 ± 10 | 0.032 | 68 ± 11 | <0.0001 | 70 ± 10 | <0.001 | 0.321 |
| 12 month | 58 ± 22 | 59 ± 23 | 66 ± 17 | 0.032 | |||
| LVESD (mm/m) | |||||||
| Baseline | 56 ± 11 | 0.089 | 55 ± 11 | <0.0001 | 59 ± 12 | <0.001 | 0.288 |
| 12 month | 42 ± 21 | 45 ± 21 | 53 ± 18 | 0.204 | |||
| LVEF (%) | |||||||
| Baseline | 24 ± 8 | <0.001 | 24 ± 7 | <0.0001 | 23 ± 7 | 0.388 | 0.283 |
| 12 month | 30 ± 11 | 29 ± 12 | 23 ± 8 | 0.001 | |||
| LA area (mm2) | |||||||
| Baseline | 48 ± 8 | 0.005 | 47 ± 9 | 0.777 | 48 ± 7 | 1.000 | 0.343 |
| 12 month | 46 ± 8 | 47 ± 7 | 49 ± 7 | 0.073 | |||
| RV dilatation | |||||||
| Baseline | 29% | 32% | 32% | 0.969 | |||
| 12 month | 26% | 32% | 42% | 0.714 | |||
| RV dysfunction—mild/moderate: | |||||||
| Baseline | 45% | 41% | 35% | 0.004 | |||
| 12 month | 30% | 38% | 45% | 0.806 | |||
| RV dysfunction—Severe | |||||||
| Baseline | 07% | 04% | 15% | 0.002 | |||
| 12 month | 06% | 06% | 13% | 0.526 | |||
| Atrial fibrillation—paroxysmal: | |||||||
| Baseline | 65% | 62% | 56% | 0.032 | |||
| 12 month | 69% | 63% | 54% | 0.025 | |||
| Atrial fibrillation—LSPersistent | |||||||
| Baseline | 35% | 38% | 44% | 0.024 | |||
| 12 month | 31% | 37% | 46% | 0.085 | |||
| . | MR improvement ≥1° at 12 months (n= 227) . | P value (within group) . | No change in MR at 12 months (n= 219) . | P value (within group) . | MR worsening ≥1° at 12 months (n= 60) . | P value (within group) . | P value (between groups) . |
|---|---|---|---|---|---|---|---|
| Prevalence of CAD | 36% | 36% | 42% | 0.581 | |||
| MR at baseline | |||||||
| Mild/moderate | 101/308 (33%) | 159/308 (52%) | 48/308 (16%) | <0.0001 | |||
| Advanced (MR 3–4) | 126/199 (63%) | 61/199 (31%) | 12/199 (6%) | <0.001 | |||
| NYHA class | |||||||
| Baseline | 3.04 ± 0.49 | <0.001 | 2.96 ± 0.52 | <0.001 | 2.96 ± 0.49 | <0.001 | 0.348 |
| 12 month | 2.29 ± 0.64 | 2.35 ± 0.65 | 2.54 ± 0.63 | 0.054 | |||
| QRS (ms) | |||||||
| Baseline | 161 ± 35 | <0.001 | 162 ± 32 | <0.0001 | 162 ± 37 | 0.012 | 0.986 |
| 12 month | 141 ± 23 | 144 ± 21 | 147 ± 40 | 0.283 | |||
| LVEDD (mm/m) | |||||||
| Baseline | 69 ± 10 | 0.032 | 68 ± 11 | <0.0001 | 70 ± 10 | <0.001 | 0.321 |
| 12 month | 58 ± 22 | 59 ± 23 | 66 ± 17 | 0.032 | |||
| LVESD (mm/m) | |||||||
| Baseline | 56 ± 11 | 0.089 | 55 ± 11 | <0.0001 | 59 ± 12 | <0.001 | 0.288 |
| 12 month | 42 ± 21 | 45 ± 21 | 53 ± 18 | 0.204 | |||
| LVEF (%) | |||||||
| Baseline | 24 ± 8 | <0.001 | 24 ± 7 | <0.0001 | 23 ± 7 | 0.388 | 0.283 |
| 12 month | 30 ± 11 | 29 ± 12 | 23 ± 8 | 0.001 | |||
| LA area (mm2) | |||||||
| Baseline | 48 ± 8 | 0.005 | 47 ± 9 | 0.777 | 48 ± 7 | 1.000 | 0.343 |
| 12 month | 46 ± 8 | 47 ± 7 | 49 ± 7 | 0.073 | |||
| RV dilatation | |||||||
| Baseline | 29% | 32% | 32% | 0.969 | |||
| 12 month | 26% | 32% | 42% | 0.714 | |||
| RV dysfunction—mild/moderate: | |||||||
| Baseline | 45% | 41% | 35% | 0.004 | |||
| 12 month | 30% | 38% | 45% | 0.806 | |||
| RV dysfunction—Severe | |||||||
| Baseline | 07% | 04% | 15% | 0.002 | |||
| 12 month | 06% | 06% | 13% | 0.526 | |||
| Atrial fibrillation—paroxysmal: | |||||||
| Baseline | 65% | 62% | 56% | 0.032 | |||
| 12 month | 69% | 63% | 54% | 0.025 | |||
| Atrial fibrillation—LSPersistent | |||||||
| Baseline | 35% | 38% | 44% | 0.024 | |||
| 12 month | 31% | 37% | 46% | 0.085 | |||
The baseline characteristic and outcome of patients with mitral regurgitation improvement as compared with those with not changed or worsened mitral regurgitation after 12 months of cardiac resynchronization therapy
| . | MR improvement ≥1° at 12 months (n= 227) . | P value (within group) . | No change in MR at 12 months (n= 219) . | P value (within group) . | MR worsening ≥1° at 12 months (n= 60) . | P value (within group) . | P value (between groups) . |
|---|---|---|---|---|---|---|---|
| Prevalence of CAD | 36% | 36% | 42% | 0.581 | |||
| MR at baseline | |||||||
| Mild/moderate | 101/308 (33%) | 159/308 (52%) | 48/308 (16%) | <0.0001 | |||
| Advanced (MR 3–4) | 126/199 (63%) | 61/199 (31%) | 12/199 (6%) | <0.001 | |||
| NYHA class | |||||||
| Baseline | 3.04 ± 0.49 | <0.001 | 2.96 ± 0.52 | <0.001 | 2.96 ± 0.49 | <0.001 | 0.348 |
| 12 month | 2.29 ± 0.64 | 2.35 ± 0.65 | 2.54 ± 0.63 | 0.054 | |||
| QRS (ms) | |||||||
| Baseline | 161 ± 35 | <0.001 | 162 ± 32 | <0.0001 | 162 ± 37 | 0.012 | 0.986 |
| 12 month | 141 ± 23 | 144 ± 21 | 147 ± 40 | 0.283 | |||
| LVEDD (mm/m) | |||||||
| Baseline | 69 ± 10 | 0.032 | 68 ± 11 | <0.0001 | 70 ± 10 | <0.001 | 0.321 |
| 12 month | 58 ± 22 | 59 ± 23 | 66 ± 17 | 0.032 | |||
| LVESD (mm/m) | |||||||
| Baseline | 56 ± 11 | 0.089 | 55 ± 11 | <0.0001 | 59 ± 12 | <0.001 | 0.288 |
| 12 month | 42 ± 21 | 45 ± 21 | 53 ± 18 | 0.204 | |||
| LVEF (%) | |||||||
| Baseline | 24 ± 8 | <0.001 | 24 ± 7 | <0.0001 | 23 ± 7 | 0.388 | 0.283 |
| 12 month | 30 ± 11 | 29 ± 12 | 23 ± 8 | 0.001 | |||
| LA area (mm2) | |||||||
| Baseline | 48 ± 8 | 0.005 | 47 ± 9 | 0.777 | 48 ± 7 | 1.000 | 0.343 |
| 12 month | 46 ± 8 | 47 ± 7 | 49 ± 7 | 0.073 | |||
| RV dilatation | |||||||
| Baseline | 29% | 32% | 32% | 0.969 | |||
| 12 month | 26% | 32% | 42% | 0.714 | |||
| RV dysfunction—mild/moderate: | |||||||
| Baseline | 45% | 41% | 35% | 0.004 | |||
| 12 month | 30% | 38% | 45% | 0.806 | |||
| RV dysfunction—Severe | |||||||
| Baseline | 07% | 04% | 15% | 0.002 | |||
| 12 month | 06% | 06% | 13% | 0.526 | |||
| Atrial fibrillation—paroxysmal: | |||||||
| Baseline | 65% | 62% | 56% | 0.032 | |||
| 12 month | 69% | 63% | 54% | 0.025 | |||
| Atrial fibrillation—LSPersistent | |||||||
| Baseline | 35% | 38% | 44% | 0.024 | |||
| 12 month | 31% | 37% | 46% | 0.085 | |||
| . | MR improvement ≥1° at 12 months (n= 227) . | P value (within group) . | No change in MR at 12 months (n= 219) . | P value (within group) . | MR worsening ≥1° at 12 months (n= 60) . | P value (within group) . | P value (between groups) . |
|---|---|---|---|---|---|---|---|
| Prevalence of CAD | 36% | 36% | 42% | 0.581 | |||
| MR at baseline | |||||||
| Mild/moderate | 101/308 (33%) | 159/308 (52%) | 48/308 (16%) | <0.0001 | |||
| Advanced (MR 3–4) | 126/199 (63%) | 61/199 (31%) | 12/199 (6%) | <0.001 | |||
| NYHA class | |||||||
| Baseline | 3.04 ± 0.49 | <0.001 | 2.96 ± 0.52 | <0.001 | 2.96 ± 0.49 | <0.001 | 0.348 |
| 12 month | 2.29 ± 0.64 | 2.35 ± 0.65 | 2.54 ± 0.63 | 0.054 | |||
| QRS (ms) | |||||||
| Baseline | 161 ± 35 | <0.001 | 162 ± 32 | <0.0001 | 162 ± 37 | 0.012 | 0.986 |
| 12 month | 141 ± 23 | 144 ± 21 | 147 ± 40 | 0.283 | |||
| LVEDD (mm/m) | |||||||
| Baseline | 69 ± 10 | 0.032 | 68 ± 11 | <0.0001 | 70 ± 10 | <0.001 | 0.321 |
| 12 month | 58 ± 22 | 59 ± 23 | 66 ± 17 | 0.032 | |||
| LVESD (mm/m) | |||||||
| Baseline | 56 ± 11 | 0.089 | 55 ± 11 | <0.0001 | 59 ± 12 | <0.001 | 0.288 |
| 12 month | 42 ± 21 | 45 ± 21 | 53 ± 18 | 0.204 | |||
| LVEF (%) | |||||||
| Baseline | 24 ± 8 | <0.001 | 24 ± 7 | <0.0001 | 23 ± 7 | 0.388 | 0.283 |
| 12 month | 30 ± 11 | 29 ± 12 | 23 ± 8 | 0.001 | |||
| LA area (mm2) | |||||||
| Baseline | 48 ± 8 | 0.005 | 47 ± 9 | 0.777 | 48 ± 7 | 1.000 | 0.343 |
| 12 month | 46 ± 8 | 47 ± 7 | 49 ± 7 | 0.073 | |||
| RV dilatation | |||||||
| Baseline | 29% | 32% | 32% | 0.969 | |||
| 12 month | 26% | 32% | 42% | 0.714 | |||
| RV dysfunction—mild/moderate: | |||||||
| Baseline | 45% | 41% | 35% | 0.004 | |||
| 12 month | 30% | 38% | 45% | 0.806 | |||
| RV dysfunction—Severe | |||||||
| Baseline | 07% | 04% | 15% | 0.002 | |||
| 12 month | 06% | 06% | 13% | 0.526 | |||
| Atrial fibrillation—paroxysmal: | |||||||
| Baseline | 65% | 62% | 56% | 0.032 | |||
| 12 month | 69% | 63% | 54% | 0.025 | |||
| Atrial fibrillation—LSPersistent | |||||||
| Baseline | 35% | 38% | 44% | 0.024 | |||
| 12 month | 31% | 37% | 46% | 0.085 | |||
When assessing the relation between three categories of MR change and response to CRT at 12 months, we found that 42% of patients with MR worsening ≥1° were CRT responders, whereas 62% of patients with no change in MR, and 67% with ≥1° of MR improvement, responded to CRT (P< 0.001).
Out of the 506 patients, improvement of MR ≥1° was present in 227(45%), no change in MR was detected in 219 (43%), and MR worsening in 60 (12%) patients. Improvement of MR was relatively more frequent in patients with advanced MR at baseline (63% of all patients with 3rd and 4th degree of MR at baseline) than with mild/moderate MR (33% of all patients with 1st and 2nd degree of MR at baseline, P< 0.001).
All baseline demographic and echocardiographic characteristics (except for the RV dysfunction and type of atrial fibrillation) were comparable between groups (Table 5). NYHA class improved and QRS duration decreased in all three groups, but these changes were greater in patients with MR improvement than in the other two groups. Greater reduction of left-atrial size was also detected in patients with MR improvement.
Regression analysis was performed using a linear model to determine predictor of MR change at 12-month follow-up. Severity of MR at baseline (regression coefficient = 0.76, R-square = 0.44, P< 0.001) and MR change at three months (regression coefficient = 0.69, R-square = 0.41, P< 0.001) were found to have strong correlation with MR (improvement/worsening) at 12 months.
Repeated measure ANOVA was performed to assess within-subjects and between-subjects (across baseline MR groups) effect on change in LVEF, LVEDD, LVESD, and LA size at baseline, 3 and 12 months. A significant within-subject and between-subjects trend was observed in LVEF and LVEDD (P< 0.001). Patients with mild/moderate and advanced MR experienced significantly better improvement compared with the no-MR group. LVESD and LA size did not show any significant between-subjects change over follow-up time periods. Cases with missing values were listwise deleted from this analysis.
Survival and need for heart failure hospitalization in patients with advanced mitral regurgitation before cardiac resynchronization therapy implantation
Survival status and need for hospitalization for HF were known in 794 patients at the last follow-up. Total of 129 (16%) patients died and 30 (3.8%) patients underwent heart transplant. There were 26 deaths in patients with no MR (24% of patients with no MR at baseline), 45 deaths in the group with mild/moderate MR (11% of patients with mild/moderate MR at baseline), and 58 deaths in patients with advanced MR at baseline (21% of all patients from that group, log-rank P< 0.001). Kaplan–Meier survival curves are presented in Figure 2A.
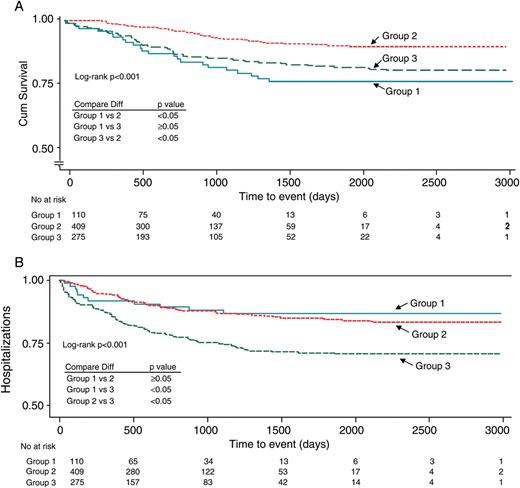
The Kaplan–Meier estimates of the time to (A) all-cause mortality and (B) heart failure hospitalization in patients with advanced mitral regurgitation as compared with patients presenting with no or mild/moderate mitral regurgitation at baseline. 1—patients with no mitral regurgitation at baseline, 2—patients with mild/moderate mitral regurgitation at baseline, 3—patients with moderate-to-severe/severe mitral regurgitation at baseline.
The all-cause mortality rate at 1 year was 10, 3, and 9%, respectively, in patients with no MR, mild/moderate MR, and advanced MR. The mean survival time was 668, 798, and 747 days in the respective groups. The cause of death was not known in many patients, thus precluding further subanalysis.
Out of the 794 patients, total of 131 patients had evidence about the HF hospitalization after CRT implantation (16%). A total of 11 (10%) patients without MR at baseline, 48 (12%) patients with mild/moderate MR, and 72 (26%) patients with advanced MR at baseline hospitalized for HF decompensation (log-rank P< 0.001). Kaplan–Meier survival curves are presented in Figure 2B.
The need of HF hospitalization at 1 year was 11, 8, and 25%, respectively, in patients with no MR, mild/moderate MR, and advanced MR at baseline. Days to hospitalization from the CRT implant date were 360 ± 374 for no MR, 685 ± 580 for mild/moderate MR, and 478 ± 450 for the advanced MR group (P< 0.001).
Discussion
Main findings
It is known that the clinical characteristics of patients treated in daily clinical practice, where the strict exclusion criteria of clinical trials are not applied, may be quite different from the profile of patients enrolled in randomized clinical trials. Our study focused on patients receiving CRT in ‘real world’ clinical practice. According to our findings, functional MR is frequent in CRT population. Although mild/moderate MR predominates, moderate–severe or severe MR occurs in 35% of CRT recipients. Patients with advanced MR are more symptomatic and tend to have more dilated and dysfunctional ventricles. Despite that, both CRT-induced clinical and echocardiographic improvement occurs in patients with severe MR. Severity of MR at baseline and degree of MR change at 3-month post-CRT follow-up, were found to be highly predictive of CRT response at 12 months. Ypenburg et al.17 reported that the extent of LV reverse remodelling at mid-term follow-up was a strong predictor of long-term outcome. Cabrera-Bueno et al.18 have examined the role of baseline MR in predicting long-term response to CRT, and they found advanced MR at baseline to be associated with non-response to CRT. However, our data showed that patients with severe MR could have more pronounced improvement after CRT. This finding is important because if this kind of response to the CRT continued in this subgroup of patients, they might be waived from mitral-valve surgery.12–14
In the general population of patients with systolic dysfunction, MR was detected in 45–75%.1,2,19 Higher prevalence of MR reported in our study is likely attributed to the severity of HF in candidates for CRT. With regard to advanced MR, some studies indicate that moderate/severe MR occurs in 12.5% and severe MR in 4.3% of patients with systolic HF.20 This observation corresponds well with our observation of 13.8 and 6.1%, respectively, in CRT patients.
This study further showed that MR improvement of ≥1° after 1 year of CRT may be expected in 45% of CRT recipients with MR at baseline. Moreover, MR improvement is relatively more frequent in patients with advanced MR at baseline. The extent of both clinical and echocardiographic improvement is greater in patients with improved MR after 12 months. In addition, the degree of post-CRT MR reduction at 3 months was identified as independent predictor of response to CRT at 12 months.
In our study population, level of baseline MR had strong association with response to CRT at follow-up. Patients with baseline MR had a worse prognosis with poorer response to CRT compared with those without MR. Our findings further indicate that extent of LV reverse remodelling varies significantly among patients with various levels of baseline MR, and patients with advanced MR had less LV reverse remodelling resulting in higher hospitalization due to HF.
Our results suggest that at 3-month follow-up following CRT, patients responding to the therapy most likely resolve their MR and do not require any further intervention. In this respect, a different timing to surgical mitral-valve replacement in this group of patients might be proposed.
General considerations
Functional MR results from multiple factors: decreased contractility, ventricular remodelling, impairment of mitral annular function, and ventricular dyssynchrony.3,21 Additionally, MR tends to progress over time 22 due to the chronic volume overload.
The vicious circle between cardiac remodelling and MR may be potentially interrupted by either reverse remodelling or decrease in MR severity. Pharmacotherapy is able to influence both targets due to impact on transmitral pressure, tethering forces, and left-ventricular geometry. However, once HF and MR become refractory to pharmacotherapy, the prognosis is poor.4,23 Since 1995,24 it was repeatedly reported that mitral-valve surgery is feasible in advanced HF and associated with improved quality of life, NYHA class, increased LVEF, improved survival, and less frequent hospital admission for HF.25,26 There is growing evidence that functional MR might be reversed or improved by CRT.6–10
Cardiac resynchronization therapy reduces MR by multiple mechanisms: acutely by resynchronization of the papillary muscles leading to a shortening of MR duration and later onset of MR,10 by augmentation of transmitral pressure gradient due to increased contractility 9 and also by modification of mitral-annulus contraction.11 Those effects are pacing dependent as the interruption of CRT causes an immediate recurrence of MR.27 Additionally, correction of atrioventricular delay in CRT eliminates pre-systolic MR when present.28 MR reduction could also be related to a resynchronization-related reverse remodelling that requires weeks/months to occur.6,7 We have shown that CRT-related MR improvement is present in one-third of CRT recipients.
Until now, it is still not well established who will benefit from CRT ‘only’ rather than from mitral-valve surgery. However, since a significant number of the patients in our series experienced improvement of their MR after CRT implant, corrective surgery could be delayed.
This observation may suggest that CRT-induced MR improvement is dependent on the presence/reduction of ventricular dyssynchrony. In fact, some studies support this hypothesis.10,11,29 Resynchronized activation of the papillary muscles and adjacent walls ensuring shortening of MR duration and later onset of MR10,11,30 is intuitively a logical explanation of MR improvement. However, our ability to quantify mechanical dyssynchrony and predict response to CRT is still limited.31 Therefore, QRS width is still used as a rough marker of ventricular asynchrony and resynchronization. In this context, an association between functional MR and baseline QRS duration was observed, particularly in patients with left bundle-branch block.32 We found only a small amount of significant association between them in our study; which may be related to the fact that QRS duration reflects only temporal but not spatiotemporal asynchrony.33 In our study, QRS narrowing was not a predictor for MR improvement at 12 months.
The other factor that could limit our ability to identify predictors of CRT-related MR improvement is the dynamic nature of functional MR. There are studies showing that exercise-induced changes of MR do not correlate with the severity of MR at rest, but with the presence/severity of ventricular dyssynchrony at baseline.34–36 As a result, some patients with mild MR at rest may develop MR worsening during exercise, thus potentially affecting the degree of reverse remodelling. Moreover, exercise-induced MR deterioration seems to be associated with a higher risk of cardiac event.37 Interestingly, some studies indicate that CRT-related changes of MR are time dependent. Particularly, that acutely, CRT reduces both MR and ventricular dyssynchrony at rest, whereas later, CRT attenuates also exercise-induced worsening of MR and ventricular dyssynchrony.38
Study limitations
Retrospective design is subject to the limitations related to a retrospective data analysis. However, all data were collected prospectively in systematic databases.
We decided to use combined definition of CRT response to distinguish patients with improvement of both symptoms and systolic function. Although modified biplane Simpson method was used for quantification of LVEF, values of left-ventricular volumes, although measured, were not recorded consistently, thus precluding their use. In this respect, increase in LVEF of ≥6 points was shown to be a good predictor of long-term outcome.12
Our original effort was also to analyse the relationship between MR and its change after CRT on one site and mechanical ventricular dyssynchrony and its correction on the other site. However, these data were not available in all patients.
One could argue that we did not distinguish the presence of ischaemic MR that could be adequately managed by surgical revascularization rather than by CRT. However, patients with ischaemic cardiomyopathy underwent complex examination before CRT and they were considered for CRT only if they were not candidates for revascularization. In addition, observation that MR reduction during CRT was similar in patients with coronary artery disease as compared with patients with idiopathic dilated cardiomyopathy suggests that the ischaemic component of MR did not modify the results significantly. Of note, this is consistent with other reports.39
The mitral insufficiency grade was subjectively assessed through quantitative and semiquantitative measurements and core lab analysis was not applied for echocardiography measures. However, highly experienced echocardiographists performed the echocardiographic evaluation at each centre and the study reflects the daily clinical practice. In addition, we calculated an intra-class correlation coefficient (ICC) to validate the within/between observer agreement in the classification of mitral-valve insufficiency. In a subset of 476 patients studied, an excellent intra-observer and inter-observer agreement (ICC 0.84 and 0.88, respectively) were observed.
Finally, the study did not analyse the morphology and the structure of the mitral valve, thus not allowing any conclusion on the mechanism by which CRT affects MR.
Conclusions
In conclusion, functional MR affects 90% of CRT candidates, with moderate–severe/severe MR being present in 35%.
CRT-induced MR improvement ≥1° may be expected in ∼30% and it is relatively more frequent in patients with advanced MR at baseline.
Clinical improvement and reverse remodelling occur also in patients with moderate–severe/severe MR. Patients with higher baseline MR severity are more likely to present with MR improvement.
Mitral regurgitation improvement at 3-month follow-up predicts CRT response and MR improvement at 12-month follow-up.
Further investigation is needed to identify other factors predicting MR reduction after CRT since it could change the timing for MR surgery.
Conflict of interest: A.N. received honoraria from Medtronic, Boston Scientific, St Jude Medical, Biotronik. A.N. is a consultant for Biosense Webster. J.D.B. is a consultant for Stereotaxis. J.K. received honoraria and a research grant from Biosense Webster, Biotronik, St Jude Medical, Medtronic and Boston Scientific. A.A. is a consultant for Biotronik, St Jude Medical, EBR, Medtronic, Biotronik, Merck and Philips.



